Abstract
It has long been recognized that generalized deficits in cognitive ability represent a core component of schizophrenia, evident prior to full illness onset and independent of medication. The possibility of genetic overlap between risk for schizophrenia and cognitive phenotypes has been suggested by the presence of cognitive deficits in first-degree relatives of patients with schizophrenia; however, until recently, molecular genetic approaches to test this overlap have been lacking. Within the last few years, large-scale genome-wide association studies (GWAS) of schizophrenia have demonstrated that a substantial proportion of the heritability of the disorder is explained by a polygenic component consisting of many common SNPs of extremely small effect. Similar results have been reported in GWAS of general cognitive ability. The primary aim of the present study is to provide the first molecular genetic test of the classic endophenotype hypothesis, which states that alleles associated with reduced cognitive ability should also serve to increase risk for schizophrenia. We tested the endophenotype hypothesis by applying polygenic SNP scores derived from a large-scale cognitive GWAS meta-analysis (~5000 individuals from 9 non-clinical cohorts comprising the COGENT consortium) to four schizophrenia case-control cohorts. As predicted, cases had significantly lower cognitive polygenic scores compared to controls. In parallel, polygenic risk scores for schizophrenia were associated with lower general cognitive ability. Additionally, using our large cognitive meta-analytic dataset, we identified nominally significant cognitive associations for several SNPs that have previously been robustly associated with schizophrenia susceptibility. Results provide molecular confirmation of the genetic overlap between schizophrenia and general cognitive ability, and may provide additional insight into pathophysiology of the disorder.
Keywords: GWAS, schizophrenia, general cognitive ability, polygenic, endophenotype
INTRODUCTION
Schizophrenia (SCZ) is a brain disorder characterized by, on average, a reduction in general cognitive abilities of >1 SD below the population mean. Given the long-standing observation of subtle cognitive abnormalities in undiagnosed and unmedicated relatives of patients with SCZ, it has been suggested that cognitive deficits may serve as an endophenotype, permitting identification of SCZ risk genes using a quantitative phenotype more closely reflecting gene function.1 Extensive family and twin data support the role of shared additive genetic factors underpinning both SCZ and cognitive deficits.2 A recent population-scale study of siblings and twins further suggested that the overlap between these phenotypes is largely genetic, but questioned the overall magnitude of the phenotypic correlation.3 However, these family-based studies have two primary limitations: 1) they lack direct molecular assays of genetic variance, and 2) they have relied upon measurement of cognitive abilities in patients with SCZ, which are subject to potential confounds relating to illness process and state.
A direct test of the endophenotype hypothesis would examine molecular genetic variants associated with cognitive performance in the general (not clinically-referred) population, in order to see if these variants are also associated with SCZ. However, this approach has not been adequately tested due to the lack of strongly replicated cognition-associated single nucleotide polymorphisms (SNPs). By contrast, a number of candidate gene studies have applied a “reverse endophenotype” approach, in which SCZ risk variants are tested for association with cognition in the general population.4,5
Large-scale genome-wide association studies (GWAS) of schizophrenia have demonstrated that a substantial proportion of the heritability of the disorder is explained by a polygenic component consisting of thousands of common SNPs of extremely small effect.6 Similarly, recent GWAS of general cognitive ability have indicated that a polygenic architecture accounts for a majority of the heritability, consistent with other normally-distributed traits in the general population, such as height.7–9 The relationship of the underlying genetic architecture between two phenotypes can be examined using polygenic score tests, in which large numbers of alleles demonstrating subtle (not statistically significant) levels of association with a given phenotype are combined to produce a genetic risk profile.10 The association of these alleles in a different cohort (measured on a different phenotype), can then be utilized to estimate the degree of molecular overlap between phenotypes.
Very recently, two reverse endophenotype studies have examined the ability of schizophrenia polygenic risk scores to predict cognitive abilities in independent cohorts.11,12 Both studies draw from the work of the Psychiatric GWAS Consortium on Schizophrenia (PGC-SCZ), a large-scale mega-analytic GWAS of 17 Caucasian cohorts encompassing >9000 SCZ cases and >12,000 controls.13 In one study12, polygenic risk scores for SCZ were significantly associated with IQ in a cohort of patients with SCZ and controls, but these associations were not observed in the smaller (n=322) healthy cohort alone. However, in a much larger study11, polygenic risk scores for SCZ were found to be significantly associated with general cognitive ability (as assessed by the first principal component of cognitive tests assessing multiple domains) in two moderately-sized (total N~1500) cohorts of psychiatrically normal older adults.
In this study, we sought to test the endophenotype hypothesis by comparing SCZ cases to controls on polygenic SNP scores derived from a large-scale meta-analysis of cognitive GWAS. We focused on general cognitive ability as the primary endophenotype for several reasons, which we have discussed in detail previously.14 While a variety of specific cognitive traits have been proposed as SCZ endophenotypes, research to date has failed to conclusively demonstrate any specific cognitive signature of schizophrenia.15 Overall, deficits in general cognitive ability follow the pattern required of an endophenotype1: they are observed in SCZ patients prior to illness onset, are largely independent of clinical state and medication status, and are familial in nature.16,17
It has been recognized for more than 100 years that most cognitive abilities in humans are not orthogonal traits, but tend to covary; the underlying factor accounting for this pattern of intercorrelation is termed general cognitive ability (or Spearman’s g).18 General cognitive ability is a robust phenotype, accounting for nearly half of the variance on the broad range of specific neurocognitive traits identifiable in humans.19 Moreover, general cognitive ability in the population is itself highly heritable,9,20,21 and heritability studies have demonstrated substantial genetic overlap across most specific cognitive domains tested.18,19,21 Crucially, general cognitive ability can be reliably estimated by extracting the first principal component of any appropriately diverse set of neurocognitive test scores, regardless of the specific components of the battery; it has been empirically demonstrated that estimates of g derived from varying batteries tend to be very highly correlated, with correlation coefficients often approaching 1.22,23
The present study represents the first empirical report of an international collaborative effort entitled, “The Cognitive Genomics Consortium (COGENT).”14 COGENT aims to bring together human genetic datasets with both: 1) high-density genome-wide genotype data and 2) phenotype data on cognitive function in individuals drawn from the general population. At the time of the first data freeze, COGENT consists of nine sites across 7 countries, with approximately 5000 individuals with available genotype and phenotype data. Although genotyping platforms and phenotype measures vary by site, genetic imputation and factor analysis of cognitive scores were used to harmonize data across sites. Because generalized cognitive ability (g) can be robustly estimated from a variety of test batteries, we were able to perform meta-analysis of GWAS associations to g across the nine COGENT cohorts. From this meta-analysis, we derived polygenic allele scores associated with general cognitive ability. These allele scores were then applied to four SCZ case-control cohorts consisting of more than 11,000 independently ascertained subjects (>5000 cases and >5000 controls), as described in detail below. We additionally performed “reverse endophenotype” analyses, examining the effects of schizophrenia risk alleles (derived from PGC-SCZ) on cognitive scores in the 9 COGENT cohorts.
MATERIALS AND METHODS
Subjects – Cognitive GWAS cohorts
Volunteers for cognitive studies were drawn from nine cohorts, for which study investigators agreed to share data as part of the Cognitive Genomics Consortium (COGENT). Details on subject recruitment procedures for each cohort are described in the Supplementary Materials; summaries of each cohort are presented in Table 1. Although screening procedures differed somewhat across cohorts, subjects were drawn from the general population, either as epidemiologically representative cohorts or as recruited control cohorts for studies of schizophrenia and/or other mental illnesses. All subjects were of Caucasian descent (as confirmed by principal components analysis of genetic data). All subjects provided written, informed consent to protocols approved by their institutional ethics boards in accordance with the Helsinki declaration.
Table 1.
Description of COGENT cohorts.
| Dataseta | Genotyping Platform |
Concordanceb | Genotypes after QC |
N | %Male | Mean Age (SD) | Lambdac |
|---|---|---|---|---|---|---|---|
| Germany | Illumina OE d | 99.50% | 1,078,289 | 594 | 51% | 54.0 (15.0) | 1.01 |
| LOGOS | Illumina OE | 99.40% | 835,287 | 802 | 100% | 22.3 (3.8) | 1.03 |
| IBG | Affymetrix 6.0 | 99.59% | 938,800 | 299 | 77% | 15.9 (1.5) | 1.00 |
| LBC1936 | Illumina 610 | 99.60% | 1,058,722 | 1005 | 51% | 69.5 (0.8) | 1.01 |
| TOP | Affymetrix 6.0 | 94.23% | 917,315 | 351 | 48% | 34.2 (9.8) | 1.01 |
| NCNG | Illumina 610 | 99.40% | 944,135 | 629 | 32% | 47.6 (18.3) | 1.00 |
| Manchester | Illumina 610 | 99.60% | 1,059,916 | 697 | 30% | 67.7 (2.8) | 1.01 |
| HBCS | Illumina 610 | 99.60% | 1,043,380 | 318 | 100% | 67.7 (2.3) | 1.00 |
| ZHH | Illumina OE | 99.40% | 1,043,785 | 201 | 47% | 39.1 (1.8) | 1.06 |
Detailed descriptions of each cohort provided in Supplementary Text.
Concordance between imputed and genotyped SNPs.
Lambda to refers λGC,, a measure of the degree of statistical inflation in genomewide association studies.
OE refers to the Illumina OmniExpress genotyping bead chip
Subjects – Schizophrenia case-control cohorts
The primary test of the endophenotype hypothesis was performed in the Molecular Genetics of Schizophrenia (MGS) European-American case-control cohort. This dataset was selected for several reasons: it is large (n>5000), publicly available, has been extensively studied,24–26 and contains an ethnic distribution that is comparable to our nine COGENT cohorts (primarily Northern European in ancestry but with a non-negligible Southern European component as well). To replicate and extend our findings, we secondarily tested three additional SCZ case-control cohorts of varying ethnicities: 1) A Japanese cohort with >1000 subjects;27 2) An Ashkenazi Jewish cohort with >2500 subjects;28 and the African-American subcohort (n>2000) of the MGS sample.25 Demographic details of these cohorts are presented in Table 2. It should be noted that increasing evidence suggests substantial common architecture of complex traits (including schizophrenia) across populations,29,30 but it would still be anticipated that replication samples would demonstrate attenuated effect sizes due to residual differences in allele frequencies and effect sizes.31
Table 2.
Description of schizophrenia case-control cohorts.
| SCZ Dataset | N Cases | N Controls | GWAS platform |
|---|---|---|---|
| MGS European-American Shi et al. 2009 | 2681 | 2653 | Affymetrix 6.0 |
| Japan Ikeda et al. 2011 | 575 | 564 | Affymetrix 5.0 |
| Ashkenazi Jewish (Israel) Guha et al. 2013 | 904 | 1640 | Illumina Omni1-Quad |
| MGS African-American Shi et al. 2009 | 1286 | 973 | Affymetrix 6.0 |
| Total | 5446 | 5830 |
Genotyping, quality control, and imputation
As described in detail in the Supplementary Materials, all COGENT subjects were genotyped on one of three microarray platforms: Affymetrix 6.0 (~900K SNPs), Illumina 610K, or Illumina OmniExpress (~770K SNPs). A standardized quality control pipeline was applied to each COGENT GWAS dataset: SNP call rate > 95%; sample call rate > 90%; SNP Hardy-Weinberg equilibrium (HWE) p>10−6; and X chromosome sex match with reported gender. For any pair of subjects with cryptic relatedness (pi-hat>.125 in PLINK32 1.07), the sample with the lower call rate was eliminated. For each dataset, a principal components analysis was performed (in SVS 7.7.4, GoldenHelix Inc., Bozeman, MT), and samples demonstrating non- Caucasian ancestry were eliminated.
After QC, all SNPs within a given cohort were strand-aligned to HapMap3 and phased using SHAPEIT33 prior to imputation with IMPUTE2.34 As recently recommended to increase imputation accuracy,35 a large, cosmopolitan HapMap3 reference panel (n=1,011 individuals from Africa, Asia, Europe, and the Americas) was utilized (except for NCNG, which was the only dataset for which imputation was not performed centrally). Because our phenotype is a quantitative trait, we sought to avoid potentially spurious findings introduced by random association of rare alleles with a few extreme scores.36 Therefore, imputed SNPs receiving a probability call >.90 were retained and converted to PLINK-format genotype calls. The imputed data were then recleaned using the same call rate and HWE criteria described above; additionally, SNPs with minor allele frequency <2.5% were dropped. For each cohort, ~3000 randomly selected genotyped SNPs were held out for concordance analysis with imputation results. As shown in Table 1, concordance exceeded 99% for seven COGENT cohorts, and ~1M SNPs were available for analysis in each cohort.
Neurocognitive assessment
Details of neurocognitive batteries for each cohort are provided in the Supplementary Materials. While the specific instruments varied across cohorts, each cohort was required to have test scores available across at least 3 domains of cognitive ability for computation of Spearman’s g,18,37 an estimate of general cognitive ability derived from principal components analysis (PCA).22,23 (For one cohort, a validated estimate of general cognitive ability derived from two subscales of the Wechsler Adult Intelligence Scale was utilized.) For each of the cohorts, available measures were entered into PCA and the first unrotated component was extracted. Any variable with more than 5% of missing data was dropped from the analysis. Normality is not a strict requirement of principal components analysis implemented for the purpose of data reduction and so no variable was subject to transformation.38 Moreover, inspection of box plots indicated that variables were generally normally distributed and no noticeable outliers were observed. In each cohort, as expected based on hundreds of prior studies,39 the first principal component significantly loaded all measures and accounted for ~40% of the variance on average.
The dependent measure for the cognitive GWAS in each cohort was this first PC score, corrected for the following (using linear regression prior to GWAS): age, sex, age*sex, age2, and age2*sex, based on consistent evidence demonstrating the presence of both linear and quadratic effects of age on general cognitive ability across the lifespan40.
Statistical analysis
Genome-wide association analysis of the quantitative cognitive phenotype was performed in each COGENT cohort using linear regression (additive model) in SVS7.7.4. As shown in Table 1, lambda (genomic control) values for each cohort were at or near 1, indicating no significant effect of subtle population structure on association results. Fixed effects meta-analysis of β weights from the linear regression analyses was performed in PLINK 1.0732 using data from all available cohorts possessing high-QC genotyped or imputed data for each given SNP. Only SNPs with data available in 3 or more cohorts were retained. By convention, a positive β weight for a given allele indicated an additive (allele-dose) relationship in the direction of higher cognitive phenotype scores.
Based on results of the meta-analysis, polygenic scores were computed in PLINK using β weights of alleles at varying statistical thresholds (nominal p<.10, .20, .30, .40, and .50) following the procedure originally described by Purcell et al.10 For each statistical threshold, the clump procedure in PLINK was utilized to prune the set of SNPs for linkage disequilibrium (using r2 threshold of .50 within a 250kb window), so as to avoid redundancy of SNPs representing a given association signal.
For each of the five statistical thresholds, the weighted allele scores of each “clumped” SNP were summed for each subject in each SCZ case-control cohort, thus creating a “cognitive polygene score.” Thus, each subject in each SCZ case-control dataset had a set of 5 cognitive polygene scores: one for each of the statistical thresholds applied to the original COGENT meta-analytic results. For each SCZ case-control dataset, five logistic regression analyses were then used to compare cases and controls on cognitive polygene scores at each threshold. Nagelkerkes’ pseudoR2 was utilized to reflect estimated percent variance in the SCZ phenotype accounted for by cognitive polygene scores at each threshold.10
RESULTS
GWAS results for general cognitive ability
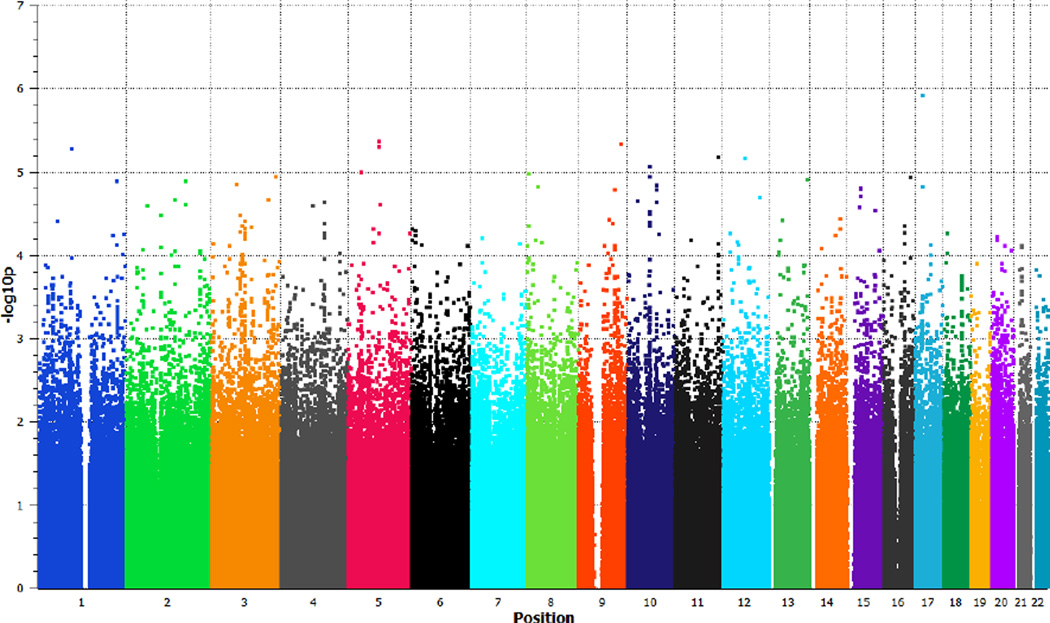
Meta-analytic results of the nine cognitive GWAS cohorts are presented as a Manhattan plot in Figure 1; no SNPs reached genomewide significance, and the overall lambda was 1.031 (see QQ plot in Supplementary Figure 1). This lambda value is higher than that for any individual cohort (Table 1), indicative of polygenic signal41, but is lower than reported in similar recent studies of cognitive ability7,9. A list of top SNPs (p<.001) emerging from the meta-analysis are presented in Supplementary Table 1.
Figure 1.
Manhattan plot depicting results of COGENT meta-analysis.
Primary test of endophenotype hypothesis – cognitive polygenic score analysis
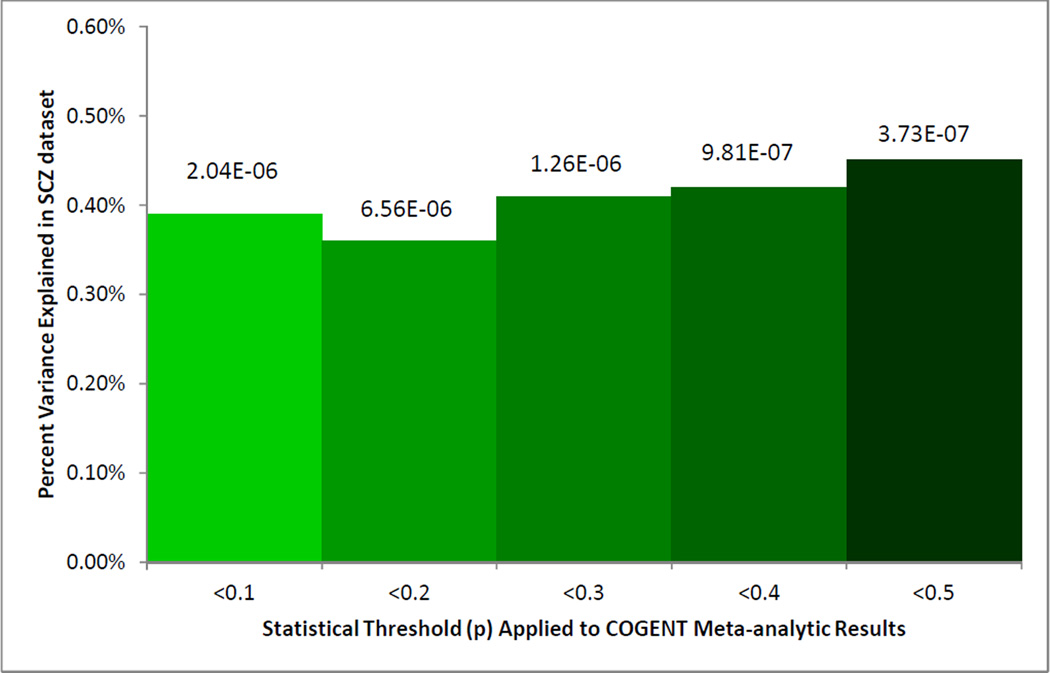
We utilized a large, publicly available, Caucasian SCZ GWAS case-control cohort to test our primary endophenotype hypothesis. Using “clumped” SNPs at 5 different thresholds (nominal p<.10, .20, .30, .40, and .50), polygenic cognitive scores were computed for each of 2886 cases and 2056 controls in the MGS/GAIN European-American cohort. As predicted, cases had significantly lower cognitive polygenic scores across each of the five thresholds (p-values ranging from 6.56*10−6 to 3.73*10−7; see Figure 2). In other words, SCZ cases had fewer alleles associated with good cognitive performance and more alleles associated with poorer cognitive performance in the COGENT meta-analysis. The overall magnitude of the polygenic effect was small (R2<.01), but comparable to the total variance explained by a similar approach applied from one cognitive cohort to another7 and from a reverse endophenotype study.11
Figure 2.
Polygenic overlap between cognitive allele scores (derived from COGENT metaanalysis thresholded at varying p-values) and schizophrenia (SCZ) case-control status in the MGS European-American cohort.
As replication, the same cognitive SNPs were tested in three smaller SCZ case-control cohorts of differing ethnicities. Because of the apparent dip in variance explained that appears at p<0.2 in the MGS EA cohort (Figure 1), we applied a p<0.3 threshold in our replication datasets. As depicted in Table 3, nominally significant results were observed in two cohorts (Japanese and Ashkenazi Jewish). Meta-analysis of these results across the four SCZ case-control cohorts yielded a strongly significant effect of cognitive polygenic scores on prediction of schizophrenia risk (p=3.6*10−7). Very similar results were observed using a clump threshold of p<0.5 (Supplementary Table 2; meta-analytic p=3.8*10−7), and somewhat stronger results were observed when the IBG cohort (the only non-adult COGENT cohort) was removed prior to calculation of polygenic allele weights (Supplementary Table 3; meta-analytic p=4.0*10−9).
Table 3.
Polygenic overlap between cognitive alleles (derived from COGENT meta-analysis using p<0.3 threshold) and schizophrenia in four case-control cohorts.
| SCZ Dataset | # overlapping SNPs | R2 for SCZ | p value | direction |
|---|---|---|---|---|
| MGS European-American | 17,237 | 0.41% | 1.3*10−6 | negative |
| Japan | 6,468 | 0.38% | 0.039 | negative |
| Ashkenazi Jewish | 15,151 | 0.16% | 0.041 | negative |
| MGS African-American | 17,382 | 0.00% | 0.958 | positive |
| Meta-analysis | 3.6*10−7 | negative |
Reverse endophenotype approach – examination of prior schizophrenia GWAS hits
As an additional examination of the relationship between cognitive GWAS results and the schizophrenia phenotype, we applied the commonly-employed “reverse endophenotype” approach described above. First, we selected all SNPs that have demonstrated genomewide significance (p<5*10−8) in large-scale (total n>5000) published SCZ GWAS obtained from the NHGRI GWAS catalog (http://www.genome.gov/gwastudies/, downloaded Jan. 10, 2013). As shown in Table 4, four of the 13 independent SNPs (excluding the major histocompatibility complex) associated with schizophrenia have nominally significant (p<.05) associations with general cognitive ability in volunteers from the general population. While effect sizes for these SNPs on cognitive phenotypes are extremely modest, the number of SNPs achieving nominal significance exceeded that expected by chance (binomial test, p=.006, two-tailed). Although extensive linkage disequilibrium in the MHC has prohibited clear discernment of the source of SCZ GWAS signal, supplementary Table 4 demonstrates results for 5 SNPs in the region derived from published SCZ GWAS.
Table 4.
Examination of cognitive associations (in COGENT meta-analysis) for SNPs identified in published GWAS for schizophrenia (excluding MHC).
| Source | SNP | Region | Gene | Risk Allele Freqa |
OR_Sza | COGENT studies |
P_COGENT meta-analysis |
|---|---|---|---|---|---|---|---|
| Ripke (PGC) Nat Gen 2011 | rs1625579 | 1p21.3 | MIR137 | 0.8 | 1.12 | 8 | 0.4789 |
| Shi (China) Nat Gen 2011 | rs10489202 | 1q24.2 | MPC2 | 0.141 | 1.23 | 9 | 0.9024 |
| Ripke (PGC) Nat Gen 2011 | rs6703335 | 1q43 | SDCCAG8 | 0.56 | 1.09 | 6 | 0.9127 |
| O’Donovan Nat Gen 2011 | rs1344706 | 2q32.1 | ZNF804A | 0.59 | 1.12 | 9 | 0.4048 |
| Ripke (PGC) Nat Gen 2011 | rs17662626 | 2q32.3 | PCGEM1 | 0.91 | 1.2 | 5 | 0.4509 |
| Bergen (Swe) Mol Psy 2012 | rs7709645 | 5q12.1 | ZSWIM6 | 0.475 | 1.11 | 9 | 0.7207 |
| Bergen (Swe) Mol Psy 2012b | rs12666575 | 7p22.3 | MAD1L1 | 0.673 | 1.12 | 8 | 0.0320 |
| Ripke (PGC) Nat Gen 2011 | rs10503253 | 8p23.2 | CSMD1 | 0.19 | 1.16 | 7 | 0.5874 |
| Shi (China) Nat Gen 2011c | rs16887244 | 8p11.23 | LSM1 | 0.683 | 1.19 | 9 | 0.0171 |
| Ripke (PGC) Nat Gen 2011b | rs7914558 | 10q24.32 | CNNM2 | 0.59 | 1.22 | 9 | 0.0368 |
| Ripke (PGC) Nat Gen 2011 | rs11191580 | 10q24.33 | NT5C2 | 0.91 | 1.2 | 9 | 0.6575 |
| Decode Nature 2009c | rs12807809 | 11q24.2 | NRGN | 0.83 | 1.15 | 8 | 0.0399 |
| Ripke (PGC) Nat Gen 2011 | rs12966547 | 18q21.2 | TCF4 | 0.58 | 1.4 | 8 | 0.7327 |
based on source publication
schizophrenia risk allele associated with lower cognitive ability
schizophrenia risk allele associated with higher cognitive ability
Reverse endophenotype approach – schizophrenia susceptibility polygenic score analysis
Finally, we performed a polygenic score analysis, comparable to the one presented in Table 2, but in the “reverse” direction. Specifically, we downloaded “clumped” SNPs derived from the publicly-available PGC13 dataset (https://pgc.unc.edu/Sharing.php#SharingOpp). Because polygenic score approaches to schizophrenia have tended to demonstrate increasing variance explained at higher p-value thresholds10, we utilized a threshold of p<.50 on the resulting clumped SNPs. These polygenic scores were then computed for each subject in each of the 9 COGENT cohorts separately; scores were then compared to cognitive ability (g) using linear regression in each cohort. As shown in Table 5, schizophrenia-derived polygenic scores were correlated with cognitive ability in the predicted direction (greater schizophrenia load associated with lower cognitive scores) in 7 of the 9 cohorts, with three cohorts demonstrating nominally significant (or nearly so) results. As with the primary analysis, total variance accounted for was small (R2 range from 0–2%) but strongly significant (p=1.4*10−4) in the combined analysis.
Table 5.
Polygenic overlap between schizophrenia risk alleles (derived from PGC meta-analysis using p<0.5 threshold) and general cognitive ability in nine COGENT cohorts.
| COGENT Dataset | # overlapping SNPs |
R2 for g | p value | Direction |
|---|---|---|---|---|
| Germany | 89,360 | 0.08% | 0.475 | Positive |
| LOGOS | 69,069 | 0.00% | 0.985 | Positive |
| IBG | 89,353 | 0.04% | 0.72 | Negative |
| LBC1936 | 96,820 | 1.17% | 0.0006 | Negative |
| TOP | 88,946 | 0.10% | 0.5481 | Negative |
| NCNG | 87,934 | 0.04% | 0.5925 | Negative |
| Manchester | 96,907 | 1.61% | 0.0007 | Negative |
| HBSC | 93,890 | 0.11% | 0.5467 | Negative |
| ZHH | 85,681 | 1.90% | 0.0532 | Negative |
| Meta-analysis | 1.4*10−4 | negative |
DISCUSSION
To our knowledge, this is the first study to present molecular genetic evidence supporting general cognitive ability as a true endophenotype for schizophrenia susceptibility. In a large Caucasian SCZ case-control cohort, we demonstrated that a set of polygenic alleles associated with lower general cognitive ability strongly predicted increased likelihood for the disorder. Consistent, though less strong, associations were observed in additional cohorts, despite the fact that they were drawn from populations of differing ethnicities.
While the statistical evidence (p-values ranging from ~10−6 to ~10−9) for association was strong, the overall amount of variance explained, even in the Caucasian SCZ cohort (~0.5%) was modest, a result that must be interpreted in the context of other studies of polygenic overlap. For example, in a recent study of cognition,7 polygenic SNP scores accounted for only ~1% of the variance in a separate cognitive cohort (these cohorts are now included in COGENT). Nevertheless, these authors were able to demonstrate that nearly half of the variation in cognitive ability could ultimately be accounted for by common SNPs. Similarly, the initial study of polygenic effects in schizophrenia identified only ~2–3% overlap between any two schizophrenia cohorts, and ~1–2% overlap between schizophrenia and bipolar cohorts.10 Again, extensive simulations demonstrated that the polygenic SNPs tagged common genetic variation accounting for approximately one-third of the total variance in SCZ risk; this estimate has been replicated, indicating that the empirically observed cross-sample allelic overlap is a substantial under-estimate of the total polygenic effect.6 Moreover, observed polygenic overlap tends to rapidly increase as a function of sample size; for example, the empirically observed variance explained by polygenic effects in schizophrenia has increased tenfold with larger sample sizes.42 While the COGENT cohort represents the largest genetic sample of cognition to date, sample size remains small relative to polygenic studies of other quantitative traits such as height.43,44
Thus, the results of the present study are consistent with a model in which a substantial fraction of the molecular basis of general cognitive ability is shared with genetic risk for schizophrenia. These results are consistent with a large body of evidence from family-based studies which use twin and sibling correlations to model genetic effects.2 While a recent population-based study using similar modeling strategies3 found only limited phenotypic overlap between intelligence and psychosis, the source of this overlap was estimated to be largely (~90%) comprised of additive genetic variation. The present study is unique in directly testing molecular genetic variation, and utilizing non-clinical volunteers for the estimation of the cognitive genetic component. Our demonstration of parallel effects when examining genomewide overlap in the reverse direction (SCZ risk alleles predicting cognitive scores, Table 5) add further confidence to our conclusions, and are also consistent with a recent genomewide reverse endophenotype study, which demonstrated significant overlap between polygenic SCZ risk alleles and cognitive decline in two aging cohorts.11
Additionally, following the conventional “reverse endophenotype” approach, multiple GWAS-identified SCZ risk SNPs (4/13, or 31%) demonstrated nominal evidence of association to general cognitive ability in a large meta-analytic cohort of non-clinical volunteers. Three of these are intronic SNPs, in MAD1LI, LSM1, and CNNM2, and the present study represents the first report of human neurocognitive correlates of variants at these loci. Little is known about the functions of these genes in the central nervous system, and the structural and functional properties of their associated proteins vary widely. The fourth nominally significant locus is less than 5kb 5’ to NRGN (and is in an LD block encompassing the gene). NRGN encodes neurogranin, a well-characterized postsynaptic protein which binds to calmodulin and thereby modulates postsynaptic calcium signaling.45 Although this locus did not show significant association with cognitive variables in a smaller prior study,46 neuroimaging studies have previously associated this locus with structural and functional variation in the frontal cortex, cingulate, and hippocampus.47–50 Although these results were significant in the aggregate (p=.006 by binomial test), it should be emphasized that the effect sizes for individual SNPs were extremely small, and p-values would not survive Bonferroni correction.
Based on the present results, it is likely that larger sample sizes will afford greater ability to predict schizophrenia using cognitive polygenic scores. This overlap can then be utilized to enhance schizophrenia gene detection using recently developed “pleiotropic enrichment” techniques.51 At the same time, the study of the genetic basis of normal variation in cognition is likely to further our understanding of the mechanisms by which schizophrenia risk genes affect the central nervous system.
Several caveats should be placed on the interpretation of this study. First, the present study did not directly evaluate the genetic source of cognitive deficits in patients with schizophrenia. It could be argued that GWAS of cognitive ability in schizophrenia cohorts would be required to test whether this putative endophenotype actually mediates the relationship between cognitive polygene score and schizophrenia risk. However, cognitive performance in patients with schizophrenia can be influenced by potential confounds such as effects of medication or acute symptomatology, which would tend to attenuate any genetic signal. Consequently, our approach of utilizing non-clinical samples was designed to maximize the potential power of GWAS.
Finally, it should be noted that no genomewide significant loci for cognition were identified in the present study, despite being the largest GWAS of cognitive ability in predominately adult cohorts. This result was anticipated based on recent large-scale GWAS results for childhood intelligence9, as well as early GWAS (with comparable sample size to the present study) of potentially comparable quantitative traits, height52 and weight53. It is also possible that power to detect genetic signals was reduced due to unavoidable heterogeneity in cognitive assessment methods across cohorts; such an interpretation is consistent with the relatively low lambda observed in our study. Future studies, ideally with prospectively collected cohorts utilizing harmonized approaches to phenotype assessment, will be required to tease out genomewide significant loci for cognitive ability.
Supplementary Material
ACKNOWLEDGEMENTS
This work has been supported by grants from the National Institutes of Health (R01 MH079800 and P50 MH080173 to A.K.M; RC2 MH089964 to T.L.; R01 MH080912 to D.C.G.; K23 MH077807 to K.E.B.; K01 MH085812 to M.C.K.). Dr. Donohoe is generously funded by the Health Research Board (Ireland) and Science Foundation Ireland. Data collection for the TOP cohort was supported by the Research Council of Norway, South-East Norway Health Authority. The NCNG study was supported by Research Council of Norway Grants 154313/V50 and 177458/V50. The NCNG GWAS was financed by grants from the Bergen Research Foundation, the University of Bergen, the Research Council of Norway (FUGE, Psykisk Helse), Helse Vest RHF and Dr Einar Martens Fund. The Helsinki Birth Cohort Study has been supported by grants from the Academy of Finland, the Finnish Diabetes Research Society, Folkhälsan Research Foundation, Novo Nordisk Foundation, Finska Läkaresällskapet, Signe and Ane Gyllenberg Foundation, University of Helsinki, Ministry of Education, Ahokas Foundation, Emil Aaltonen Foundation. For the LBC cohort, phenotype collection was supported by Research Into Ageing (continues as part of Age UK The Disconnected Mind project). Genotyping was funded by the UK Biotechnology and Biological Sciences Research Council (BBSRC). The work was undertaken by The University of Edinburgh Centre for Cognitive Ageing and Cognitive Epidemiology, part of the cross council Lifelong Health and Wellbeing Initiative (G0700704/84698). Funding from the BBSRC, Engineering and Physical Sciences Research Council (EPSRC), Economic and Social Research Council (ESRC), and MRC is gratefully acknowledged. We are grateful to investigators, led by Pablo Gejman, who have made data for the MGS cohort publicly available through dbGAP (http://www.ncbi.nlm.nih.gov/projects/gap): /cgi-bin/study.cgi?study_id=phs000167.v1.p1; /cgi-bin/study.cgi?study_id=phs000021.v2.p1.
Footnotes
CONFLICT OF INTEREST
The authors report no conflict of interest related to the present work.
REFERENCES
- 1.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 2.Toulopoulou T, Goldberg TE, Mesa IR, Picchioni M, Rijsdijk F, Stahl D, et al. Impaired intellect and memory: a missing link between genetic risk and schizophrenia? Arch Gen Psychiatry. 2010;67:905–913. doi: 10.1001/archgenpsychiatry.2010.99. [DOI] [PubMed] [Google Scholar]
- 3.Fowler T, Zammit S, Owen MJ, Rasmussen F. A population-based study of shared genetic variation between premorbid IQ and psychosis among male twin pairs and sibling pairs from Sweden. Arch Gen Psychiatry. 2012;69:460–466. doi: 10.1001/archgenpsychiatry.2011.1370. [DOI] [PubMed] [Google Scholar]
- 4.Almasy L. The role of phenotype in gene discovery in the whole genome sequencing era. Hum Genet. 2012;131:1533–1540. doi: 10.1007/s00439-012-1191-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bilder RM, Howe A, Novak N, Sabb FW, Parker DS. The genetics of cognitive impairment in schizophrenia: a phenomic perspective. Trends Cogn Sci. 2011;15:428–435. doi: 10.1016/j.tics.2011.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lee SH, DeCandia TR, Ripke S, Yang J, et al. Schizophrenia Psychiatric Genome-Wide Association Study Consortium (PGC-SCZ), International Schizophrenia Consortium (ISC) Estimating the proportion of variation in susceptibility to schizophrenia captured by common SNPs. Nat Genet. 2012;44:247–250. doi: 10.1038/ng.1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Davies G, Tenesa A, Payton A, Yang J, Harris SE, Liewald D, et al. Genome-wide association studies establish that human intelligence is highly heritable and polygenic. Mol Psychiatry. 2011;16:996–1005. doi: 10.1038/mp.2011.85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Plomin R, Haworth CM, Meaburn EL, Price TS, Davis OS Wellcome Trust Case Control Consortium 2. Common DNA markers can account for more than half of the genetic influence on cognitive abilities. Psychol Sci. 2013;24:562–568. doi: 10.1177/0956797612457952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Benyamin B, Pourcain B, Davis OS, Davies G, Hansell NK, Brion MJ, et al. Childhood intelligence is heritable, highly polygenic and associated with FNBP1L. Mol Psychiatry. 2013 Jan 29; doi: 10.1038/mp.2012.184. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.International Schizophrenia Consortium. Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature. 2009;460:748–752. doi: 10.1038/nature08185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntosh AM, Gow A, Luciano M, Davies G, Liewald DC, Harris SE, et al. Polygenic risk for schizophrenia is associated with cognitive change between childhood and old age. Biol Psychiatry. 2013;73:938–943. doi: 10.1016/j.biopsych.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 12.Terwisscha van Scheltinga AF, Bakker SC, van Haren NE, Derks EM, Buizer-Voskamp JE, Cahn W, et al. Schizophrenia genetic variants are not associated with intelligence. Psychol Med. 2013 Feb 15;:1–8. doi: 10.1017/S0033291713000196. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schizophrenia Psychiatric Genome-Wide Association Study (GWAS) Consortium. Genome-wide association study identifies five new schizophrenia loci. Nat Genet. 2011;43:969–976. doi: 10.1038/ng.940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donohoe G, Deary IJ, Glahn DC, Malhotra AK, Burdick KE. Neurocognitive phenomics: examining the genetic basis of cognitive abilities. Psychol Med. 2012 Nov 30;:1–10. doi: 10.1017/S0033291712002656. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 15.Dickinson D, Ragland JD, Gold JM, Gur RC. General and specific cognitive deficits in schizophrenia: Goliath defeats David? Biol Psychiatry. 2008;64:823–827. doi: 10.1016/j.biopsych.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Keefe RS, Harvey PD. Cognitive impairment in schizophrenia. Handb Exp Pharmacol. 2012;213:11–37. doi: 10.1007/978-3-642-25758-2_2. [DOI] [PubMed] [Google Scholar]
- 17.Keshavan MS, Kulkarni S, Bhojraj T, Francis A, Diwadkar V, Montrose DM, Seidman LJ, Sweeney J. Premorbid cognitive deficits in young relatives of schizophrenia patients. Front Hum Neurosci. 2010;3:62. doi: 10.3389/neuro.09.062.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deary IJ, Penke L, Johnson W. The neuroscience of human intelligence differences. Nat Rev Neurosci. 2010;11:201–211. doi: 10.1038/nrn2793. [DOI] [PubMed] [Google Scholar]
- 19.Deary IJ, Johnson W, Houlihan LM. Genetic foundations of human intelligence. Hum Genet. 2009;126:215–232. doi: 10.1007/s00439-009-0655-4. [DOI] [PubMed] [Google Scholar]
- 20.Plomin R. The genetics of g in human and mouse. Nat Rev Neurosci. 2001;2:136–141. doi: 10.1038/35053584. [DOI] [PubMed] [Google Scholar]
- 21.Glahn DC, Almasy L, Blangero J, Burk GM, Estrada J, Peralta JM, et al. Adjudicating neurocognitive endophenotypes for schizophrenia. Am J Med Genet B Neuropsychiatr Genet. 2007;144B:242–249. doi: 10.1002/ajmg.b.30446. [DOI] [PubMed] [Google Scholar]
- 22.Johnson W, Bouchard TJ, Jr, Krueger RF, McGue M, Gottesman II. Just one g: Consistent results from three test batteries. Intelligence. 2004;34:95–107. [Google Scholar]
- 23.Johnson W, Nijenhuis JT, Bouchard TJ., Jr Still just 1 g: Consistent results from five test batteries. Intelligence. 2008;36:81–95. [Google Scholar]
- 24.Sanders AR, Duan J, Levinson DF, Shi J, He D, Hou C, et al. No significant association of 14 candidate genes with schizophrenia in a large European ancestry sample: implications for psychiatric genetics. Am J Psychiatry. 2008;165:497–506. doi: 10.1176/appi.ajp.2007.07101573. [DOI] [PubMed] [Google Scholar]
- 25.Shi J, Levinson DF, Duan J, Sanders AR, Zheng Y, Pe'er I, et al. Common variants on chromosome 6p22.1 are associated with schizophrenia. Nature. 2009;460:753–757. doi: 10.1038/nature08192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J, et al. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry. 2011;168:302–316. doi: 10.1176/appi.ajp.2010.10060876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ikeda M, Aleksic B, Kinoshita Y, Okochi T, Kawashima K, Kushima I, et al. Genome-wide association study of schizophrenia in a Japanese population. Biol Psychiatry. 2011;69:472–478. doi: 10.1016/j.biopsych.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 28.Guha S, Rees E, Darvasi A, Ivanov D, Ikeda M, Bergen SE, et al. Implication of a rare deletion at distal 16p11.2 in schizophrenia. JAMA Psychiatry. 2013;70:253–260. doi: 10.1001/2013.jamapsychiatry.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fu J, Festen EA, Wijmenga C. Multi-ethnic studies in complex traits. Hum Mol Genet. 2011;20:R206–R213. doi: 10.1093/hmg/ddr386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.de Candia TR, Lee SH, Yang J, Browning BL, Gejman PV, Levinson DF, et al. Additive genetic variation in schizophrenia risk is shared by populations of african and European descent. Am J Hum Genet. 2013;93:463–470. doi: 10.1016/j.ajhg.2013.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.N'Diaye A, Chen GK, Palmer CD, Ge B, Tayo B, Mathias RA, et al. Identification, replication, and fine-mapping of Loci associated with adult height in individuals of african ancestry. PLoS Genet. 2011;7:e1002298.s. doi: 10.1371/journal.pgen.1002298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Delaneau O, Marchini J, Zagury JF. A linear complexity phasing method for thousands of genomes. Nat Methods. 2011;9:179–181. doi: 10.1038/nmeth.1785. [DOI] [PubMed] [Google Scholar]
- 34.Howie B, Marchini J, Stephens M. Genotype imputation with thousands of genomes. G3 (Bethesda) 2011;1:457–470. doi: 10.1534/g3.111.001198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Howie B, Fuchsberger C, Stephens M, Marchini J, Abecasis GR. Fast and accurate genotype imputation in genome-wide association studies through pre-phasing. Nat Genet. 2012;44:955–959. doi: 10.1038/ng.2354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Montgomery SB, Lappalainen T, Gutierrez-Arcelus M, Dermitzakis ET. Rare and common regulatory variation in population-scale sequenced human genomes. PLoS Genet. 2011;7:e1002144. doi: 10.1371/journal.pgen.1002144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Spearman C. General intelligence objectively determined and measured. Am J Psychology. 1904;15:201–293. doi: 10.1037/0022-3514.86.1.96. [DOI] [PubMed] [Google Scholar]
- 38.Jackson JE. A User's Guide to Principal Components. Hoboken, NJ, USA: John Wiley & Sons, Inc.; 2006. [Google Scholar]
- 39.Carroll JB. Human Cognitive Abilities: A Survey of Factor-Analytic Studies. Cambridge, UK: Cambridge University Press; 1993. [Google Scholar]
- 40.Verhaeghen P, Salthouse TA. Meta-analyses of age-cognition relations in adulthood: estimates of linear and nonlinear age effects and structural models. Psychol Bull. 1997;122:231–249. doi: 10.1037/0033-2909.122.3.231. [DOI] [PubMed] [Google Scholar]
- 41.Yang J, Weedon MN, Purcell S, Lettre G, Estrada K, Willer CJ, et al. Genomic inflation factors under polygenic inheritance. Eur J Hum Genet. 2011;19:807–812. doi: 10.1038/ejhg.2011.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ripke S, O'Dushlaine C, Chambert K, Moran JL, Kähler AK, Akterin S, et al. Genome-wide association analysis identifies 13 new risk loci for schizophrenia. Nat Genet. 2013 Aug 25; doi: 10.1038/ng.2742. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yang J, Benyamin B, McEvoy BP, Gordon S, Henders AK, Nyholt DR, et al. Common SNPs explain a large proportion of the heritability for human height. Nat Genet. 2010;42:565–569. doi: 10.1038/ng.608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lango Allen H, Estrada K, Lettre G, Berndt SI, Weedon MN, Rivadeneira F, et al. Hundreds of variants clustered in genomic loci and biological pathways affect human height. Nature. 2010;467:832–838. doi: 10.1038/nature09410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Papaleo F, Lipska BK, Weinberger DR. Mouse models of genetic effects on cognition: relevance to schizophrenia. Neuropharmacology. 2012;62:1204–1220. doi: 10.1016/j.neuropharm.2011.04.025. [DOI] [PubMed] [Google Scholar]
- 46.Donohoe G, Walters J, Morris DW, Da Costa A, Rose E, Hargreaves A, et al. A neuropsychological investigation of the genome wide associated schizophrenia risk variant NRGN rs12807809. Schizophr Res. 2011;125:304–306. doi: 10.1016/j.schres.2010.10.019. [DOI] [PubMed] [Google Scholar]
- 47.Krug A, Krach S, Jansen A, Nieratschker V, Witt SH, Shah NJ, et al. The effect of neurogranin on neural correlates of episodic memory encoding and retrieval. Schizophr Bull. 2013;39:141–150. doi: 10.1093/schbul/sbr076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ohi K, Hashimoto R, Yasuda Y, Nemoto K, Ohnishi T, Fukumoto M, et al. Impact of the genome wide supported NRGN gene on anterior cingulate morphology in schizophrenia. PLoS One. 2012;7:e29780. doi: 10.1371/journal.pone.0029780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rose EJ, Morris DW, Fahey C, Robertson IH, Greene C, O'Doherty J, et al. The effect of the neurogranin schizophrenia risk variant rs12807809 on brain structure and function. Twin Res Hum Genet. 2012;15:296–303. doi: 10.1017/thg.2012.7. [DOI] [PubMed] [Google Scholar]
- 50.Pohlack ST, Nees F, Ruttorf M, Witt SH, Nieratschker V, Rietschel M, Flor H. Risk variant for schizophrenia in the neurogranin gene impacts on hippocampus activation during contextual fear conditioning. Mol Psychiatry. 2011;16:1072–1073. doi: 10.1038/mp.2011.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Andreassen OA, Djurovic S, Thompson WK, Schork AJ, Kendler KS, O'Donovan MC, et al. Improved detection of common variants associated with schizophrenia by leveraging pleiotropy with cardiovascular-disease risk factors. Am J Hum Genet. 2013;92:197–209. doi: 10.1016/j.ajhg.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weedon MN, Lettre G, Freathy RM, Lindgren CM, Voight BF, Perry JR, et al. A common variant of HMGA2 is associated with adult and childhood height in the general population. Nat Genet. 2007;39:1245–1245. doi: 10.1038/ng2121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Frayling TM, Timpson NJ, Weedon MN, Zeggini E, Freathy RM, Lindgren CM, et al. A common variant in the FTO gene is associated with body mass index and predisposes to childhood and adult obesity. Science. 2007;316:889–894. doi: 10.1126/science.1141634. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.