Abstract
To elucidate the influence of growth temperature and of stage of maturity on lipid synthesis in seeds, oat plants (Avena sativa nuda L., variety NOS) were fed with 14CO2 at different stages after flowering, and the 14C-incorporation into the grain lipids was determined at 2, 24, and 48 hours after the end of 14CO2-application. By changing growth temperature from 12 C to 28 C after the application of 14CO2 to intact plants, a higher 14C-labeling of saturated fatty acids was found at the higher temperature. At 28 C, palmitic and stearic acids contained 23% and 9% respectively of total fatty acid-14C shortly after the 14CO2-application, whereas at 12 C the corresponding values were 19% and 4%, respectively. Within 2 days 14C-activity of saturated fatty acids decreased at both temperatures, but to a lesser degree at 28 C. The higher 14C-labeling of saturated fatty acids and its lower decrease within 2 days at 28 C clearly show a direct influence of temperature on fatty acid biosynthesis in oat grains.
At all stages of grain growth, oleic acid had the highest 14C-activity of all fatty acids shortly after the 14CO2-application. However, 14C activity of oleic acid rapidly decreases in favor of linoleic acid. With increasing maturity, the intensity of lipid synthesis in the grains decreases; simultaneously, the relative amount of 14C-saturated fatty acids increases primarily at the expense of 14C-oleic acid. These tendencies, which were observed in oat plants grown at day temperatures of 12 C during seed development, seem to be paralleled by lipid synthesis in younger grains grown at day temperatures of 28 C. This indicates an indirect influence of growth temperature on lipid synthesis in oat grains during maturation.
Full text
PDF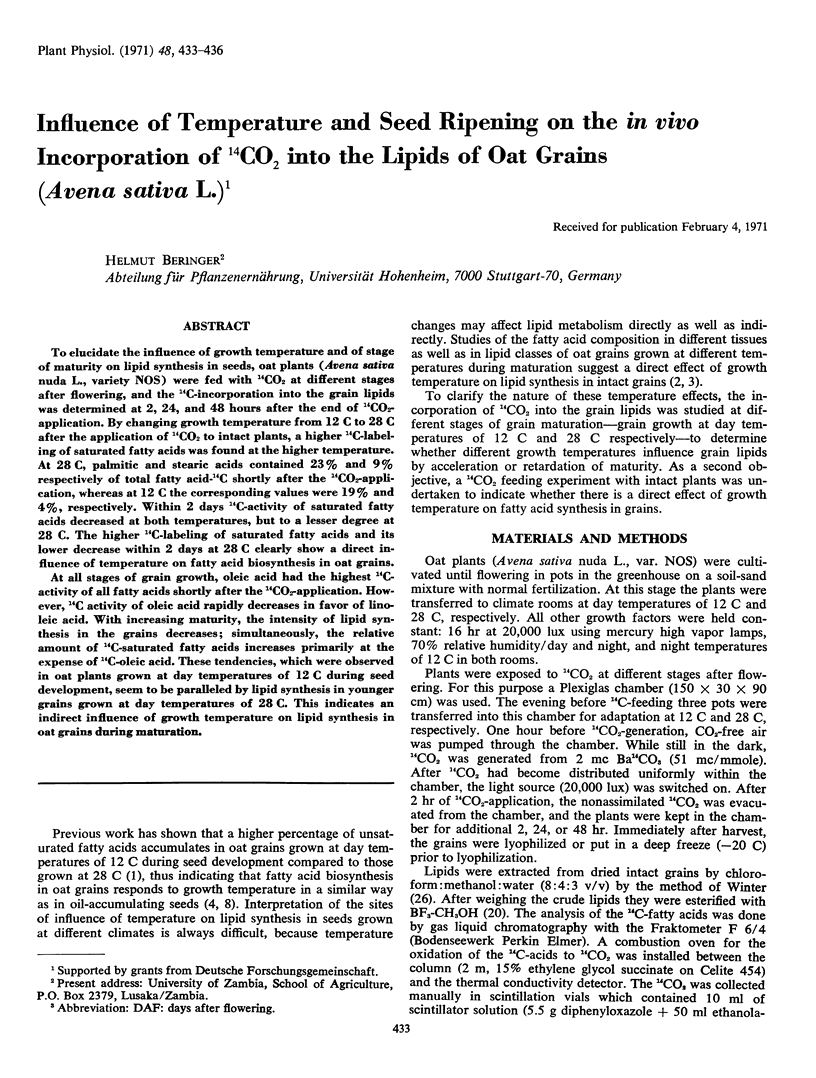

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Dompert W., Beringer H. Fettsynthese in Sonnenblumen-Früchten bei unterschiedlicher Sauerstoff-Konzentration. Naturwissenschaften. 1970 Jan;57(1):40–40. doi: 10.1007/BF00593560. [DOI] [PubMed] [Google Scholar]
- Dutton H. J., Mounts T. L. Desaturation of fatty acids in seeds of higher plants. J Lipid Res. 1966 Mar;7(2):221–225. [PubMed] [Google Scholar]
- Dybing C. D., Zimmerman D. C. Fatty Acid accumulation in maturing flaxseeds as influenced by environment. Plant Physiol. 1966 Nov;41(9):1465–1470. doi: 10.1104/pp.41.9.1465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurr M. I., Robinson M. P., James A. T. The mechanism of formation of polyunsaturated fatty acids by photosynthetic tissue. The tight coupling of oleate desaturation with phospholipid synthesis in Chlorella vulgaris. Eur J Biochem. 1969 May 1;9(1):70–78. doi: 10.1111/j.1432-1033.1969.tb00577.x. [DOI] [PubMed] [Google Scholar]
- Harris P., James A. T. Effect of low temperature on fatty acid biosynthesis in seeds. Biochim Biophys Acta. 1969 Jul 29;187(1):13–18. doi: 10.1016/0005-2760(69)90127-1. [DOI] [PubMed] [Google Scholar]
- Harris P., James A. T. The effect of low temperatures on fatty acid biosynthesis in plants. Biochem J. 1969 Apr;112(3):325–330. doi: 10.1042/bj1120325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hawke J. C., Stumpf P. K. Fat metabolism in higher plants. 28. The biosynthesis of saturated and unsaturated fatty acids by preparations from barley seedlings. J Biol Chem. 1965 Dec;240(12):4746–4752. [PubMed] [Google Scholar]
- Howling D., Morris L. J., James A. T. The influence of chain length on the dehydrogenation of saturated fatty acids. Biochim Biophys Acta. 1968 Jan 10;152(1):224–226. doi: 10.1016/0005-2760(68)90027-1. [DOI] [PubMed] [Google Scholar]
- Inkpen J. A., Quackenbush F. W. Desaturation of palmitate and stearate by cell-free fractions from soybean cotyledons. Lipids. 1969 Nov;4(6):539–543. doi: 10.1007/BF02531038. [DOI] [PubMed] [Google Scholar]
- MCMAHON V., STUMPF P. K. SYNTHESIS OF LINOLEIC ACID BY PARTICULATE SYSTEM FROM SAFFLOWER SEEDS. Biochim Biophys Acta. 1964 Jun 15;84:359–361. doi: 10.1016/0926-6542(64)90065-4. [DOI] [PubMed] [Google Scholar]
- Nagai J., Bloch K. Enzymatic desaturation of stearyl acyl carrier protein. J Biol Chem. 1966 Apr 25;241(8):1925–1927. [PubMed] [Google Scholar]
- Nichols B. W. Fatty acid metabolism in the chloroplast lipids of green and blue-green algae. Lipids. 1968 Jul;3(4):354–360. doi: 10.1007/BF02530939. [DOI] [PubMed] [Google Scholar]
- Rinne R. W. Biosynthesis of Fatty acids by a soluble extract from developing soybean cotyledons. Plant Physiol. 1969 Jan;44(1):89–94. doi: 10.1104/pp.44.1.89. [DOI] [PMC free article] [PubMed] [Google Scholar]


