Abstract
Perturbations in mitochondrial health may foster age-related losses of aerobic capacity (VO2peak) and skeletal muscle size. However, limited data exist regarding mitochondrial dynamics in aging human skeletal muscle and the influence of exercise. The purpose of this study was to examine proteins regulating mitochondrial biogenesis and dynamics, VO2peak, and skeletal muscle size before and after aerobic exercise training in young men (20 ± 1 y) and older men (74 ± 3 y). Exercise-induced skeletal muscle hypertrophy occurred independent of age, whereas the improvement in VO2peak was more pronounced in young men. Aerobic exercise training increased proteins involved with mitochondrial biogenesis, fusion, and fission, independent of age. This is the first study to examine pathways of mitochondrial quality control in aging human skeletal muscle with aerobic exercise training. These data indicate normal aging does not influence proteins associated with mitochondrial health or the ability to respond to aerobic exercise training at the mitochondrial and skeletal muscle levels.
Key Words: Mitofusin, FIS1, Mitochondrial dynamics, PGC-1α, Sarcopenia, Hypertrophy.
Introduction
Age-related skeletal muscle loss has been associated with reduced muscle function (1) and aerobic capacity (2), two strong predictors of morbidity and mortality (3–5) in older adults. Although the loss of muscle mass with advancing age is characterized by reduced muscle fiber size and number, the etiology of age-related atrophy is not well understood. Recent investigations imply that age-related decrements in aerobic capacity and skeletal muscle mass may be linked to the disruption of mitochondrial health (ie, biogenesis, dynamics, function) (6,7).
Peroxisome proliferator-activated receptor-γ coactivator 1α (PGC-1α), a primary regulator of mitochondrial biogenesis, may be lower in aging human skeletal muscle (8,9) and therefore potentially involved with reduced mitochondrial protein synthesis (10), DNA copy number (11,12), content, and function (11,13). Although not obligatory for exercise-induced mitochondrial biogenesis, PGC-1α is an integral factor in co-ordinating pathways for mitochondrial homeostasis and has been implicated in mitochondrial dynamics (ie, fusion and fission) (14). Mitochondrial fusion (mitofusion) and fission (mitofission) are not completely understood in aging human skeletal muscle but are thought to be key components in regulating mitochondrial quality and function. By promoting mitochondrial protein turnover, fusion and fission facilitate the maintenance of mitochondrial and skeletal muscle health by avoiding the accumulation of protein damage that can evoke the stimulation of apoptotic and catabolic pathways (15–17). Mitofusion connects mitochondrial membranes of separate mitochondria to exchange and dilute damaged components (18), whereas mitofission partitions severely damaged sections of mitochondria for removal by mitochondrial specific autophagy (19,20). In mitofusion knockout animals and humans with low mitofusion messenger RNA expression, mitochondrial function and skeletal muscle mass are reduced with an increased prevalence of metabolic disorders (21,22). Reports of altered mitofusion (mitofusion-1 [MFN1], -2 [MFN2] and optic atrophy protein-1) and mitofission (fission protein-1 [FIS1] and dynamin-related protein-1) messenger RNA expression are equivocal in older adults (23,24); therefore, clarification is needed to determine the role of mitochondrial dynamics in maintaining mitochondrial and skeletal muscle quality and function.
Although disturbances in mitochondrial health are associated with increasing age and loss of skeletal muscle mass, it is important to note the observation of mitochondrial dysfunction in aging humans is highly ambiguous with an equivalent number of studies finding marginal to no differences between young and old cohorts. Physical activity and chronic aerobic exercise are well known to increase surrogates of mitochondrial mass and oxidative capacity (25,26), rendering this prescription an attractive treatment to improve mitochondrial health and potentially skeletal muscle size in older adults. However, many studies implementing acute stimuli in human and animal models have recently demonstrated age-related deficiencies in mitochondrial biogenesis and anabolic potential. These data have created speculations that adaptations to chronic exercise are impaired in older adults (younger than 80 y) but rarely has young and older human skeletal muscle been directly compared after the same aerobic exercise training (AET) program (9,27). To our knowledge, it remains to be determined if a controlled exercise training program can influence proteins regulating mitochondrial quality in human skeletal muscle from young and older adults. Thus, the primary purpose was to examine mitochondrial quality control proteins and skeletal muscle size before and after AET in young men (YM) and older men (OM).
Methods
Subjects
Seven YM (20±1 y) and six OM (74±3 y) were recruited from the university and surrounding community (Table 1). Prior to enrollment, YM completed a detailed medical and exercise history questionnaire, whereas OM underwent a physical examination that included a description of medical and exercise history, blood chemistry profile, pulmonary function test, and a resting and maximal exercise electrocardiogram. Volunteers were excluded based on the following criteria: (a) body mass index > 32 kg/m2; (b) type 1 or type 2 diabetes; (c) uncontrolled hypertension; (d) active cancer, cancer in remission, or having received treatment for cancer in the previous 5 years; (e) coronary artery disease; (f), cardiovascular disease; (g) abnormal thyroid function; (h) physically active defined as one who has completed regular aerobic or resistance exercise more than one time per week for 20 minutes or longer during the previous year; (i) 3% change in body weight over the previous 6 months; (j) chronic use of nonsteroidal anti-inflammatory drugs, beta-adrenergic blocking or antagonist agents or tobacco; and (k) any condition that presents a limitation to exercise training (eg, severe arthritis, neuromuscular disorder, chronic obstructive pulmonary disease, and Alzheimer’s disease). Some OM were consuming medications during the entirety of the investigation, three for blood pressure (diuretic and/or calcium influx inhibitor), four for cholesterol (statins), and five for prostate health. No differences, before or after training, were observed for OM consuming medications compared with those who were not taking medications. Written informed consent was obtained from each volunteer for all procedures approved by the Institutional Review Board at Ball State University.
Table 1.
Subject Characteristics and Details of the Exercise Training Program
| YM | OM | |
|---|---|---|
| Number of subjects | 7 | 6 |
| Age (y) | 20±1 | 74±3 |
| Body mass index (kg/m2) | 26±2 | 26±1 |
| Average (kp) | 1.93±0.11 | 1.20±0.04* |
| Average (RPM) | 81±1 | 70±1* |
| Total number of contractions | 138,000±2,2380 | 118,460±3,055* |
| Total mechanical work performed (MJ) | 16.4±1.1 | 8.8±4.6* |
Notes: kp = kilopond; RPM = revolutions per minute; MJ = mega joules. Exercise was performed on a Monark cycle ergometer. kp and RPM values represent the 12-week average. Number of muscle contractions per leg is a product of recorded RPM and time for the entire aerobic exercise training program. *p < .05 versus young.
Experimental Design and Methodology
Eligible subjects completed a series of baseline measurements for the determination of aerobic capacity, whole-muscle size, and content of mitochondrial proteins from a muscle biopsy sample. After completion of baseline analyses, participants performed 12 weeks of AET. Following the intervention, all subjects repeated the testing procedures conducted at baseline.
Aerobic Capacity
YM and OM performed a graded exercise test to exhaustion on an electronically braked cycle ergometer for the assessment of aerobic capacity (ie, VO2peak). The older subjects completed the graded exercise test with electrocardiogram and physician monitoring. Details of the graded exercise test for YM and OM have been described previously (28).
Whole-Muscle Size
Proton magnetic resonance images of the leg were measured using a Siemens Symphony 1.5 Tesla imaging system at standard settings (repetition time/echo time [TR/TE] = 2000/9ms) as we have previously described (28–30). Bilateral scans were obtained after 1 hour of supine rest to avoid the influence of potential fluid shifts (31). Subjects were positioned with an adjustable foot restraint for fixation of joint angles and thus muscle lengths. Contiguous, 1-cm interleaved, serial scans were obtained from the greater trochanter of the femur to the articular surface of the tibia, as estimated from frontal or sagittal scout images. After electronic data transfer to a personal computer (Macintosh Power PC), images were coded. The same investigator performed measurements and was blind to the time point associated with each stack of respective images. Average muscle cross-sectional area (CSA) was taken as the average of each slice from the first distal image containing the rectus femoris and the last proximal image not containing the gluteal muscle using National Institutes of Health Image J 1.63b. The average CSA (cm2) was taken as the average of all analyzed slices for the vastii and rectus femoris and then summed for total quadriceps femoris.
Skeletal Muscle Biopsy
Tissue was obtained from the vastus lateralis following local anesthetic (Lidocaine HCl 1%) using a 5-mm Bergstrom needle with suction (32). The muscle sample was cleansed of excess blood and adipose tissue, immediately frozen and stored in liquid nitrogen. Subjects restricted physical activity for 48 hours before the pretraining biopsy, whereas the post-training biopsy sample was obtained ~48 hours after the last exercise session and restricted physical activity.
AET Protocol
Subjects performed 12 weeks of progressive aerobic training on a cycle ergometer (Monark Ergomedic 828E). Body weight was measured prior to every exercise session to ensure weight maintenance. Exercise intensity was standardized for YM and OM relative to their maximal aerobic capacity by utilizing a percentage of their heart rate reserve, which is based on the resting heart rate and maximum heart rate achieved during the graded exercise test (ie, % heart rate reserve) as previously outlined (29). A total of 42 exercise sessions were completed where duration (20–45min), intensity (60%–80% heart rate reserve), and frequency (3–4 sessions/wk) of exercise were progressively increased throughout the 12 weeks to optimize the training response. The last 5 weeks of the exercise program consisted of four 45-minute sessions at 80% heart rate reserve/wk. The training program was identical to the protocol our laboratory has previously published with a cohort of older women (29,30,33), and an itemized outline describing the weekly progression of exercise intensity, duration, and frequency has been previously published (33). A member of our laboratory group supervised each exercise session in its entirety to record training data and ensure proper exercise intensity. Both YM and OM completed all 42 exercise sessions at the prescribed exercise intensity and duration for a 100% adherence rate. Detailed characteristics of the AET program in terms of average pedaling cadence (revolutions per minute), resistance (kiloponds), and total work completed (megajoules) are reported in Table 1.
Western Blotting
Proteins were identified utilizing Western blotting as we have previously described (30,34). Briefly, homogenized samples were centrifuged for 5 minutes at 100g to remove cellular debris. Proteins (20 µg) were diluted in 2× SDS sample buffer (1:1), heated to 95°C for 5 minutes, and resolved on a 4%–20% gradient gel (Pierce) using SDS–polyacrylamide gel electrophoresis for ~90 minutes at 100V (Mini Protean 3 system, Bio-Rad Laboratories, Hercules, CA). Subsequently, proteins were transferred to polyvinylidene difluoride membrane (Immobilon-P, Millipore, Bedford, MA) for 2 hours. The membrane was blocked with 5% milk–tris-buffered saline with 0.1% tween-20 solution and then incubated with a primary antibody overnight at 4°C. Primary antibodies are described in Supplemental Table 1 and were diluted 1:1000 with 5% milk–tris-buffered saline with 0.1% tween-20. Blots were incubated with horseradish peroxidase–conjugated secondary antibody diluted 1:4000 with 5% milk–tris-buffered saline with 0.1% tween-20 and exposed to an enhanced chemiluminescent substrate (GE Amersham ECL Plus Western Blotting Detection System). Images were captured using a chemiluminescent imaging system (FluorChem SP, Alpha Innotech). Verification of equal protein loading was accomplished by use of a housekeeping protein (Pan-Actin, Cell Signaling). Sizes of the immunodetected proteins were confirmed by molecular weight markers SeeBlue and Magic Mark (Invitrogen). Each subject’s pre- and post-training samples were analyzed on the same blot, and samples were loaded in an alternate fashion of YM and OM to control for intra- and interassay variability. All data for proteins of interest were expressed relative to pan-actin.
Statistics
Data are expressed as mean and standard error. Statistical significance for differences between group means for all variables was assessed with a two-way (group × intervention) analysis of variance with repeated measures. A Bonferroni post hoc analysis was performed to make pairwise comparisons. Group means from the exercise training data (eg, kiloponds, revolutions per minute, number of contractions, and total work) were analyzed using a paired two-tailed student’s t test. Significance was accepted as p < .05.
Results
Mitochondrial Content and Biogenesis
To assess the influence of age and AET on markers of mitochondrial content, we measured the primary regulator of mitochondrial biogenesis (PGC-1α), metabolic enzymes (citrate synthase [CS] and β-hydroxylacyl Co A dehydrogenase [βHAD]), and proteins within the electron transport chain (succinate dehydrogenase [SDH] and cytochrome c oxidase, subunit IV [COXIV]). Before training, no age-related differences (p > .05) were present for these select mitochondrial proteins.
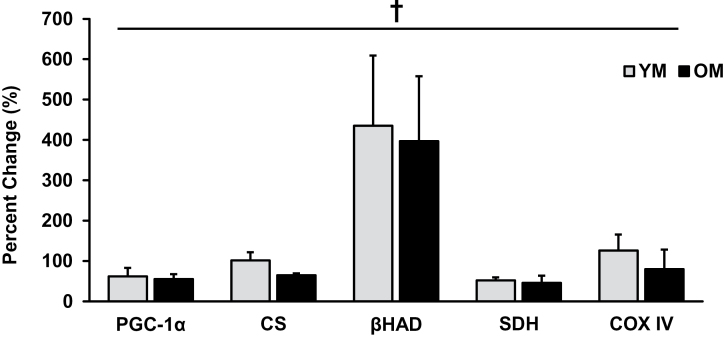
Each protein increased after training with no age-related differences in the training response. Specifically, PGC-1α protein content increased (p < .05) after AET in YM (62% ± 21%) and OM (55% ± 12%). AET increased (p < .05) levels of CS (YM: 102% ± 20%; OM: 65% ± 5%) and βHAD (YM: 435% ± 174%; OM: 397% ± 161%). Additionally, YM and OM had elevated (p < .05) levels of SDH (YM: 52% ± 7%; OM: 46% ± 18%) and COXIV (YM: 126% ± 39%; OM: 80% ± 48%) after AET (Figure 1).
Figure 1.
Each protein representing mitochondrial biogenesis increased (p < .05) with aerobic exercise training, independent of age, suggesting mitochondrial adaptations are not impaired with age. † = main effect for time; p < .05. PGC = proliferator-activated receptor-γ coactivator 1α; CS = citrate synthase; βHAD = β-hydroxylacyl Co A dehydrogenase; SDH = succinate dehydrogenase; COX IV = cytochrome c oxidase, subunit IV; YM = young men; OM = older men.
Mitochondrial Quality Control
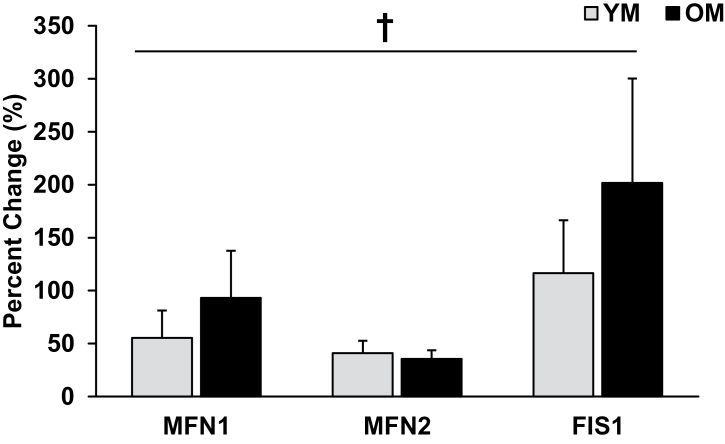
We are the first to measure proteins associated with mitofusion (MFN1, MFN2, and optic atrophy protein-1) and mitofission (FIS1) to gain insight on mitochondrial dynamics within aging skeletal muscle before and after AET (Figure 2). Prior to training, there were no age-related differences in mitofusion or mitofission proteins. We observed that YM and OM increased (p < .05) MFN1 (YM: 55% ± 26%; OM: 93% ± 44%) and MFN2 (YM: 41% ± 12%; OM: 36% ± 8%), whereas optic atrophy protein-1 was unaltered with AET. FIS1 protein content was greater (p < .05) after AET in YM (117% ± 50%) and OM (201% ± 98%). A marker of mitochondrial antioxidant capacity, manganese superoxide dismutase, tended (p = .06) to be elevated in OM compared with YM before training, and the training-induced increase tended (p = .07) to be higher in OM (369% ± 87%) than in YM (220% ± 27%).
Figure 2.
Markers of mitofusion (MFN1, MFN2) and mitofission (FIS1) increased (p < .05) with AET, independent of age. Elevated content of proteins related to mitochondrial dynamics suggests enhanced control of mitochondrial protein turnover and quality. † = main effect for time; p < .05. YM = young men; OM = older men.
Cardiorespiratory and Exercise Capacity
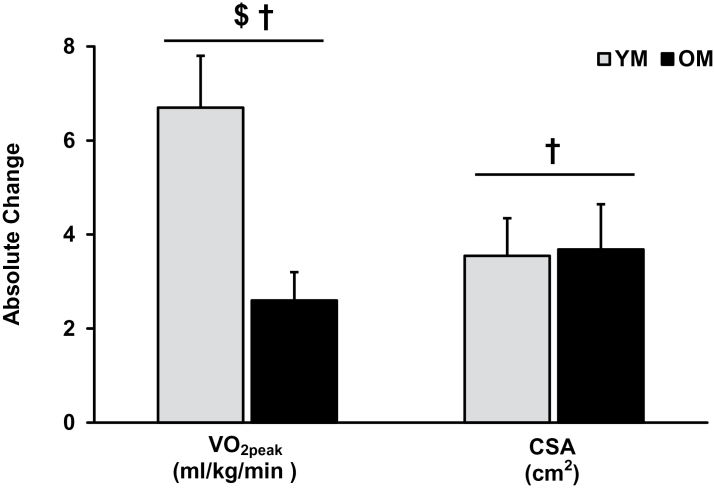
VO2peak (ml/kg/min) was higher (p < .05) in YM and was increased (p < .05) after AET in YM and OM. The increased VO2peak was greater (p < .05) in YM (6.7±1.1 ml/kg/min) compared with OM (2.6±0.6 ml/kg/min; Figure 3). Similar statistical differences were observed for peak values of oxygen pulse, ventilation, and workload (Table 2). Peak oxygen uptake efficiency was lower (p < .05) in the OM prior to training and increased (p < .05) after AET, independent of age. Maximum heart rate was lower (p < .05) in OM but not altered with AET in either YM or OM.
Figure 3.
Aerobic capacity (VO2peak) and skeletal muscle CSA increased after 12 weeks of aerobic exercise training in YM and OM. The absolute change in aerobic capacity (ml/kg/min) was greater (p < .05) in the YM vs. OM whereas the absolute increase in CSA was independent of age. † = main effect for time; $ = interaction; p < .05. YM = young men; OM = older men; CSA = cross-sectional area.
Table 2.
Peak Values From the Graded Exercise Test Before (PRE) and After (POST) the Aerobic Training Intervention
| YM | OM | |||||||
|---|---|---|---|---|---|---|---|---|
| PRE | POST | Abs. Δ | % Δ | PRE | POST | Abs. Δ | % Δ | |
| VO2 (ml/kg/min)*†‡ | 40.5±2.8 | 47.2±2.7 | 6.7±1.1 | 17±3 | 21.0±1.0 | 23.6±1.0 | 2.6±0.6 | 13±3 |
| Heart rate (bpm)* | 199±1 | 194±2 | — | — | 147±6 | 149±7 | — | — |
| O2 pulse (ml/beat)*†‡ | 16.9±0.8 | 20.1±1.0 | 3.2±0.5 | 19±3 | 12.1±0.7 | 13.5±0.8 | 1.4±0.3 | 12±3 |
| VE (L/min)*†‡ | 115±8 | 133±8 | 18±3.4 | 16±4 | 80±4 | 86±2 | 6±2 | 8±3 |
| OUE (L/min)*† | 3.5±0.3 | 3.9±0.2 | 0.4±0.2 | 13±5 | 1.9±0.1 | 2.2±0.1 | 0.3±0.1 | 16±4 |
| Workload (Watts)*† | 261±12 | 313±13 | 52±5 | 20±2 | 142±6 | 172±10 | 30±8 | 21±6 |
Notes: YM = young men; OM = older men; bpm = beats per minute; O2 pulse = VO2/heart rate; VE = ventilation; OUE = oxygen uptake efficiency (VO2peak/Log10VE); absolute (Abs. Δ) and percent (% Δ) change are presented when statistical differences occurred with training.*Main effect for age.
†Main effect for time.
‡Interaction.
Whole-Muscle Size
Before training, YM had a larger (74±2 vs 63±4cm2; p < .05) quadriceps femoris CSA than OM, whereas CSA was increased (p < .05) with AET, independent of age. The absolute change in quadriceps femoris CSA with AET is shown in Figure 3.
Discussion
The focus of this investigation was to examine the regulation of mitochondrial biogenesis and dynamics, aerobic capacity (VO2peak), and skeletal muscle size (CSA) in YM (20±1 y) and OM (74±3 y) before and after 12 weeks of AET. Limited data exist directly comparing the impact of the same exercise prescription on mitochondrial and skeletal muscle adaptations in different age groups, whereas no studies have examined the influence of AET on mitochondrial dynamics in humans. In the current investigation, AET increased aerobic capacity, skeletal muscle size, and markers of mitochondrial biogenesis and dynamics in both young and older cohorts. These data indicate that OM maintain the ability to respond favorably to aerobic exercise at the mitochondrial, skeletal muscle, and whole-body levels, highlighting that AET should be considered a valuable tool to help offset the age-related loss of skeletal muscle mass and aerobic capacity.
To gain further insight into molecular regulation of mitochondrial health, we examined an assortment of proteins involved in biogenesis (PGC-1α), substrate metabolism (CS and βHAD), and oxidative phosphorylation (SDH and COXIV). Before training, these markers of mitochondrial biogenesis and content were not different between groups. Proteins affiliated with mitochondrial fusion (MFN1 and MFN2) and fission (FIS1) were also not different in OM compared with YM. There may be a relationship between the maintenance of mitochondrial quality control mechanisms and the preservation of surrogates for mitochondrial mass (eg, CS and SDH), but further research is needed. Mitofusion and fission proteins act as morphological governors, removing portions of mitochondria with mutations and/or damage to be degraded (21,35) and assisting in the synthesis of new organelles to maintain a mitochondrial domain or density within myofibers to meet energetic demands. Collectively, markers of mitofusion and mitofission are not different between YM and OM and appear to maintain mitochondrial protein content (eg, CS and SDH) during the normal, slow process of age-related atrophy (<1%/y) in OM (74 y).
The observation of no apparent age-related differences for select mitochondrial proteins is not surprising as research investigating aging mitochondria in human skeletal muscle is equivocal, with many observing no age-specific impairments, whereas numerous others find age-related mitochondrial deficiencies. The discrepancies between investigations are not completely understood, but we propose that mitochondrial content and regulation are more likely to be impaired in those individuals older than 80 years (36–38), suffering from severe muscle loss, chronic diseases, or orthopedic issues, which may limit normal physical activity (39–42). When removing these confounding variables, our data suggest that apparently healthy, free-living OM (74 y) do not display measureable effects of age on key proteins associated with mitochondrial biogenesis or dynamics compared with their young, sedentary counterparts.
We have demonstrated that mitochondrial biogenesis (ie, PGC-1α, CS, βHAD, SDH, and COXIV) and skeletal muscle hypertrophy occur within 12 weeks of AET, independent of age. PGC-1α was increased after AET, which is supported by cross-sectional analysis demonstrating elevated levels of PGC-1α protein in older endurance-trained subjects compared with a sedentary control group (12). In mice with overexpressed PGC-1α, the age-related loss of muscle mass, aerobic capacity, and mitochondrial function were eliminated, highlighting the importance of PGC-1α in maintaining a healthy skeletal muscle phenotype (43). Consistently, AET increased markers of mitochondrial biogenesis, muscle mass, and aerobic capacity in YM and OM. These data suggest that healthy OM (74 y) match the robust mitochondrial adaptations to AET as observed in their young (20 y) counterparts, more than 50 years younger.
An emerging role for PGC-1α is the regulation of mitochondrial dynamics (14). In this study, markers of mitochondrial fusion (MFN1 and MFN2) and fission (FIS1) were elevated after AET, independent of age. Recent data have revealed that acute exercise (44,45) and short-term training (46,47) increase markers of mitochondrial dynamics in young individuals, supporting our data that suggest chronic exercise positively affects proteins associated with mitochondrial morphology and function in young and older adults. Animal models also demonstrate that acute and chronic exercise can alter markers of mitochondrial content and dynamics (48,49); however, the current investigation is the first to empirically establish the benefits of AET on mitochondrial quality control pathways in human muscle samples obtained from young and older individuals.
Improvements in mitochondrial health may also play a potential role in the exercise-induced increase of aerobic capacity and skeletal muscle size (6). AET has been traditionally characterized by improved aerobic capacity but less commonly acknowledged for its ability to induce skeletal muscle hypertrophy in sedentary individuals as has been previously established by our laboratory and others (28–30,50–56). Interestingly, our study is the first to report a similar increase of skeletal muscle CSA in YM and OM after the same AET program. The absolute increase of skeletal muscle CSA is similar to the skeletal muscle hypertrophy observed after the same 12-week AET program in older women (30). These findings are intriguing due to recent research speculating impaired training adaptations in older adults due to an attenuated metabolic response after an acute anabolic stimulus (ie, anabolic resistance) (57). The current data do not support the notion of anabolic resistance in OM (74 y) after 12 weeks of AET and are reinforced by a study establishing increased basal levels of mixed-muscle protein synthesis after AET, independent of age (58). Taken together with our previous observations of reduced catabolic messenger RNA expression (ie, FOXO3A, myostatin) following AET (30), it appears that aerobic exercise may promote a positive protein balance favorable for skeletal muscle hypertrophy in sedentary individuals, independent of age. More research is needed to determine the connection of such molecular characteristics that occur concomitantly with skeletal muscle hypertrophy. Recent investigations have provided valuable insight on how an isoform of PGC-1α (ie, PGC-1α4) may reduce FOXO3A and myostatin messenger RNA expression and induce skeletal muscle hypertrophy (59); however, further investigation is warranted to examine the impact of various exercise modalities. Additionally, further consideration of the acute exercise response in relation to chronic exercise adaptation is necessary to gain an understanding of how AET can overcome age-related anabolic resistance within 12 weeks.
Our findings demonstrate that AET performed on a cycle ergometer increased skeletal muscle size and markers of mitochondrial biogenesis and quality control to a similar extent in YM and OM. This is the first study to demonstrate increased markers of mitochondrial dynamics and biogenesis in YM and OM after 12 weeks of AET, providing plausible mechanisms for improving skeletal muscle and metabolic health after regular aerobic exercise. Although there may be latent limitations in studying a modest number of participants (six OM and seven YM), investigations with larger sample sizes would be welcomed to expand on these data that suggest that AET can positively impact the aging phenotype observed at the whole-body, whole-muscle, and subcellular levels in sedentary individuals. Although AET should be considered a practical intervention to combat the age-related decline of both aerobic capacity and skeletal muscle mass, more research is needed to improve our comprehension and translation of the molecular regulation to applied, clinical outcomes with advancing age.
Supplementary Material
Supplementary material can be found at: http://biomedgerontology.oxfordjournals.org/
Funding
This research is supported by National Institute of Health Grant AG032127.
Acknowledgments
The authors would like to thank J. Matthew Hinkley and Tina Vannatta for assistance with exercise training and data collection.
References
- 1. Doherty TJ. Invited review: Aging and sarcopenia. J Appl Physiol. 2003;95:1717–1727 [DOI] [PubMed] [Google Scholar]
- 2. Proctor DN, Joyner MJ. Skeletal muscle mass and the reduction of VO2max in trained older subjects. J Appl Physiol. 1997;82:1411–1415 [DOI] [PubMed] [Google Scholar]
- 3. Kokkinos P, Myers J, Faselis C, et al. Exercise capacity and mortality in older men: a 20-year follow-up study. Circulation. 2010;122:790–797 [DOI] [PubMed] [Google Scholar]
- 4. Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. N Engl J Med. 2002;346:793–801 [DOI] [PubMed] [Google Scholar]
- 5. Metter EJ, Schrager M, Ferrucci L, Talbot LA. Evaluation of movement speed and reaction time as predictors of all-cause mortality in men. J Gerontol A Biol Sci Med Sci. 2005;60:840–846 [DOI] [PubMed] [Google Scholar]
- 6. Johnson ML, Robinson MM, Nair KS. Skeletal muscle aging and the mitochondrion. Trends Endocrinol Metab. 2013;24:247–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Coen PM, Jubrias SA, Distefano G, et al. Skeletal muscle mitochondrial energetics are associated with maximal aerobic capacity and walking speed in older adults. J Gerontol A Biol Sci Med Sci. 2013;68:447–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ling C, Poulsen P, Carlsson E, et al. Multiple environmental and genetic factors influence skeletal muscle PGC-1alpha and PGC-1beta gene expression in twins. J Clin Invest. 2004;114:1518–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghosh S, Lertwattanarak R, Lefort N, et al. Reduction in reactive oxygen species production by mitochondria from elderly subjects with normal and impaired glucose tolerance. Diabetes. 2011;60:2051–2060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Rooyackers OE, Adey DB, Ades PA, Nair KS. Effect of age on in vivo rates of mitochondrial protein synthesis in human skeletal muscle. Proc Natl Acad Sci U S A. 1996;93:15364–15369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Short KR, Bigelow ML, Kahl J, et al. Decline in skeletal muscle mitochondrial function with aging in humans. Proc Natl Acad Sci U S A. 2005;102:5618–5623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lanza IR, Short DK, Short KR, et al. Endurance exercise as a countermeasure for aging. Diabetes. 2008;57:2933–2942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Conley KE, Jubrias SA, Esselman PC. Oxidative capacity and ageing in human muscle. J Physiol. 2000;526(Pt 1):203–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Soriano FX, Liesa M, Bach D, Chan DC, Palacín M, Zorzano A. Evidence for a mitochondrial regulatory pathway defined by peroxisome proliferator-activated receptor-gamma coactivator-1 alpha, estrogen-related receptor-alpha, and mitofusin 2. Diabetes. 2006;55:1783–1791 [DOI] [PubMed] [Google Scholar]
- 15. Romanello V, Sandri M. Mitochondrial biogenesis and fragmentation as regulators of muscle protein degradation. Curr Hypertens Rep. 2010;12:433–439 [DOI] [PubMed] [Google Scholar]
- 16. Wasilewski M, Scorrano L. The changing shape of mitochondrial apoptosis. Trends Endocrinol Metab. 2009;20:287–294 [DOI] [PubMed] [Google Scholar]
- 17. Green DR, Galluzzi L, Kroemer G. Mitochondria and the autophagy-inflammation-cell death axis in organismal aging. Science. 2011;333:1109–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ishihara N, Eura Y, Mihara K. Mitofusin 1 and 2 play distinct roles in mitochondrial fusion reactions via GTPase activity. J Cell Sci. 2004;117(Pt 26):6535–6546 [DOI] [PubMed] [Google Scholar]
- 19. Seo AY, Joseph AM, Dutta D, Hwang JC, Aris JP, Leeuwenburgh C. New insights into the role of mitochondria in aging: mitochondrial dynamics and more. J Cell Sci. 2010;123(Pt 15):2533–2542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Twig G, Elorza A, Molina AJ, et al. Fission and selective fusion govern mitochondrial segregation and elimination by autophagy. EMBO J. 2008;27:433–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Chen H, Vermulst M, Wang YE, et al. Mitochondrial fusion is required for mtDNA stability in skeletal muscle and tolerance of mtDNA mutations. Cell. 2010;141:280–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bach D, Pich S, Soriano FX, et al. Mitofusin-2 determines mitochondrial network architecture and mitochondrial metabolism. A novel regulatory mechanism altered in obesity. J Biol Chem. 2003;278:17190–17197 [DOI] [PubMed] [Google Scholar]
- 23. Crane JD, Devries MC, Safdar A, Hamadeh MJ, Tarnopolsky MA. The effect of aging on human skeletal muscle mitochondrial and intramyocellular lipid ultrastructure. J Gerontol A Biol Sci Med Sci. 2010;65:119–128 [DOI] [PubMed] [Google Scholar]
- 24. Bori Z, Zhao Z, Koltai E, et al. The effects of aging, physical training, and a single bout of exercise on mitochondrial protein expression in human skeletal muscle. Exp Gerontol. 2012;47:417–424 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Holloszy JO. Biochemical adaptations in muscle. Effects of exercise on mitochondrial oxygen uptake and respiratory enzyme activity in skeletal muscle. J Biol Chem. 1967;242:2278–2282 [PubMed] [Google Scholar]
- 26. Holloszy JO, Coyle EF. Adaptations of skeletal muscle to endurance exercise and their metabolic consequences. J Appl Physiol. 1984;56:831–838 [DOI] [PubMed] [Google Scholar]
- 27. Short KR, Vittone JL, Bigelow ML, et al. Impact of aerobic exercise training on age-related changes in insulin sensitivity and muscle oxidative capacity. Diabetes. 2003;52:1888–1896 [DOI] [PubMed] [Google Scholar]
- 28. Harber MP, Konopka AR, Undem MK, et al. Aerobic exercise training induces skeletal muscle hypertrophy and age-dependent adaptations in myofiber function in young and older men. J Appl Physiol. 2012;113:1495–1504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Harber MP, Konopka AR, Douglass MD, et al. Aerobic exercise training improves whole muscle and single myofiber size and function in older women. Am J Physiol Regul Integr Comp Physiol. 2009;297:R1452–R1459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Konopka AR, Douglass MD, Kaminsky LA, et al. Molecular adaptations to aerobic exercise training in skeletal muscle of older women. J Gerontol A Biol Sci Med Sci. 2010;65:1201–1207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Berg HE, Tedner B, Tesch PA. Changes in lower limb muscle cross-sectional area and tissue fluid volume after transition from standing to supine. Acta Physiol Scand. 1993;148:379–385 [DOI] [PubMed] [Google Scholar]
- 32. Bergstrom J. Muscle electrolytes in man. Scand J Clin Lab Invest. 1962;68:1–110 [Google Scholar]
- 33. Konopka AR, Trappe TA, Jemiolo B, Trappe SW, Harber MP. Myosin heavy chain plasticity in aging skeletal muscle with aerobic exercise training. J Gerontol A Biol Sci Med Sci. 2011;66:835–841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Harber MP, Crane JD, Douglass MD, et al. Resistance exercise reduces muscular substrates in women. Int J Sports Med. 2008;29:719–725 [DOI] [PubMed] [Google Scholar]
- 35. Twig G, Hyde B, Shirihai OS. Mitochondrial fusion, fission and autophagy as a quality control axis: the bioenergetic view. Biochim Biophys Acta. 2008;1777:1092–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bua E, Johnson J, Herbst A, et al. Mitochondrial DNA-deletion mutations accumulate intracellularly to detrimental levels in aged human skeletal muscle fibers. Am J Hum Genet. 2006;79:469–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Chabi B, Mousson de Camaret B, Chevrollier A, Boisgard S, Stepien G. Random mtDNA deletions and functional consequence in aged human skeletal muscle. Biochem Biophys Res Commun. 2005;332:542–549 [DOI] [PubMed] [Google Scholar]
- 38. Lezza AM, Boffoli D, Scacco S, Cantatore P, Gadaleta MN. Correlation between mitochondrial DNA 4977-bp deletion and respiratory chain enzyme activities in aging human skeletal muscles. Biochem Biophys Res Commun. 1994;205:772–779 [DOI] [PubMed] [Google Scholar]
- 39. Safdar A, Hamadeh MJ, Kaczor JJ, Raha S, Debeer J, Tarnopolsky MA. Aberrant mitochondrial homeostasis in the skeletal muscle of sedentary older adults. PLoS One. 2010;5:e10778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Boffoli D, Scacco SC, Vergari R, Solarino G, Santacroce G, Papa S. Decline with age of the respiratory chain activity in human skeletal muscle. Biochim Biophys Acta. 1994;1226:73–82 [DOI] [PubMed] [Google Scholar]
- 41. Barreiro E, Rabinovich R, Marin-Corral J, Barberà JA, Gea J, Roca J. Chronic endurance exercise induces quadriceps nitrosative stress in patients with severe COPD. Thorax. 2009;64:13–19 [DOI] [PubMed] [Google Scholar]
- 42. Joseph AM, Adhihetty PJ, Buford TW, et al. The impact of aging on mitochondrial function and biogenesis pathways in skeletal muscle of sedentary high- and low-functioning elderly individuals. Aging Cell. 2012;11:801–809 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Wenz T, Rossi SG, Rotundo RL, Spiegelman BM, Moraes CT. Increased muscle PGC-1alpha expression protects from sarcopenia and metabolic disease during aging. Proc Natl Acad Sci U S A. 2009;106:20405–20410 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44. Cartoni R, Léger B, Hock MB, et al. Mitofusins ½ and ERRalpha expression are increased in human skeletal muscle after physical exercise. J Physiol. 2005;567(Pt 1):349–358 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Slivka DR, Dumke CL, Tucker TJ, Cuddy JS, Ruby B. Human mRNA response to exercise and temperature. Int J Sports Med. 2012;33:94–100 [DOI] [PubMed] [Google Scholar]
- 46. Perry CG, Lally J, Holloway GP, Heigenhauser GJ, Bonen A, Spriet LL. Repeated transient mRNA bursts precede increases in transcriptional and mitochondrial proteins during training in human skeletal muscle. J Physiol. 2010;588(Pt 23):4795–4810 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Slivka DR, Dumke CL, Hailes WS, Cuddy JS, Ruby BC. Substrate use and biochemical response to a 3,211-km bicycle tour in trained cyclists. Eur J Appl Physiol. 2012;112:1621–1630 [DOI] [PubMed] [Google Scholar]
- 48. Koltai E, Hart N, Taylor AW, et al. Age-associated declines in mitochondrial biogenesis and protein quality control factors are minimized by exercise training. Am J Physiol Regul Integr Comp Physiol. 2012;303:R127–R134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ding H, Jiang N, Liu H, et al. Response of mitochondrial fusion and fission protein gene expression to exercise in rat skeletal muscle. Biochim Biophys Acta. 2010;1800:250–256 [DOI] [PubMed] [Google Scholar]
- 50. Izquierdo M, Häkkinen K, Ibáñez J, Kraemer WJ, Gorostiaga EM. Effects of combined resistance and cardiovascular training on strength, power, muscle cross-sectional area, and endurance markers in middle-aged men. Eur J Appl Physiol. 2005;94:70–75 [DOI] [PubMed] [Google Scholar]
- 51. Izquierdo M, Ibañez J, HAkkinen K, Kraemer WJ, Larrión JL, Gorostiaga EM. Once weekly combined resistance and cardiovascular training in healthy older men. Med Sci Sports Exerc. 2004;36:435–443 [DOI] [PubMed] [Google Scholar]
- 52. Schwartz RS, Shuman WP, Larson V, et al. The effect of intensive endurance exercise training on body fat distribution in young and older men. Metabolism. 1991;40:545–551 [DOI] [PubMed] [Google Scholar]
- 53. Hudelmaier M, Wirth W, Himmer M, Ring-Dimitriou S, Sänger A, Eckstein F. Effect of exercise intervention on thigh muscle volume and anatomical cross-sectional areas–quantitative assessment using MRI. Magn Reson Med. 2010;64:1713–1720 [DOI] [PubMed] [Google Scholar]
- 54. Sillanpää E, Laaksonen DE, Häkkinen A, et al. Body composition, fitness, and metabolic health during strength and endurance training and their combination in middle-aged and older women. Eur J Appl Physiol. 2009;106:285–296 [DOI] [PubMed] [Google Scholar]
- 55. Lovell DI, Cuneo R, Gass GC. Can aerobic training improve muscle strength and power in older men? J Aging Phys Act. 2010;18:14–26 [DOI] [PubMed] [Google Scholar]
- 56. McPhee JS, Williams AG, Degens H, Jones DA. Inter-individual variability in adaptation of the leg muscles following a standardised endurance training programme in young women. Eur J Appl Physiol. 2010;109:1111–1118 [DOI] [PubMed] [Google Scholar]
- 57. Breen L, Phillips SM. Skeletal muscle protein metabolism in the elderly: Interventions to counteract the ‘anabolic resistance’ of ageing. Nutr Metab (Lond). 2011;8:68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Short KR, Vittone JL, Bigelow ML, Proctor DN, Nair KS. Age and aerobic exercise training effects on whole body and muscle protein metabolism. Am J Physiol Endocrinol Metab. 2004;286:E92–101 [DOI] [PubMed] [Google Scholar]
- 59. Ruas JL, White JP, Rao RR, et al. A PGC-1α isoform induced by resistance training regulates skeletal muscle hypertrophy. Cell. 2012;151: 1319–1331 [DOI] [PMC free article] [PubMed] [Google Scholar]