Abstract
The repair and regeneration of large bone defects, including the formation of functional vasculature, represents a highly challenging task for tissue engineering and regenerative medicine. Recent studies have shown that vascularization and ossification can be stimulated by mild heat stress (MHS), which would offer the option to enhance the bone regeneration process by relatively simple means. However, the mechanisms of MHS-enhanced angiogenesis and osteogenesis, as well as potential risks for the treated cells are unclear. We have investigated the direct effect of MHS on angiogenesis and osteogenesis in a co-culture system of human outgrowth endothelial cells (OECs) and primary osteoblasts (pOBs), and assessed cytotoxic effects, as well as the levels of various heat shock proteins (HSPs) synthesized under these conditions. Enhanced formation of microvessel-like structures was observed in co-cultures exposed to MHS (41°C, 1 h), twice per week, over a time period of 7–14 days. As shown by real-time polymerase chain reaction (PCR), the expression of vascular endothelial growth factor (VEGF), angiopoietin-1 (Ang-1), angiopoietin-2 (Ang-2), and tumor necrosis factor-alpha was up-regulated in MHS-treated co-cultures 24 h post-treatment. At the protein level, significantly elevated VEGF and Ang-1 concentrations were observed in MHS-treated co-cultures and pOB mono-cultures compared with controls, indicating paracrine effects associated with MHS-induced angiogenesis. MHS-stimulated co-cultures and OEC mono-cultures released higher levels of Ang-2 than untreated cultures. On the other hand MHS treatment of co-cultures did not result in a clear effect regarding osteogenesis. Nevertheless, real-time PCR demonstrated that MHS increased the expression of mitogen-activated protein kinase, interleukin-6, and bone morphogenetic protein 2, known as HSP-related molecules in angiogenic and osteogenic regulation pathways. In agreement with these observations, the expression of some selected HSPs also increased at both the mRNA and protein levels in MHS-treated co-cultures.
Introduction
Regenerative medicine and bone tissue engineering applications to treat critical-sized bone defects are still facing severe problems. One major obstacle lies in the need to develop a construct with a functional vasculature. Thus, various strategic approaches to enhance the vascularization of tissue constructs have been developed in recent years. Nevertheless, these approaches have to take into account clinical feasibility, preferring technically simplified but safe methods, and this implies a detailed knowledge of the potential risks of the individual treatments. In this context, physical stress such as hypoxic treatment or hyperthermia as physiological stress factors are known to induce regenerative processes in the body or to enhance the therapeutic potential of cells. Such physical treatment might be more controllable compared with gene therapeutic approaches or the systemic application of pharmacological compounds. Mild heat stress (MHS) might be considered a relatively simple form of treatment to trigger bone regeneration and vascularization, although the mechanisms and the potential effects on cell function are relatively obscure.
Several studies have previously demonstrated that physical stresses, such as hypoxia stress, shear stress, or oxidative stress, can promote angiogenesis.1–5 In particular, hypoxic preconditioning of human mesenchymal stem cells results in promotion of blood vessel formation and improved their therapeutic potential after implantation.2,3 Furthermore, investigators have shown that through hypoxia-inducible factor-1 in combination with other transcription factors, triggered by hypoxia, angiogenesis and osteogenesis can be promoted in mice.1 A recent study involving the activation of Toll-like receptor-2 with novel endogenous ligands has confirmed that oxidative stress promotes angiogenesis.3 Moreover, shear stress applied to the surfaces of endothelial cells promotes the formation of microvessel network in a 3D in vitro model.4 Temperature as a general physical and physiological factor influences growth processes in the cellular response to MHS. During fever, the body temperature of homeothermic animals only increases by 1°C–2°C.6,7 Several studies have investigated low-level heat-stress conditioning as a method for promoting in vitro and in vivo tissue regeneration. Cells of the human microvascular line HMEC-1 exposed to repeated MHS increase their propensity for vascular tube formation in vitro.8 In addition, low-level heat stress (HS) induces positive responses for tissue regeneration not only in skin,9 but also in muscle.10
Positive effects of MHS have also been reported for bone regeneration, with hTERT-immortalized mesenchymal stem cells exposed to MHS exhibiting an enhanced osteoblastic differentiation.11 Local hyperthermia shows effects on active bone resorption and new bone formation in rats.12 Furthermore, MHS induces the proliferation and differentiation of human bone marrow stromal cells and MG-63 cells in vitro.13 The conditioned culture media acquired from heat-treated osteoblasts enhances osteocalcin secretion and mineralization in bone marrow mesenchymal cells.14 Taken together, these data suggest that physiological MHS conditioning has a positive effect on bone regeneration.
However, the mechanisms and signaling pathways for heat-induced angiogenesis and bone formation are still unclear. During heat treatment, the heat shock response (HSR) is employed as a highly conserved cellular defense mechanism. As the results of HSR, the cell synthesizes a small set of highly conserved proteins, called heat shock proteins (HSPs). HSPs function as molecular chaperones and are involved in a variety of cellular activities, such as cell-cycle regulatory and anti-apoptotic activities,6 tissue regeneration,15,16 and differentiation by regulating caspase activity and stabilizing proteins associated with differentiation.17 The potential importance of HSP27 for migration and its role in vascular endothelial growth factor (VEGF)-dependent angiogenesis has previously been reported.18,19 The possible association of HSP70 with VEGF signaling and angiogenesis in the heart has been reported by Gong et al.7 HSP90α can be secreted by endothelial cells and acts as a positive regulator of angiogenesis and tube formation via stabilizing matrix metalloproteinase-2.20 Moreover, the effect of HSP70 on angiogenesis and osteogenesis via the regulation of bone morphogenetic protein (BMP) activity and interleukin-6 (IL-6) expression has also been demonstrated in a previous study (Yao et al., 2009),41 suggesting that a relationship exists between HSPs and angiogenesis/osteogenesis. However, the role of HSPs in strategic approaches for tissue engineering is far from being completely understood.
Thus, we have investigated the effects of MHS treatment on angiogenesis and osteogenesis in a co-culture model of outgrowth endothelial cells (OECs) isolated from peripheral blood and osteogenic cells that are used to mimic the physiological angiogenic process during bone regeneration. This model was used in previous studies to assess prevascularization strategies, the effects of biomaterial modification on bone tissue engineering, and the effect of the morphogen sonic hedgehog (SHH), to promote and accelerate angiogenesis.21,22
In the present work, we show that MHS-increased angiogenesis in a co-culture system is mediated via an increase in angiogenic factors produced by the osteoblasts. Moreover, we have confirmed that some HSPs and HSP-related angiogenic and osteogenic factors are involved in vascularization and bone regeneration processes.
Materials and Methods
Isolation and expansion of OEC
Mono-nuclear cells were isolated from peripheral blood buffy coats by Ficoll/histopaque (Sigma-Aldrich) density gradients Fuchs et al.23 Mono-nuclear cells at a density of 5×106/well were seeded on 24-culture-well plates coated with collagen type-I (BD Biosciences) and cultured in EGM-2 BulletKit (endothelial cell growth medium-2 BulletKit, CC-3162; Lonza) supplemented with 5% fetal calf serum (FCS; GIBCO Life Technologies) and 1% Penicillin/Streptomycin (Pen/Strep liquid; GIBCO Life Technologies). The cells were fed with fresh medium thrice per week. After 3–4 weeks, single colonies of late OECs with a cobblestone-like morphology appeared on the plates. When cells achieved confluence, OECs were collected and expanded over several passages at a splitting ratio of 1:2 on fibronectin-coated (Millipore) 24-well plates. OEC for these experiments were used in passages 9–13. OEC from the different passages were characterized before by flow cytometry and several other methods as already described in previous publications,23,24 indicating a high purity of (95%) for endothelial markers such as CD31, CD146, and VE-cadherin and a high phenotypic stability during the expansion.
Isolation of primary osteoblasts
Human primary osteoblasts (pOBs) were isolated from human cancellous bone fragments from donors as previously published.25,26 Bone explants were washed four to five times with phosphate-buffered saline (PBS) with 1% Pen/Strep and incubated in 1 mg/mL collagenase type IV (C-5138; Sigma-Aldrich) for 1 h at 37°C for enzymatic digestion. The bone fragments were washed four to five times in PBS with 1% Pen/Strep, subsequently placed on six-well plates, and further cultured in Dulbecco's-modified Eagle's medium (DMEM-Ham F12; Gibco) containing 20% FCS and 1% Pen/Strep for about 2–4 weeks. After approaching subconfluence, cells were transferred into T75 culture flasks and cultured in DMEM-Ham F12 containing only 10% FCS and 1% Pen/Strep. For this study, cells were passaged at a ratio of 1:2 up to the third passage. For the experiments, pOB were used in passage 2 to 3 with osteogenic characteristics as described in earlier reports.25
Co-culture of OECs and pOBs
Co-cultures of OECs and pOBs were prepared as previously described.26 In the first step, 300,000 pOB cells/well were seeded on 24-culture-well plates coated with fibronectin (Millipore) and cultivated in DMEM-Ham F12 containing only 10% FCS and 1% Pen/Strep. After 24 h, 200,000 OECs/well were added and co-cultivated in EGM-2 with 5% FCS, 1% Pen/Strep, and supplements from kits. At least three different donors of OECs and pOBs were used for each co-culture experiment.
MHS treatment of co-cultures consisting of pOBs and OECs
Cells and co-cultures were prepared as previously described in 24-well plates. After 1 day of co-cultivation, cells were exposed to MHS (41°C, 1 h) repeated twice a week over 14 days. In addition, non-HS treated cells were cultured with the conditioned culture medium collected from cells exposed to HS (HSM). MHS-treated co-cultures were further processed for immunofluorescent staining, gene expression analysis, and protein analysis.
Immunofluorescent staining
For immunofluorescent staining, cells were seeded on Thermanox coverslips. After 1 or 2 weeks of co-cultivation, cells were fixed with 3.7% paraformaldehyde (MERCK). Cells were washed thrice with PBS and then permeabilized for 5 min by using 0.1% Triton® X-100 (Sigma-Aldrich). After three washes in PBS, the cells were incubated with anti-human CD31 (M0823; Dako) diluted 1:50 in 1% bovine serum albumin (Sigma-Aldrich) in PBS and incubated for 1 h at room temperature. Cells were washed again with PBS and then labeled with the corresponding secondary antibody, namely Alexa 488 anti-mouse (A-11029; MoBiTec) diluted 1:1000 in 1% bovine serum albumin in PBS, for 45 min in the dark at room temperature. After three washes with PBS, cell nuclei were counterstained with 1 μg/mL Hoechst. Finally, the stained samples cells were mounted with Gelmount (Biomeda) and visualized by using a confocal laser scanning microscope (LeicaTCS-NT) (Leica Microsystems).
Assay for caspase-3/7 activity
OECs and pOBs were plated (as described earlier) into 96-well plates at a density of 5×104 cells/well. After 24 h, the cells were treated at 41°C for 1 h. After 2 h of recovery time, the cells were lysed with Caspase-Glo 3/7 Reagent (Promega) according to the manufacturer's instructions. The Caspase 3/7 activity of each sample was determined by measuring the luminescence at 485Ex/527Em nm with a microtiter plate reader.
The CellTiter 96 Aqueous One Solution Assay (MTS)
According to the CellTiter 96 Aqueous One Solution Assay (Promega) protocol, 11,000 OECs or pOBs were plated in 96-well plates. After incubation for 48 h at 37°C, cells were treated with MHS. After 2 days of culture at 37°C, 20 μL of CellTiter 96 AQueous One Solution (Promega) was added, and the plate was incubated at 37°C, under 5% CO2 for 1.5 h. Medium (100 μL) from each well was transferred to an enzyme-linked immunosorbent assay (ELISA) 96-well plate. The absorbance for MTS was measured and quantified by a microplate reader (GENios plus) at 492 nm wavelength and the corresponding software.
Quantitative real-time polymerase chain reaction
Total mRNA was isolated from mono- and co-cultures with an RNeasy Mini Kit according to the manufacturer's protocol (Qiagen). Transcription of cDNA from RNA was performed by using the Omniscript Reverse Transcription Kit (Qiagen). To quantify relative gene expression by quantitative real-time polymerase chain reaction (qRT-PCR), we used the Applied Biosystems 7300 Real-Time PCR System (Applera Deutschland GmbH). The qRT-PCR primers were provided by Qiagen GmbH (Table 1). The total 25 μL probe included 12.5 μL QuantiTect™ SYBR® Green PCR Master Mix, 2.5 μL QuantiTect SYBR Green primer assay, 5 μL RNase free water, and 5 μL cDNA (1 ng/μL) for one reaction. The relative expression of analyzed genes was determined by the Applied Biosystems Sequence Detection Software v.1.2.2 by using d-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) or ribosomal protein 13A (RPL13A) as an endogenous standard. Gene expression was compared by the ΔΔCt method by setting untreated cultures to 1 (reference value) as indicated in the relevant figures.
Table 1.
Primer Information for Primers Used in Real-Time Polymerase Chain Reaction
| Gene name | Primer assay name catalog number | Catalog number |
|---|---|---|
| ALP | Hs_ALPL_1_SG QuantiTect Primer Assay | QT00012957 |
| Ang-1 | Hs ANGPT1_1 SG QuantiTect Primer Assay | QT00046865 |
| Ang-2 | Hs ANGPT2_1 SG QuantiTect Primer Assay | QT00100947 |
| BMP-2 | Hs_BMP2_1_SG_QuantiTectPrimerAssay | QT00012544 |
| Col1A1 | Hs_COL1A1_1_SG QuantiTect Primer Assay | QT00037793 |
| GAPDH | Hs_GAPDH_1_SG QuantiTect Primer Assay | QT00079247 |
| HSP70 | Hs_HSPA1A_2_SG QuantiTect Primer Assay | QT01671873 |
| HSP90 | Hs_HSP90AA1_3_SG QuantiTect Primer Assay | QT01848273 |
| HSP27 | Hs_HSPB1_1_SG QuantiTect Primer Assay | QT00233457 |
| IL-6 | Hs_IL6_1_SG QuantiTect Primer Assay | QT00083720 |
| MAPK-1 | Hs_MAPK1_1_SG QuantiTect Primer Assay | QT00065933 |
| Osteocalcin | Hs_BGLAP_1_SG_QuantiTect PrimerAssay | QT00232771 |
| Osteopontin | Hs_SPP1_1_SG_QuantiTect PrimerAssay | QT01008798 |
| RPL13A | Hs_RPL13A_1_SG QuantiTect Primer Assay | QT00089915 |
| TNF-α | Hs_TNF_3_SG QuantiTect Primer Assay | QT01079561 |
| VEGF | Hs_VeGFA_2_SG QuantiTect Primer Assay | QT01036861 |
Enzyme-linked immunosorbent assay
ELISA was carried out according to the manufacturer's recommendations for the ELISA DuoSets® kit (R&D Systems). Culture supernatants from differently treated cells were collected after 3 days, 7 days, 10 days, and 14 days after heat treatment. The streptavidin-horseradish peroxidase (HRP) colorimetric reaction was performed to visualize the concentration of the various growth factors. A microplate reader (GENios plus; Tecan) was used to measure the optical density of each well at 450 nm wavelength. Results are depicted as the percentage ratio to the control (control=100%) or as concentrations standardized by the protein content of individual samples.
Alkaline phosphatase quantification in the cell lysate
Quantification of alkaline phosphatase (ALP) activity within the cell lysates of MHS-treated and nontreated co-cultures and pOB mono-cultures was performed by using p-nitrophenyl phosphate (pNPP; Sigma-Aldrich). Briefly, the cells were washed with PBS and lysed with 0.1% Triton X-100 in 0.1 M Tris buffer pH 7.2 in an Eppendorf tube and mixed at 4°C for 45 min. In triplicate, 20 μL of the cell lysate of each sample were incubated in a 96-well plate with 60 μL of the substrate solution (0.2% pNPP in 1 M diethanolamine and 0.5 mM MgCl2, pH 9.8) for 45 min at 37°C. The reaction was stopped by the addition of 80 μL of stop solution (0.2 mM EDTA in NaOH, pH 8.0). Absorbance was measured at 405 nm by using a microplate reader. Absolute ALP activity was standardized to the protein content, and results were depicted as mM pNP/mg protein.
Protein extraction and quantification assay
Cell protein was extracted according to our previously published protocol.22 After trypsinization and centrifugation, cells were lysed with 0.1% Triton-X in 0.1 M Tris buffer pH 7.2. Cell lysates were mixed for 45 min at 4°C. After a centrifugation step, supernatants were transferred to new tubes and stored at −20°C until use. A bicinchoninic acid (BCA) protein Assay Reagent Kit was used to determine the protein concentration according to the manufacturer's instructions (Pierce; Thermo Fischer).
Sodium dodecyl sulfate polyacrylamide electrophoresis and western blot
Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western blot were carried out according to our previously published protocol Dohle et al.22 Proteins were separated on the basis of their molecular weight by SDS-PAGE (10% resolving gel and 4.5% stacking gel with a thickness of 0.75 mm). Protein extracts were mixed with 4×RotiLoad-1 loading buffer and incubated for 10 min at 95°C before being loaded into the gel. The proteins were separated at 25 mA for 1.5 h in SDS-running buffer (25 mM Tris, 192 mM glycine, and 0.1% SDS). An aliquot of 10 μL of a protein molecular weight standard was loaded onto the gel. After separation by SDS-PAGE, proteins were transferred from the polyacrylamide gels to nitrocellulose membranes by electroblotting by using a mini-transfer chamber filled with SDS transfer buffer (25 mM Tris-HCl, pH 8.0, 100 mM glycine, and 25% methanol) for 1 h at 350 mA. The membrane was blocked with 5% nonfat milk in PBS containing 0.2% Tween (blocking solution) for 2 h at room temperature and incubated separately with blocking solution containing primary antibody diluted 1:1000 overnight at 4°C. The membrane was washed thrice with 1×PBS 0.2% Tween and then incubated with the HRP-conjugated secondary antibody diluted 1:2000 in blocking solution for 1 h at room temperature. Enhanced chemiluminescent detection reagents were used to detect the antibody. The membrane was stripped with stripping buffer (100 mM glycine-HCl pH 2.8) and tested with an antibody against ERK-2 to assess equal protein loading per lane.
Image quantification
Image processing was performed by using software ImageJ 1.43 as previously published.27 Statistical analysis was performed with MS-Excel (Student's t-test, paired, two-tailed distribution).
Statistical analysis
MS-Excel (Microsoft Office; Microsoft) was used in statistical analysis, and statistically significant differences were evaluated by using the paired Student's t-test (p-value *p<0.05 and **p<0.01).
Results
MHS enhances the formation of capillary-like structures in a co-culture system of pOBs and OECs
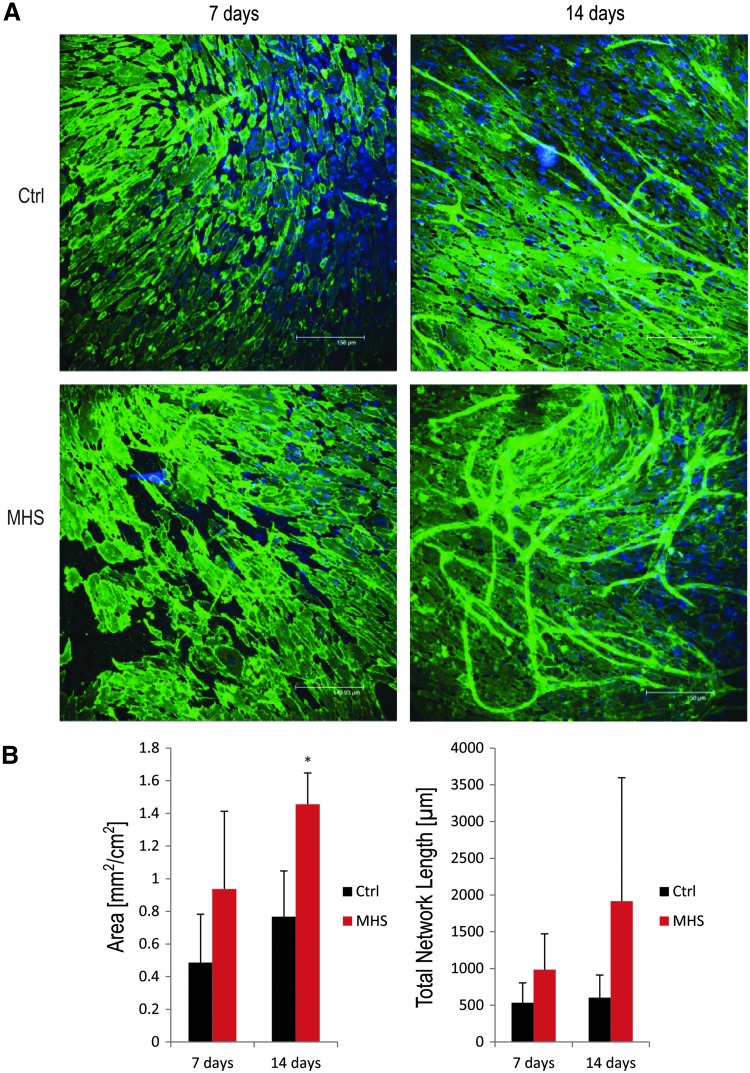
In order to investigate the effects of MHS on the formation of capillary-like structures in a co-culture system of pOBs and OECs, co-cultures consisting of pOBs and OECs were treated with direct HS at 41°C for 1 h twice weekly. After 7 and 14 days of cultivation, the microvessel-like structures were analyzed by using immunohistochemical staining with the endothelial marker PECAM CD31. An enhancement of the formation of capillary-like structures could be observed morphologically in MHS-treated co-cultures compared with untreated cultures (Fig. 1A).
FIG. 1.
Effect of mild heat stress (MHS) on co-cultures of primary osteoblasts (pOBs) and outgrowth endothelial cells (OECs). (A) Immunofluorescent staining for CD31 analysis of the effects of MHS on the formation of capillary-like structures in a co-culture system. After 1 day of co-cultivation, cells were treated under MHS conditions (at 41°C for 1 h) twice a week. After 7 and 14 days, co-cultures were stained immunohistochemically for the endothelial marker CD31, and the formation of capillary-like structures was detected by using a Leica confocal laser scanning microscope. (B) Quantification of the formation of capillary-like structures in a co-culture system. Angiogenic structures were quantified by comparing the total area and length of angiogenic structures in mild heat-stressed co-cultures after 7 and 14 days (n=3; nine images in total were analyzed for each treatment). (*p<0.05).
This morphological phenomenon was further investigated via quantitative analysis of capillary-like structures in MHS-treated co-cultures. The area of angiogenic structures and total skeleton length in the MHS-treated co-cultures were analyzed quantitatively after 7 and 14 days of heat stimulation.
After 14 days, MHS treatment resulted in a statistically significant increase in the area of angiogenic structures in the co-cultures. Similar positive effects in angiogenesis resulting from MHS treatment were observed for the length of the angiogenic structures after 14 days, as well as for the earlier time point of the co-culture (7 days) with no statistically significant difference, but positive effects in the area and length of angiogenic structures compared with the control.
In addition, enhanced microvessel-like structures could also be observed in co-cultures in nonheat-shocked cells treated with conditioned culture medium collected from cells exposed to MHS (41°C, 1 h) medium (HSM) twice per week over 14 days (Supplementary Fig. S1; Supplementary Data are available online at www.liebertpub.com/tec). These assays were performed to analyze whether the proangiogenic effects were mediated by the paracrine factors via an influence of MHS on the release of angiogenic growth factors.
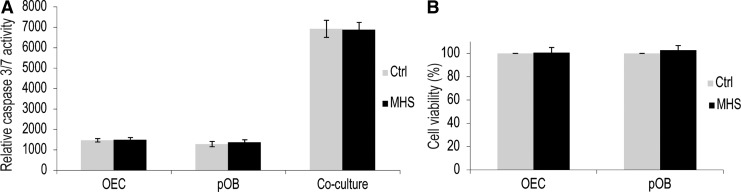
MHS did not induce apoptosis as shown by caspase-Glo 3/7 assay and MTS
MHS can increase angiogenesis in pOB and OEC co-culture systems (see above). However, heat-induced apoptosis might negatively affect cell viability and interfere with the beneficial effects on the neovascularization. In MHS-treated cells, no morphological evidence of apoptosis was observed for the conditions chosen in the experimental settings of the present studies. Thus, for example, in the fluorescent images, in which the nuclei gave a blue signal, there was no sign of nuclear fragmentation. Furthermore, we investigated apoptotic cell death in OECs and pOBs based on the activation of the apopotose-related enzymes caspase 3/7 by using the Caspase-Glo 3/7 assay kit. Caspase 3/7 activity did not increase in mono- and co-cultures after 1 h of MHS stimulation compared with untreated cultures (Fig. 2A).
FIG. 2.
Effects of MHS on apoptosis and cell viability. (A) Caspase 3/7 activity of mono- and co-culture consisting of pOBs and OECs treated with MHS. Cells were treated at 41°C for 1 h (grey bar) and compared with cells under control conditions (black bar). Caspase 3/7 activity was measured by the Caspase-Glo 3/7 assay kit. (B) Cell viability of pOB and OEC mono-cultures treated with MHS. MTS assays were performed to measure the survival rate of mono-cultures of OECs and pOBs after treatment with MHS. Data are expressed as the mean in percent compared with controls after exposure for 24 h±standard deviation (n=3).
In order to compare the effect of MHS on cell viability in mono-culture of pOBs and OECs, an MTS assay was used. The results of the MTS assay revealed that the cell viability of MHS-treated pOBs and OECs was equivalent to that of the untreated control (Fig. 2B). MHS-treated mono- and co-cultures consisting of OECs and pOBs did not cause cytotoxic effects interfering with the positive effects reported in this study.
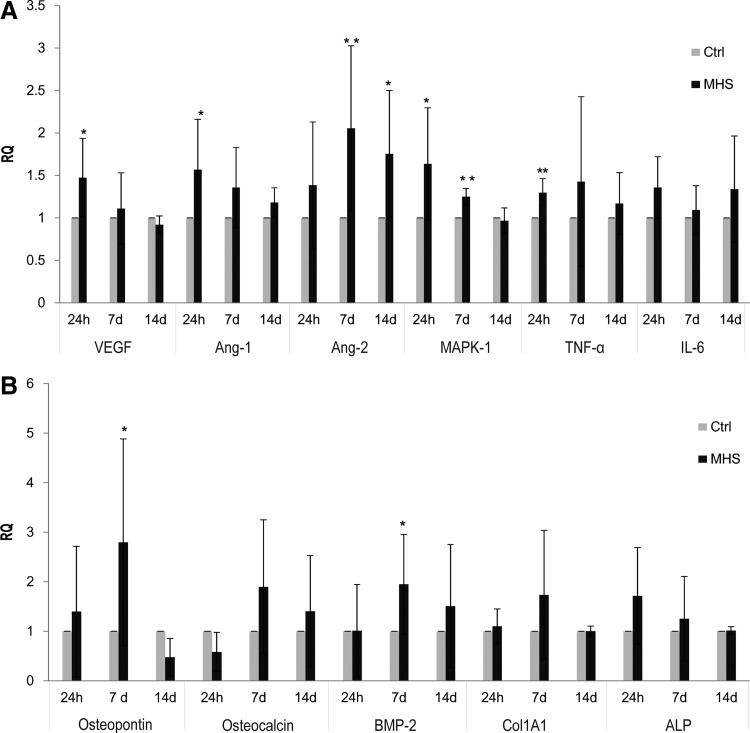
Effects of MHS treatment on angiogenic and osteogenic factors at the mRNA level
To analyze the molecular mechanisms leading to the angiogenic and osteogenic activation via heat stimulation of the co-culture, several angiogenic and osteogenic factors, as well as HSP-related angiogenic molecules were investigated by using semi-qRT-PCR. The gene expression of VEGF, angiopoietin-1 (Ang-1), mitogen-activated protein kinase 1 (MAPK1), and tumor necrosis factor-alpha (TNF-α) exhibited a statistically significant up-regulation in MHS-treated co-cultures in response to 1 h of stimulation and investigated 24 h post-treatment. However, Ang-2 was significantly up-regulated after 7 and 14 days of cultivation compared with untreated co-cultures (Fig. 3A). In addition, in order to assess the effect of MHS treatment on osteogenic differentiation, several osteogenic factors were analyzed by using qRT-PCR. As depicted in Figure 3B, the expression of osteopontin and BMP-2 was significantly up-regulated in MHS-treated co-cultures after 7 days of cultivation. At the same time, the relative gene expression of IL-6, osteocalcin, collagen type 1 alpha 1 (Col1A1), and ALP showed the tendency to increase slightly after MHS stimulation in the cultures investigated after 24 h and 7 days (Fig. 3A, B).
FIG. 3.
Effects of MHS on expression of angiogeneic and osteogenic factors at the mRNA level. Co-cultures consisting of pOBs and OECs were treated for 1 h at 41°C (twice a week) and incubated for 24 h, 7 days, and 14 days. Quantitative real-time polymerase chain reaction (qRT-PCR) was performed to examine differences in the expression of angiogenic factors (A) and osteogenic factors (B) in MHS-treated co-cultures compared with control co-cultures. d-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was chosen as the endogenous control. (*p<0.05, **p<0.01) n=3.
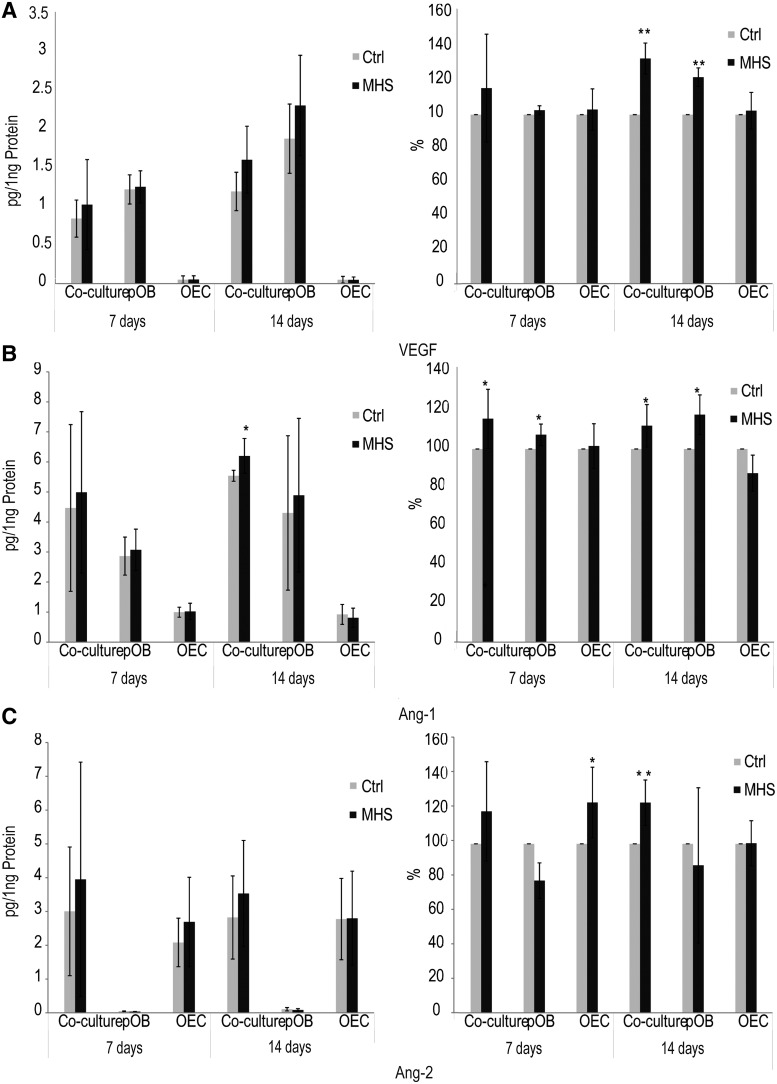
Up-regulation of angiogenic factors in response to MHS at the protein level
Growth factors VEGF, Ang-1, and Ang-2 play important roles in the regulation of the angiogenic process. The secretion of these molecules was analyzed at the protein level in MHS-treated and untreated mono- and co-cultures of pOBs and OECs by ELISA. The culture supernatants from MHS-treated and untreated mono- and co-cultures were collected after 1 and 2 weeks for analysis by ELISA. Results from ELISA for the angiogenic factors VEGF, Ang-1, and Ang-2 in MHS-treated cultures are shown as absolute values (left) and are additionally presented as the relative change (control=100%) (right) compared with untreated cells (Fig. 4).
FIG. 4.
Effects of MHS on the release of angiogenic and osteogenic factors in mono- and co-culture systems at the protein level by using enzyme-linked immunosorbent assay (ELISA). The concentrations of vascular endothelial growth factor (VEGF) (A), angiopoietin-1 (Ang-1) (B), and Ang-2 (C) in the supernatants of co-cultures consisting of pOBs and OECs were measured by ELISA after 1 and 2 weeks of cultivation and stimulation at 41°C for 1 h (twice a week) compared with untreated control co-cultures. Results are represented as absolute values (left) and are additionally shown as ratio in percent (control=100%) (right). (*p<0.05) n=4.
The free VEGF level was significantly increased in MHS-treated pOB mono- and co-culture supernatants compared with untreated culture supernatants after 2 weeks (Fig. 4A). In addition, MHS-stimulated pOB mono-cultures and co-cultures released significant higher levels of Ang-1 than untreated cultures after both 7 and 14 days in vitro (Fig. 4B). Significantly increased concentrations of Ang-2 were also detected in MHS-treated OEC mono-cultures after 1 week of cultivation and in co-culture supernatants after 2 weeks of cultivation compared with untreated cultures (Fig. 4C). In general, in the pOB mono-cultures, higher VEGF levels were found than in co-cultures. VEGF and Ang-1 was not detected in OEC mono-cultures, nor was Ang-2 measureable in pOB mono-cultures (Fig. 4A–C). These data are consistent with reports from previous studies from our group with regard to the cell type-related production of specific molecules involved in the regulation of angiogenesis.
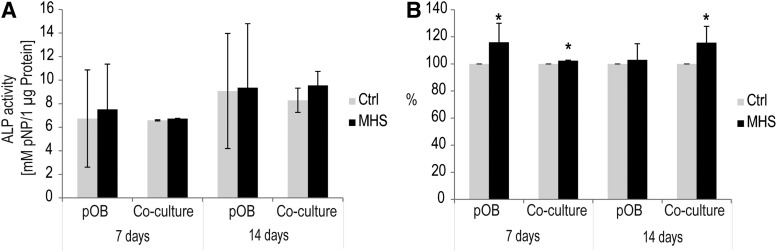
Consequences of MHS treatment on osteoblastic differentiation
During bone formation, matrix mineralization is mainly mediated through the activity of the essential osteoblastic differentiation marker, ALP. In order to confirm the potential positive effect of MHS on osteoblastic differentiation, the activity of ALP after 7 and 14 days of culture was examined in MHS-treated and untreated pOB mono-cultures and co-cultures. ALP is shown as absolute concentrations given as mM/mg protein (Fig. 5A) and as relative change in percent in relation to the control (Fig. 5B) to identify influences of MHS stimulation in pOB mono- or co-culture, respectively. Mono-cultures of pOBs exposed to MHS revealed significantly higher levels of ALP activity during 7 days of incubation compared with the untreated controls. In addition, the level of ALP activity was significantly increased in MHS-treated co-cultures after 7 or 14 days of cultivation compared with untreated co-cultures (Fig. 5B, p<0.05). Nevertheless, analyzing the ALP activity in relation to the total protein content resulted in no significant effects (Fig. 5A) of MHS on ALP-activity.
FIG. 5.
Effect of MHS on osteoblastic differentiation of mono-culture of pOBs and co-culture consisting of pOBs and OECs as measured by the levels of alkaline phosphatase (ALP). (A, B) ALP activity within the cell lysates of MHS-treated (black bar) co-cultures and pOB mono-cultures compared with untreated cultures (gray bar) after 7 and 14 days. Results are presented as averages. The absolute ALP activity was defined as mM pNP/μg protein (A) and as ratios (B) with regard to control (control=100%). (*p<0.05) n=3.
Furthermore, the effect of MHS on the mineralization of mono-cultures and co-cultures after 7 and 14 days was investigated by using Alizarin red quantification (data not shown). Although the increase was not statistically significant, the data revealed a consistent trend (7.5% and 3% to control after 7 days, and 25%–17% to control after 14 days in pOB and co-culture, respectively).
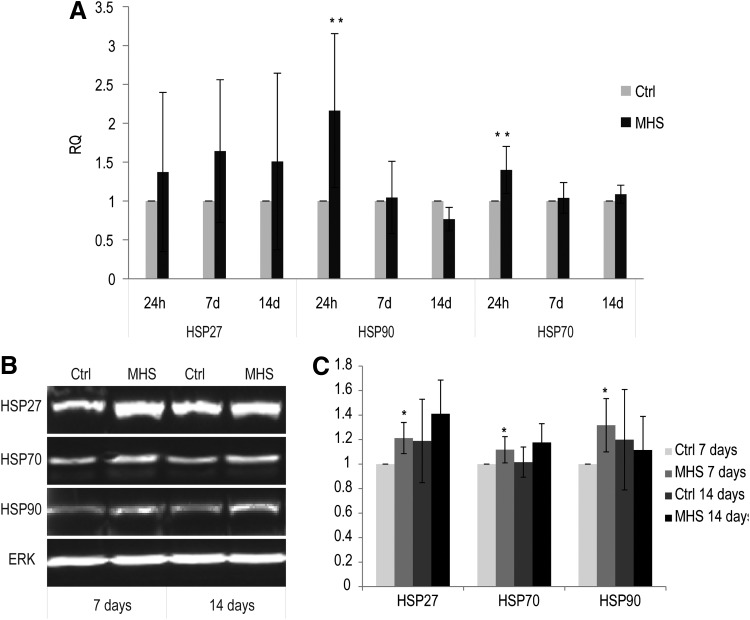
Up-regulation of HSPs at the mRNA and protein levels in MHS-treated co-cultures
Heat-treated co-cultures significantly increased their expression of HSP90 and HSP70 genes, after 1 h of MHS treatment and investigated 24 h post-treatment, compared with untreated co-cultures. In general, the expression of HSP27 at the mRNA level increased concomitantly in MHS-treated co-cultures, compared with nontreated cultures after 24 h, 7 days, and 14 days of cultivation (Fig. 6A).
FIG. 6.
Effects of MHS on expression of heat shock proteins (HSPs) at the mRNA and protein levels. (A) Analysis of the expression of HSPs at the mRNA level by using qRT-PCR; Co-cultures consisting of pOBs and OECs were treated for 1 h at 41°C (twice a week) and incubated for 24 h, 7 days, and 14 days. qRT-PCR was performed to examine differences in the expression of HSPs in MHS-treated co-cultures compared with control co-cultures. GAPDH was taken as an endogenous control. (B) Analysis of the expression of HSPs at the protein level by using sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) and western blot. ERK-2 was used as a loading control. (C) Quantification of HSP amounts. Control co-cultures cultivated for 7 days were set to 1. (*p<0.05, **p<0.01) n=3.
The effect of MHS on the expression of HSPs was further analyzed at the protein level by using SDS-PAGE and western blotting (Fig. 6B, C). The protein amount of HSPs was analyzed in response to the various treatments and standardized to ERK, used as an internal control in co-culture cell lysates. These experiments revealed that the amount of protein of HSP27, HSP70, and HSP90 significantly increased in MHS-treated co-cultures at 7 days of cultivation compared with untreated co-cultures (Fig. 6B, C, p<0.05). Nevertheless, although the protein amount of HSP27 and HSP70 increased in MHS-treated co-cultures after 14 days of cultivation (Fig. 6B, C) in comparison to untreated co-cultures, this was not statistically significant. In addition, similar expression of HSPs was detected in both pOB and OEC mono-cultures (data not shown).
Discussion
MHS is one of the common physical stresses possibly enhancing tissue repair. On the basis of a co-culture system consisting of pOBs and OECs, we have shown that the formation of microvessel-like structures in vitro can be supported by MHS. This effect of MHS is associated with the induction of angiogenic factors and HSPs that are involved in the up-regulation of angiogenesis. In addition, we observed no obvious negative effects of MHS treatment on the viability of the investigated cell types in this study. Thus, mild physical stress factors such as MHS could be of considerable therapeutic potential to enhance tissue regeneration and might act as an additional means to enhance bone repair, both in situ and in a tissue engineering strategy.
Prevascularization strategies based on the inclusion of endothelial cells and primitive prevascular structures into tissue constructs make use of the natural interaction of endothelial cells with angiogenic factor-producing cells, either present or recruited to the regenerative niche. Several studies in the past have already reported that these prevascularization strategies might also be applied to bone tissue vascularization. Co-culture system consisting, for example, of pOBs and OECs, support the formation of vascular structures in vitro and in vivo.26,28 In addition, they can also serve as advanced in vitro model systems to analyze the potential effects of growth factors, morphogens from the embryonic phase,21 or biomaterial design for bone vascularization and regeneration.28
During recent years, much effort has been invested to enhance vascularization by extrinsic factors, which also implement the use of suitable delivery systems. Nevertheless, extrinsic factors are associated with safety concerns and undesired side effects.
In contrast to methods based on extrinsic factors, physical treatments might thus offer some advantages in terms of costs and general manageability of the treatment.
Rattan and Ali8 have demonstrated that after stimulation by MHS, human microsvacsular cells (HMEC-1) possess a higher tube-formation ability than macrovascular cells in vitro. In our experimental setting, using a co-culture system with osteogenic cells, MHS leads to the enhanced formation of vascular structures by blood-derived OECs. According to our observations and reports from the literature, OEC can be considered endothelial cells with microvascular characteristics.29
VEGF is one key growth factor in the promotion of blood vessel formation, and it also controls bone formation via mediating the communication between endothelial cells and bone cells.30 In this co-culture context, the osteoblast provides both proangiogenic matrix components such as collagen and VEGF, which guides endothelial proliferation and formation of vascular sprouts. In addition, as shown in this and in previous studies, osteoblasts or mesenchymal stem cells are also involved in the stabilization of vascular structures by producing Ang-1 or might even act as pericyte-like cells.31 This stabilization of newly formed vessels is one of the key processes in the generation of a functional vasculature in tissue engineering and regenerative medicine. We have previously shown that treatment of co-cultures with the morphogen SHH supports angiogenesis, but does not interfere with vessel-stabilizing factors. In addition, osteogenic differentiation is also stimulated simultaneously by SHH.22,24 Nevertheless, SHH is a potent and multi-facetted molecule that also carries potential risks. The present study shows that a potentially safer stress treatment, namely MHS, can also up-regulate both VEGF and Ang-1 in pOB mono-cultures and in co-cultures of OECs and pOBs and thus offers an additional means to support prevascularization. The effect of MHS on these key regulatory molecules in angiogenesis was proved at the gene regulatory level using real-time PCR, as well at the protein level.
In terms of the molecular pathways associated with MHS in driving angiogenesis, it is known that whole-body hyperthermia induces increased levels of VEGF, which also correlates with the up-regulation of HSP70 in cardiac tissue Gong et al.7 In our experiments, we observed a significant up-regulation of this molecule at gene expression and protein level in the co-cultures. Moreover, thermal stress preconditioning with or without growth factors was shown to induce the up-regulation of VEGF in a murine preosteoblastic cell line, MC3T3-E1.32 In addition, HSP70 expression might be associated with VEGF signaling and angiogenesis Wang et al.1 VEGF levels in the cell culture supernatant of pOB were increased, with the released VEGF from MHS-stimulated pOBs probably being absorbed by OECs, thus leading to the activation of OECs in the co-culture. This might also explain why the increase in VEGF release observed in MHS-treated co-cultures was lower than that in the mono-cultures.
MHS might be an advantageous medical therapy to induce and to improve angiogenesis during implantation. In addition, MHS might accelerate the formation of prevascular tissues in vitro with reduced effective preculture time. Nevertheless, MHS treatment did not result in a clear effect on osteogenic differentiation in the co-culture model, although some osteogenic differentiation markers, such as osteopontin, and BMP-2 were significantly up-regulated as shown by real-time PCR in co-culture systems (Fig. 3B). On the other hand, the determination of the ALP activity and the effect on calcification did not result in consistent effects of MHS on osteogenic differentiation. Nevertheless, some previous studies showed that HS induces a positive effect on bone growth and remodeling in vitro and in vivo.13,33 Recently, conditions involving heating and growth factors in combination resulted in the inhibition of osteoclast differentiation and bone-resorptive processes in the bone microenvironment.32
The close relationship between HSP synthesis and tissue regeneration under HS conditions has also been reported.15,16 In terms of the role of HSPs in bone repair, HSP27 and HSP70 have been shown to be highly expressed in osteoblasts of new bone-generating areas, along with type I collagen, as well as in rat tibia and in preosteoblasts after identical heating conditions in vitro.32,34,35 HSP27, in particular, plays an important role in bone regeneration through the up-regulation of TGF-β,36 estrogen,37 endothelin-1,38 and prostaglandins.39 In summary, this indicates that HSPs are crucial for tissue regeneration. In our study, the increased expression of HSPs appears to be correlated with angiogenesis. It can also be postulated that the up-regulated expression of HSPs after the MHS treatment of co-cultures should increase stress tolerance after implantation of tissue constructs. The preconditioning of tissue-engineered constructs with HS has been reported to cause a significant increase in the number of surviving osteoblast-like cells through induced HSPs.40 Therefore, MHS treatment might support a beneficial and safe implantation of tissue constructs with high stress resistance. In addition, we observed no negative effects on cell viability in our experimental setting. However, the expression of HSPs at the protein level in the co-culture systems at 14 days is not significantly increased under MHS-treatment conditions; this might be attributable to the higher constitutive expression of these HSPs in co-cultures after 14 days in vitro.
In conclusion, MHS has a stimulating effect on the processes of angiogenesis in co-cultures consisting of OECs and pOBs. MHS influences the activation of OECs in co-culture by increasing the secretion of VEGF and Ang-1 in pOBs, while the effects on the osteogenic differentiation markers need to be further refined in future experiments. In addition, the enhancement of HSP expression may involve chaperons involved in the angiogenic and osteogenic pathways. The advantages of the MHS system lie in the ability to enhance tissue regeneration without additional exogenous molecular factors. In addition, the heat-treated co-cultures might provide increasing stress tolerance to the tissue, a benefit for further implantation.
Supplementary Material
Acknowledgments
The authors thank BMBF (German-Chinese Young investigator group grant number 0315033) for financial support; Eva Dohle, Marlen Kolbe, Xin Jiang, and Bin Ma for their friendly help; and Barbara Pavic and Stephanie Hünerkopf for their excellent technical assistance.
Disclosure Statement
No competing financial interests exist.
References
- 1.Wang Y., Wan C., Deng L., Liu X., Cao X., Gilbert S.R., et al. . The hypoxia-inducible factor alpha pathway couples angiogenesis to osteogenesis during skeletal development. J Clin Invest 117,1616, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rosova I., Dao M., Capoccia B., Link D., and Nolta J.A.Hypoxic preconditioning results in increased motility and improved therapeutic potential of human mesenchymal stem cells. Stem Cells 26,2173, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.West X.Z., Malinin N.L., Merkulova A.A., Tischenko M., Kerr B.A., Borden E.C., et al. . Oxidative stress induces angiogenesis by activating TLR2 with novel endogenous ligands. Nature 467,972, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ueda A., Koga M., Ikeda M., Kudo S., and Tanishita K.Effect of shear stress on microvessel network formation of endothelial cells with in vitro three-dimensional model. Am J Physiol Heart Circ Physiol 287,H994, 2004 [DOI] [PubMed] [Google Scholar]
- 5.Li T.S., Hamano K., Suzuki K., Ito H., Zempo N., and Matsuzaki M.Improved angiogenic potency by implantation of ex vivo hypoxia prestimulated bone marrow cells in rats. Am J Physiol Heart Circ Physiol 283,H468, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Park H.G., Han S.I., Oh S.Y., and Kang H.S.Cellular responses to mild heat stress. Cell Mol Life Sci 62,10, 2005 [DOI] [PubMed] [Google Scholar]
- 7.Gong B., Asimakis G.K., Chen Z., Albrecht T.B., Boor P.J., Pappas T.C., et al. . Whole-body hyperthermia induces up-regulation of vascular endothelial growth factor accompanied by neovascularization in cardiac tissue. Life Sci 79,1781, 2006 [DOI] [PubMed] [Google Scholar]
- 8.Rattan S.I., and Ali R.E.Hormetic prevention of molecular damage during cellular aging of human skin fibroblasts and keratinocytes. Ann N Y Acad Sci 1100,424, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Harder Y., Contaldo C., Klenk J., Banic A., Jakob S.M., and Erni D.Improved skin flap survival after local heat preconditioning in pigs. J Surg Res 119,100, 2004 [DOI] [PubMed] [Google Scholar]
- 10.Riederer I., Negroni E., Bigot A., Bencze M., Di Santo J., Aamiri A., et al. . Heat shock treatment increases engraftment of transplanted human myoblasts into immunodeficient mice. Transplant Proc 40,624, 2008 [DOI] [PubMed] [Google Scholar]
- 11.Norgaard R., Kassem M., and Rattan S.I.Heat shock-induced enhancement of osteoblastic differentiation of hTERT-immortalized mesenchymal stem cells. Ann N Y Acad Sci 1067,443, 2006 [DOI] [PubMed] [Google Scholar]
- 12.Ogawa H.[Effects of the localized thermal enhancement on new bone formation following mechanical expansion of the rat sagittal suture]. Nihon Kyosei Shika Gakkai Zasshi 49,485, 1990 [PubMed] [Google Scholar]
- 13.Shui C., and Scutt A.Mild heat shock induces proliferation, alkaline phosphatase activity, and mineralization in human bone marrow stromal cells and Mg-63 cells in vitro. J Bone Miner Res 16,731, 2001 [DOI] [PubMed] [Google Scholar]
- 14.Ye C.P., Heng B.C., Liu H., Toh W.S., and Cao T.Culture media conditioned by heat-shocked osteoblasts enhances the osteogenesis of bone marrow-derived mesenchymal stromal cells. Cell Biochem Funct 25,267, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Hebb M.O., Myers T.L., and Clarke D.B.Enhanced expression of heat shock protein 27 is correlated with axonal regeneration in mature retinal ganglion cells. Brain Res 1073,146, 2006 [DOI] [PubMed] [Google Scholar]
- 16.Laplante A.F., Moulin V., Auger F.A., Landry J., Li H., Morrow G., et al. . Expression of heat shock proteins in mouse skin during wound healing. J Histochem Cytochem 46,1291, 1998 [DOI] [PubMed] [Google Scholar]
- 17.Lanneau D., de Thonel A., Maurel S., Didelot C., and Garrido C.Apoptosis versus cell differentiation: role of heat shock proteins HSP90, HSP70 and HSP27. Prion 1,53, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Evans I.M., Britton G., and Zachary I.C.Vascular endothelial growth factor induces heat shock protein (HSP) 27 serine 82 phosphorylation and endothelial tubulogenesis via protein kinase D and independent of p38 kinase. Cell Signal 20,1375, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Kato K., Tokuda H., Adachi S., Matsushima-Nishiwaki R., Yamauchi J., Natsume H., et al. . Role of heat shock protein 27 in transforming growth factor-beta-stimulated vascular endothelial growth factor release in osteoblasts. Int J Mol Med 27,423, 2011 [DOI] [PubMed] [Google Scholar]
- 20.Song X., and Luo Y.The regulatory mechanism of Hsp90alpha secretion from endothelial cells and its role in angiogenesis during wound healing. Biochem Biophys Res Commun 398,111, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Dohle E., Fuchs S., Kolbe M., Hofmann A., Schmidt H., and Kirkpatrick C.J.Sonic hedgehog promotes angiogenesis and osteogenesis in a coculture system consisting of primary osteoblasts and outgrowth endothelial cells. Tissue Eng Part A 16,1235, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dohle E., Fuchs S., Kolbe M., Hofmann A., Schmidt H., and Kirkpatrick C.J.Comparative study assessing effects of sonic hedgehog and VEGF in a human co-culture model for bone vascularisation strategies. Eur Cell Mater 21,144, 2011 [DOI] [PubMed] [Google Scholar]
- 23.Fuchs S., Hermanns M.I., and Kirkpatrick C.J.Retention of a differentiated endothelial phenotype by outgrowth endothelial cells isolated from human peripheral blood and expanded in long-term cultures. Cell Tissue Res 326,79, 2006 [DOI] [PubMed] [Google Scholar]
- 24.Kolbe M., Dohle E., Katerla D., Kirkpatrick C.J., and Fuchs S.Enrichment of outgrowth endothelial cells in high and low colony-forming cultures from peripheral blood progenitors. Tissue Eng Part C Methods 16,877, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hofmann A., Konrad L., Gotzen L., Printz H., Ramaswamy A., and Hofmann C.Bioengineered human bone tissue using autogenous osteoblasts cultured on different biomatrices. J Biomed Mater Res A 67,191, 2003 [DOI] [PubMed] [Google Scholar]
- 26.Fuchs S., Hofmann A., and Kirkpatrick C.J.Microvessel-like structures from outgrowth endothelial cells from human peripheral blood in 2-dimensional and 3-dimensional co-cultures with osteoblastic lineage cells. Tissue Eng 13,2577, 2007 [DOI] [PubMed] [Google Scholar]
- 27.Fuchs S., Jiang X., Schmidt H., Dohle E., Ghanaati S., Orth C., et al. . Dynamic processes involved in the pre-vascularization of silk fibroin constructs for bone regeneration using outgrowth endothelial cells. Biomaterials 30,1329, 2009 [DOI] [PubMed] [Google Scholar]
- 28.Fuchs S., Ghanaati S., Orth C., Barbeck M., Kolbe M., Hofmann A., et al. . Contribution of outgrowth endothelial cells from human peripheral blood on in vivo vascularization of bone tissue engineered constructs based on starch polycaprolactone scaffolds. Biomaterials 30,526, 2009 [DOI] [PubMed] [Google Scholar]
- 29.Jiang A., Pan W., Milbauer L.C., Shyr Y., and Hebbel R.P.A practical question based on cross-platform microarray data normalization: are BOEC more like large vessel or microvascular endothelial cells or neither of them? J Bioinform Comput Biol 5,875, 2007 [DOI] [PubMed] [Google Scholar]
- 30.Dai J., and Rabie A.B.VEGF: an essential mediator of both angiogenesis and endochondral ossification. J Dent Res 86,937, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Sundberg C., Kowanetz M., Brown L.F., Detmar M., and Dvorak H.F.Stable expression of angiopoietin-1 and other markers by cultured pericytes: phenotypic similarities to a subpopulation of cells in maturing vessels during later stages of angiogenesis in vivo. Lab Invest 82,387, 2002 [DOI] [PubMed] [Google Scholar]
- 32.Chung E., and Rylander M.N.Response of preosteoblasts to thermal stress conditioning and osteoinductive growth factors. Cell Stress Chaperones 17,203, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yoshida K., Uoshima K., Oda K., and Maeda T.Influence of heat stress to matrix on bone formation. Clin Oral Implants Res 20,782, 2009 [DOI] [PubMed] [Google Scholar]
- 34.Tiffee J.C., Griffin J.P., and Cooper L.F.Immunolocalization of stress proteins and extracellular matrix proteins in the rat tibia. Tissue Cell 32,141, 2000 [DOI] [PubMed] [Google Scholar]
- 35.Rylander M.N., Feng Y., Zimmermann K., and Diller K.R.Measurement and mathematical modeling of thermally induced injury and heat shock protein expression kinetics in normal and cancerous prostate cells. Int J Hyperthermia 26,748, 2010 [DOI] [PubMed] [Google Scholar]
- 36.Hatakeyama D., Kozawa O., Niwa M., Matsuno H., Ito H., Kato K., et al. . Upregulation by retinoic acid of transforming growth factor-beta-stimulated heat shock protein 27 induction in osteoblasts: involvement of mitogen-activated protein kinases. Biochim Biophys Acta 1589,15, 2002 [DOI] [PubMed] [Google Scholar]
- 37.Cooper L.F., and Uoshima K.Differential estrogenic regulation of small M(r) heat shock protein expression in osteoblasts. J Biol Chem 269,7869, 1994 [PubMed] [Google Scholar]
- 38.Tokuda H., Niwa M., Ito H., Oiso Y., Kato K., and Kozawa O.Involvement of stress-activated protein kinase/c-Jun N-terminal kinase in endothelin-1-induced heat shock protein 27 in osteoblasts. Eur J Endocrinol 149,239, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Kozawa O., Otsuka T., Hatakeyama D., Niwa M., Matsuno H., Ito H., et al. . Mechanism of prostaglandin D(2)-stimulated heat shock protein 27 induction in osteoblasts. Cell Signal 13,535, 2001 [DOI] [PubMed] [Google Scholar]
- 40.Tavassol F., Kampmann A., Lindhorst D., Schumann P., Kokemuller H., Bormann K.H., et al. . Prolongated survival of osteoblast-like cells on biodegradable scaffolds by heat shock preconditioning. Tissue Eng Part A 17,1935, 2011 [DOI] [PubMed] [Google Scholar]
- 41.Yao Y., Watson AD., Ji S., and Boström KI.Heat shock protein 70 enhances vascular bone morphogenetic protein-4 signaling by binding matrix Gla protein. Circ Res 105,575, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.