Abstract
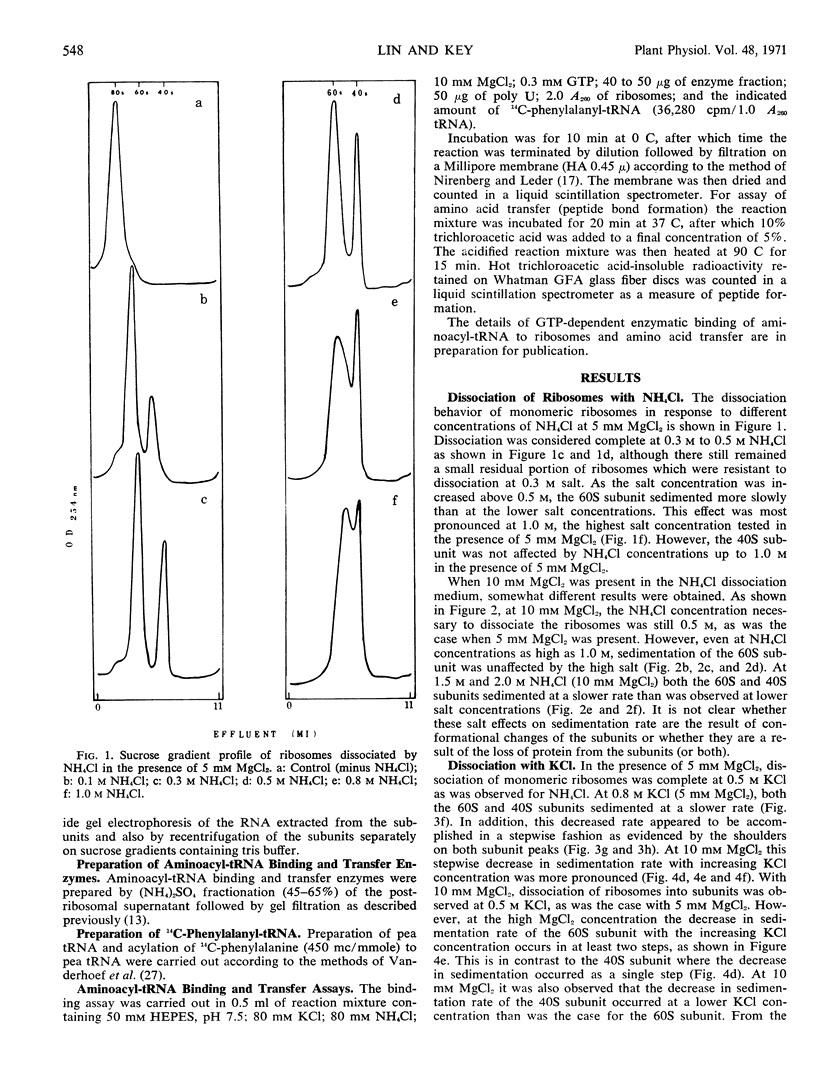
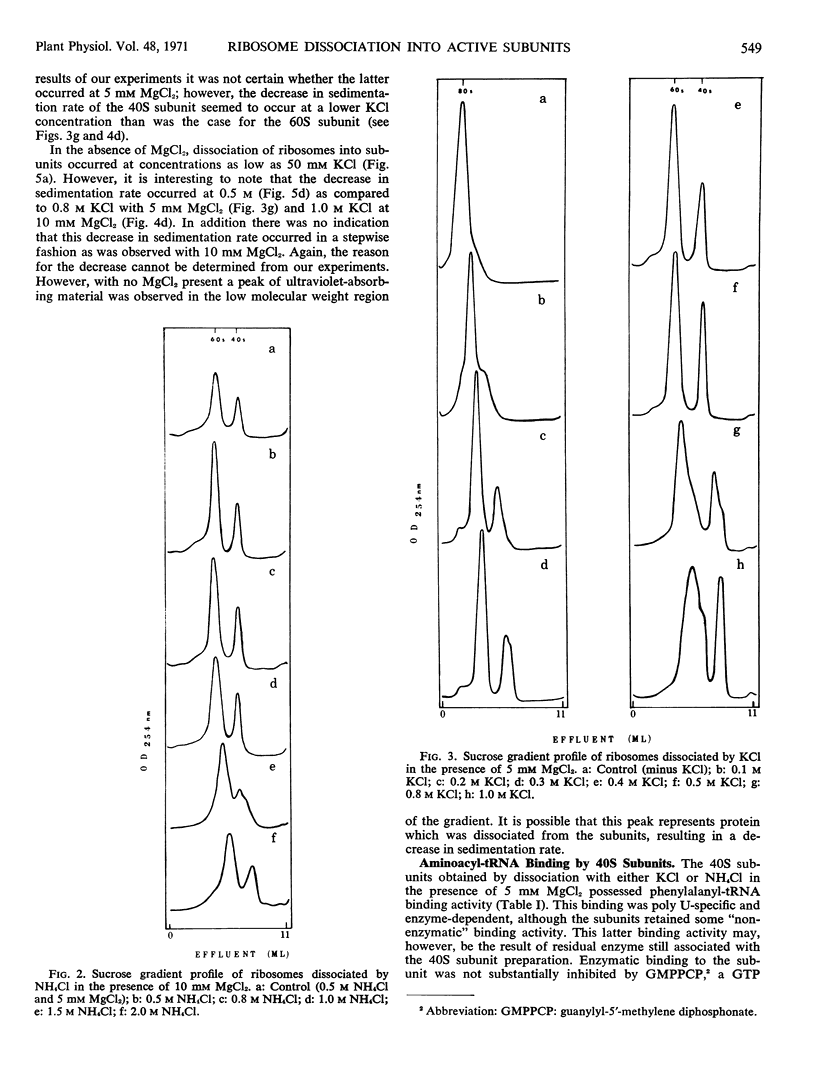
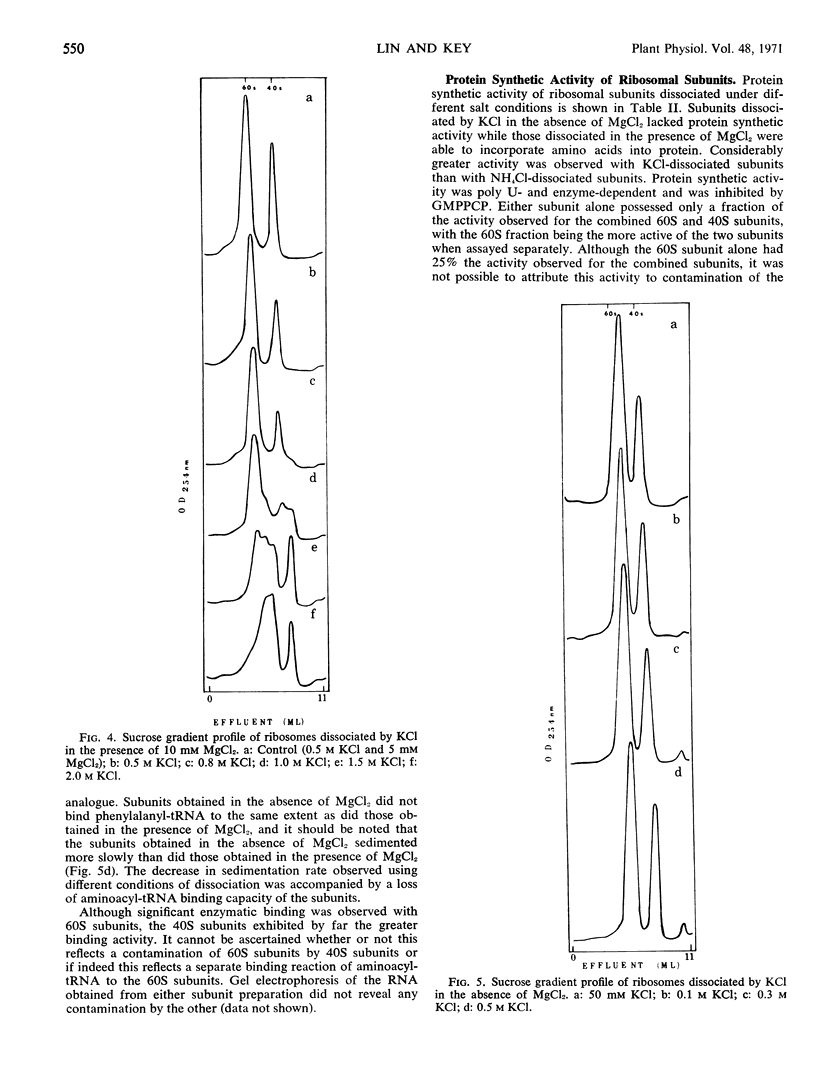
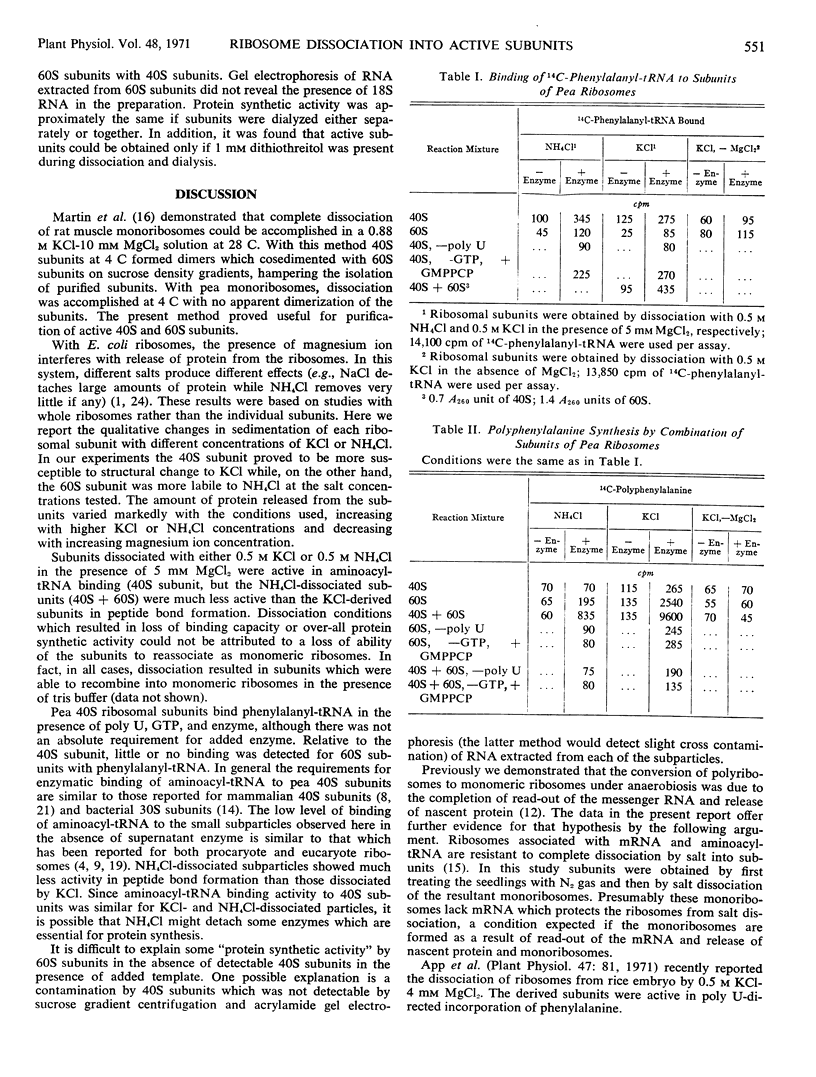
The dissociation of N2 gas-induced monomeric ribosomes from the pea root was studied by varying the concentration of KCl (or NH4Cl) and MgCl2 in the presence of dithiothreitol. These monoribosomes were shown to dissociate completely into subunits at 0.5m KCl or NH4Cl in the presence of 5 mm MgCl2. The 40S subunits were more susceptible to structural change in KCl than were the 60S subunits. On the other hand, the 60S subunits appeared to be more labile to NH4Cl.
The activity of the subunits relative to aminoacyl-tRNA binding and peptide bond formation was investigated using subunits derived from 0.5 m KCl (or NH4Cl) in the absence and presence of 5 mm MgCl2. The 40S subunits were active in aminoacyl-tRNA binding only when dissociated in the presence of MgCl2. The 40S and 60S subunits combined in the presence of poly U were active in incorporation of 14C-phenylalanine from 14C-phenylalanyl-tRNA only when dissociation was achieved in the presence of 5 mm MgCl2. The KCl-dissociated subunits were much more active in protein synthesis than NH4Cl-dissociated subunits.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atsmon A., Spitnik-Elson P., Elson D. Detachment of ribosomal proteins by salt. II. Some properties of protein-deficient particles formed by the detachment of ribosomal proteins. J Mol Biol. 1969 Oct 14;45(1):125–135. doi: 10.1016/0022-2836(69)90215-0. [DOI] [PubMed] [Google Scholar]
- BAYLEY S. T. PHYSICAL STUDIES ON RIBOSOMES FROM PEA SEEDLINGS. J Mol Biol. 1964 Feb;8:231–238. doi: 10.1016/s0022-2836(64)80132-7. [DOI] [PubMed] [Google Scholar]
- BONNER J., TS'O P. O., VINOGRAD J. Microsomal nucleoprotein particles from pea seedlings. J Biophys Biochem Cytol. 1956 Jul 25;2(4):451–466. doi: 10.1083/jcb.2.4.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonanou S., Cox R. A., Higginson B., Kanagalingam K. The production of biologically active subparticles from rabbit reticulocyte ribosomes. Biochem J. 1968 Nov;110(1):87–98. doi: 10.1042/bj1100087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castles J. J., Wool I. G. Polyuridylic acid directed binding of phenylalanyl transfer ribonucleic acid to mammalian 40S ribosomal subunits. Biochemistry. 1970 Apr 28;9(9):1909–1916. doi: 10.1021/bi00811a008. [DOI] [PubMed] [Google Scholar]
- Dickman S. R., Bruenger E. Purification and properties of dog pancreas ribosomes and subunits. Biochemistry. 1969 Aug;8(8):3295–3303. doi: 10.1021/bi00836a025. [DOI] [PubMed] [Google Scholar]
- GROS F., HIATT H., GILBERT W., KURLAND C. G., RISEBROUGH R. W., WATSON J. D. Unstable ribonucleic acid revealed by pulse labelling of Escherichia coli. Nature. 1961 May 13;190:581–585. doi: 10.1038/190581a0. [DOI] [PubMed] [Google Scholar]
- Gould H. J., Arnstein H. R., Cox R. A. The dissociation of reticulocyte polysomes into subunits and the location of messenger RNA. J Mol Biol. 1966 Feb;15(2):600–618. doi: 10.1016/s0022-2836(66)80130-4. [DOI] [PubMed] [Google Scholar]
- Hamada K., Yang P., Heintz R., Schweet R. Some properties of reticulocyte ribosomal subunits. Arch Biochem Biophys. 1968 May;125(2):598–603. doi: 10.1016/0003-9861(68)90618-8. [DOI] [PubMed] [Google Scholar]
- Igarashi K., Kaji A. Evidence for one functional phenylalanyl-tRNA binding site on the 30S ribosomal subunit. Proc Natl Acad Sci U S A. 1969 Feb;62(2):498–505. doi: 10.1073/pnas.62.2.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingle J., Key J. L. A comparative evaluation of the synthesis of DNA-like RNA in excised and intact plant tissues. Plant Physiol. 1965 Nov;40(6):1212–1219. doi: 10.1104/pp.40.6.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leder P., Skogerson L. E., Roufa D. J. Translocation of mRNA codons. II. Properties of an anti-translocase antibody. Proc Natl Acad Sci U S A. 1969 Mar;62(3):928–933. doi: 10.1073/pnas.62.3.928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin C. Y., Key J. L. Dissocation and reassembly of polyribosomes in relation to protein synthesis in the soybean root. J Mol Biol. 1967 Jun 14;26(2):237–247. doi: 10.1016/0022-2836(67)90294-x. [DOI] [PubMed] [Google Scholar]
- Lucas-Lenard J., Haenni A. L. Requirement of granosine 5'-triphosphate for ribosomal binding of aminoacyl-SRNA. Proc Natl Acad Sci U S A. 1968 Feb;59(2):554–560. doi: 10.1073/pnas.59.2.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin T. E., Hartwell L. H. Resistance of active yeast ribosomes to dissociation by KCl. J Biol Chem. 1970 Mar 25;245(6):1504–1506. [PubMed] [Google Scholar]
- Martin T. E., Rolleston F. S., Low R. B., Wool I. G. Dissociation and reassociation of skeletal muscle ribosomes. J Mol Biol. 1969 Jul 14;43(1):135–149. doi: 10.1016/0022-2836(69)90084-9. [DOI] [PubMed] [Google Scholar]
- NIRENBERG M., LEDER P. RNA CODEWORDS AND PROTEIN SYNTHESIS. THE EFFECT OF TRINUCLEOTIDES UPON THE BINDING OF SRNA TO RIBOSOMES. Science. 1964 Sep 25;145(3639):1399–1407. doi: 10.1126/science.145.3639.1399. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y., Lipmann F. Comparison of guanosine triphosphate split and polypeptide synthesis with a purified E. coli system. Proc Natl Acad Sci U S A. 1966 Jan;55(1):212–219. doi: 10.1073/pnas.55.1.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestka S., Nirenberg M. Regulatory mechanisms and protein synthesis. X. Codon recognition on 30 S ribosomes. J Mol Biol. 1966 Oct 28;21(1):145–171. doi: 10.1016/0022-2836(66)90085-4. [DOI] [PubMed] [Google Scholar]
- Phillips M., Hersh R. T. Dissociation studies on the 80 S ribosome from Pisum sativum. Exp Cell Res. 1970 Aug;61(2):365–370. doi: 10.1016/0014-4827(70)90459-3. [DOI] [PubMed] [Google Scholar]
- Rao P., Moldave K. Interaction of polypeptide chain elongation factors with rat liver ribosomal subunits. J Mol Biol. 1969 Dec 28;46(3):447–457. doi: 10.1016/0022-2836(69)90188-0. [DOI] [PubMed] [Google Scholar]
- Ravel J. M. Demonstration of a guanosine triphosphate-dependent enzymatic binding of aminoacyl-ribonucleic acid to Escherichia coli ribosomes. Proc Natl Acad Sci U S A. 1967 Jun;57(6):1811–1816. doi: 10.1073/pnas.57.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SCHLESSINGER D., GROS F. STRUCTURE AND PROPERTIES OF ACTIVE RIBOSOMES OF ESCHERICHIA COLI. J Mol Biol. 1963 Oct;7:350–359. doi: 10.1016/s0022-2836(63)80029-7. [DOI] [PubMed] [Google Scholar]
- Spitnik-Elson P., Atsmon A. Detachment of ribosomal proteins by salt. I. Effect of conditions on the amount of protein detached. J Mol Biol. 1969 Oct 14;45(1):113–124. doi: 10.1016/0022-2836(69)90214-9. [DOI] [PubMed] [Google Scholar]
- Tissieres A., Schlessinger D., Gros F. AMINO ACID INCORPORATION INTO PROTEINS BY ESCHERICHIA COLI RIBOSOMES. Proc Natl Acad Sci U S A. 1960 Nov;46(11):1450–1463. doi: 10.1073/pnas.46.11.1450. [DOI] [PMC free article] [PubMed] [Google Scholar]


