Abstract
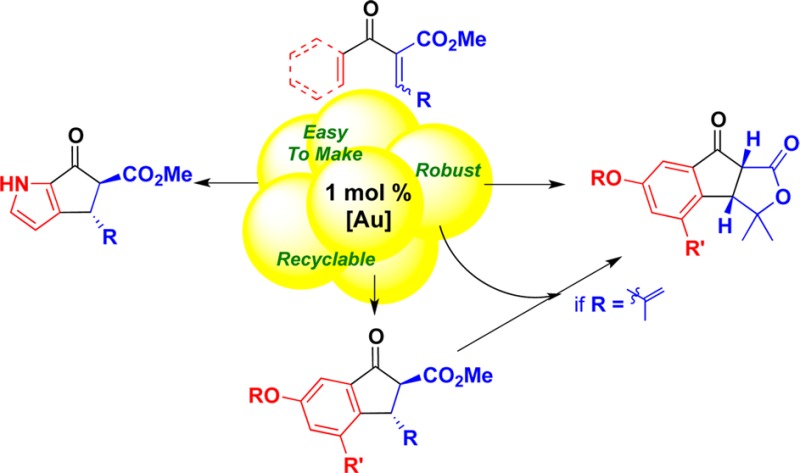
A heterogeneous gold catalyst with remarkable activity for promoting the electrophilic reactions of aryl vinyl ketones and aryl dienyl ketones is described. The catalyst is easy to prepare, is robust, and can be recycled. Low loadings are effective for different types of cationic reactions, including Nazarov cyclizations, lactonizations, and [1,2] shifts.
Catalysis of pericyclic reactions using coinage metals (Cu, Ag, Au) is a rapidly evolving field. In this context, catalysis of the Nazarov electrocyclization of aryl and vinyl enones has been studied with numerous Cu(II) complexes1 and with Ag(I).2−4 Homogeneous Au(I) and Au(III) complexes have been reported to promote both iso-Nazarov cyclizations5 and the cyclization of more typical divinyl ketone substrates.6,7 When considering effective catalysts for the Nazarov cyclization of less reactive aryl enones,1c the Ir(III) complexes developed in our group demonstrate unique reactivity, promoting Nazarov cyclization and novel electrophilic chemistry of otherwise inert aryl and heteroaryl enones.8,9 However, multistep reaction sequences are necessary to prepare the dicationic and tricationic Ir(III) complexes.
Several reports have detailed the in situ generation of a catalytically active [Au] species from gold(III) chloride (AuCl3) and silver(I) salts for catalysis of arene and alkene functionalization reactions,10−15 as well as Nazarov cyclization.6,7 However, the identity of the active catalyst in these reactions is unknown.16 We describe here a related gold catalyst that is generated in situ from a combination of AuCl3 and silver(I) hexafluoroantimonate (AgSbF6). This catalyst is convenient to prepare, heterogeneous, recyclable, and comparable in activity to the Ir(III) complexes for the catalysis of Nazarov cyclization and other electrophilic chemistry of aryl vinyl ketones.
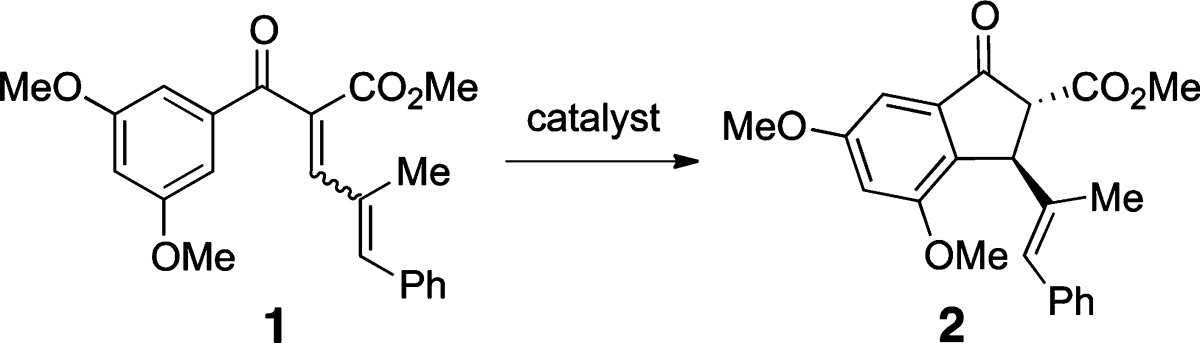
The catalyst was identified during a screen performed to identify gold(III) complexes able to promote the Nazarov cyclization of aryl enone 1, a polarized,17,18 but relatively unreactive substrate (Table 1). The addition of AgSbF6 to AuCl3 was intended to abstract gold-bound chloride ions to generate an unsaturated, cationic Au(III) species.10 The suspension obtained upon addition of AgSbF6 (2–3 mol %) to AuCl3 (1 mol %) was found to be highly active, promoting the cyclization of 1 at room temperature within 0.5 h with >99% yield (entries 1 and 2). In an earlier study, cyclization of a similar substrate using Cu(ClO4)2 required heating at 45 °C for 8 h to achieve cyclization (80% yield).17
Table 1. Gold-Catalyzed Nazarov Cyclizations Aryl Enone 1a.
| entry | catalyst | temp (°C) | time (h) | yield (%)b |
|---|---|---|---|---|
| 1 | AuCl3/AgSbF6 (1:3) | rt | 0.5 | >99 |
| 2 | AuCl3/AgSbF6 (1:2) | rt | 0.5 | >99 |
| 3 | AuCl3/AgSbF6 (1:1) | rt | 0.5 | –d |
| 4 | AuCl3/AgSbF6 (1:2) recycled once | rt | 0.5 | >99 |
| 5 | AuCl3/AgSbF6 (1:2) recycled twice | rt | 0.5 | 97 |
| 6 | (AuCl)2dppe/AgSbF6 | rt | 24 | NR |
| 7 | AuCl3/Na[BArF4] (1:2) | rt | 0.5 | >99 |
| 8 | AuCl3/AgSbF6 (1:2) + Proton-spongee | rt | 1 | >99 |
| 9 | AuCl3c | 60 | 24 | –d |
| 10 | AgSbF6c | 60 | 24 | –d |
Reaction conditions: 1 mol % of AuCl3 was used; catalyst was premixed for 0.5 h prior to reaction in dichloromethane or toluene; reactions containing AgSbF6 were run under minimal light conditions.
Isolated yields.
15 mol % was used.
Incomplete reactions monitored by thin layer chromatography (<50% conversion).
100 mol % was used. NR = no reaction, dppe =1,2-bis(diphenylphosphino)ethane, [BArF4]− = tetrakis[3,5-bis(trifluoromethyl)phenyl]borate, Proton-sponge =1,8-bis(dimethylamino)naphthalene.
A 1:1 mixture of AuCl3 and AgSbF6 shows diminished activity with incomplete conversion over 0.5 h (entry 3). The dark precipitate generated from the 1:2 mixture can be recovered after the reaction by filtration through Celite and reused in the presence of Celite for catalysis with negligible loss in activity. The filtrate is not catalytically active. Subsequent cyclizations were performed with the recovered heterogeneous substance, giving >99% after the first recycle (entry 4) and 97% after the second recycle (entry 5).
Cyclization does not occur upon exposure to a combination of (AuCl)2dppe (dppe = 1,2-bis(diphenylphosphino)ethane) with AgSbF6 (Au/Ag = 1:1) (entry 6). However, a CH2Cl2 mixture of AuCl3/Na[BArF4] ([BArF4]− = tetrakis[3,5-bis(trifluoromethyl)phenyl]borate) in a 1:2 ratio (entry 7) has the same catalytic activity as the optimal catalyst formed from AuCl3/AgSbF6 (1:2), indicating that silver does not play a significant role in the catalysis. A CH2Cl2 mixture of AuCl3/AgSbF6 (1:2) with Proton-sponge (100 mol %; Proton-sponge =1,8-bis(dimethylamino)naphthalene) gives a quantitative yield of cyclized product but requires a slightly longer reaction time (entry 8). Thus, trace amounts of H+ may aid in the catalysis, but the effect is not great. Finally, neither AuCl3 (15 mol %) nor AgSbF6 (15 mol %) used alone exhibits high activity (entries 9 and 10), giving incomplete conversions after 24 h at 60 °C.
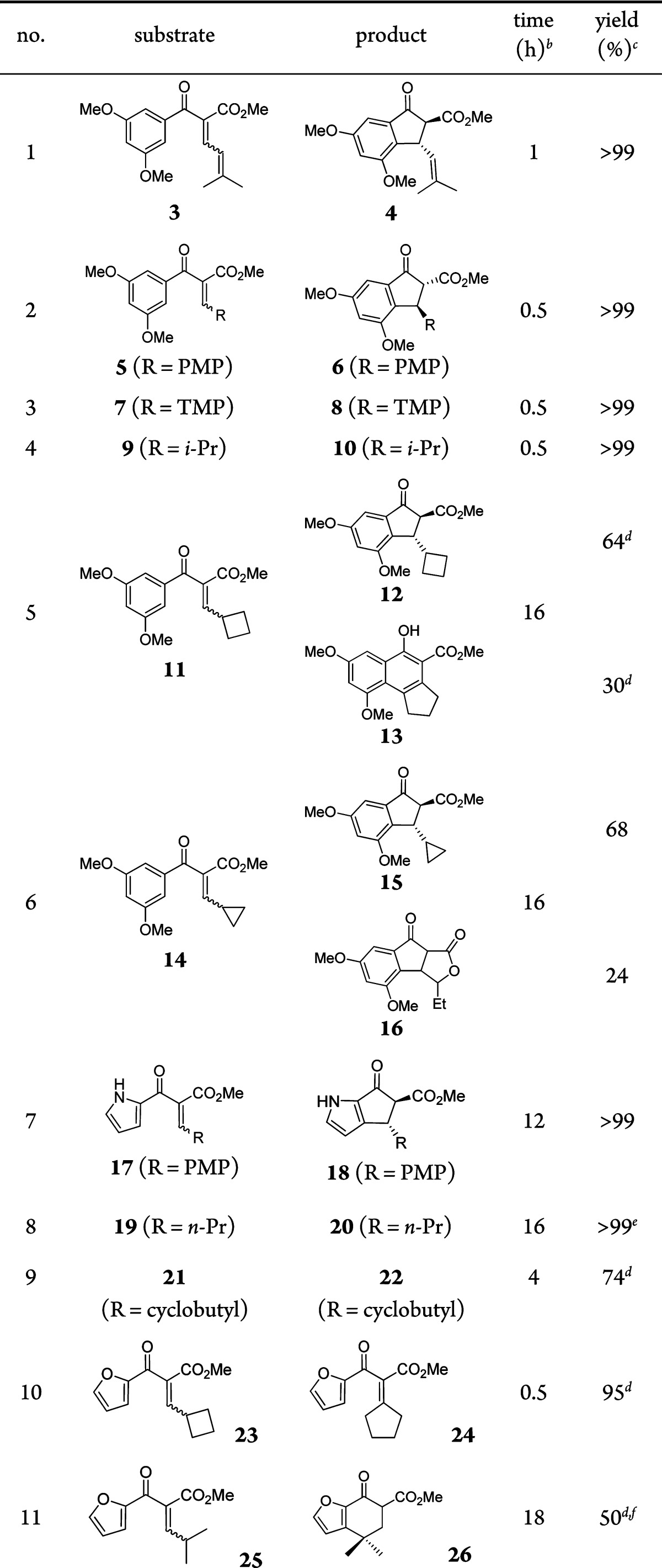
To determine the reaction scope and look for new reactivity patterns, the aryl vinyl and aryl dienyl ketones shown in Table 2 were exposed to the electrophilic heterogeneous gold catalyst (Au(III) loading = 1 mol %). We observed rapid and quantitative cyclizations of aryl dienone 3 as well as dienones having aromatic β-substituents such as PMP (p-methoxyphenyl) 5 and TMP (2,4,6-trimethoxyphenyl) 7 (entries 2 and 3, respectively). While aryl enone 9 provides Nazarov cyclopentenone 10 (entry 4), aryl γ-cyclobutyl enone 11 affords electrocyclized product 12 and unexpected naphthalenol 13 (entry 5). Similarly, the aryl γ-cyclopropyl enone 14 provides both electrocyclized product 15 and tricycle 16 (entry 6).
Table 2. Cyclization of Aryl and Heteroaryl Enones with the Heterogeneous Gold Catalyst [Au]a.
Reaction conditions: AuCl3 (1 mol %)/AgSbF6 (2 mol %), premixed prior to reaction, CH2Cl2 or toluene, rt unless otherwise indicated, minimal light conditions.
Reactions monitored using thin layer chromatography.
Isolated yields.
Reactions at 80 °C.
Reaction at 60 °C.
10 mol % catalyst. PMP = para-methoxyphenyl, TMS = trimethylsilyl, TMP = 2,4,6-trimethoxyphenyl.
The gold catalyst is effective for electrocyclizations of unprotected pyrroles 17, 19, and 21, promoting efficient cyclization using 1 mol % of the gold complex under mild conditions (entries 7–9). In comparison, cyclization of 19 using Sc(OTf)3 (5 mol %) and LiClO4 (1 equiv) requires heating at 80 °C for 8.5 h.18
The electrophilic nature of the heterogeneous gold catalyst ([Au]) is evident in its ability to promote [1,2] rearrangement chemistry, which was previously only possible using a tricationic Ir(III) complex.9 For example, rearrangement of 23 produced tetrasubstituted enone 24 (entry 10), and furyl enone 25 rearranges and cyclizes to afford 26 in 50% yield (entry 11).19 These results also suggest that product 13 is produced from 11 via a [1,2]-alkyl shift/Friedel–Crafts annulation,9 followed by aromatization upon air oxidation.
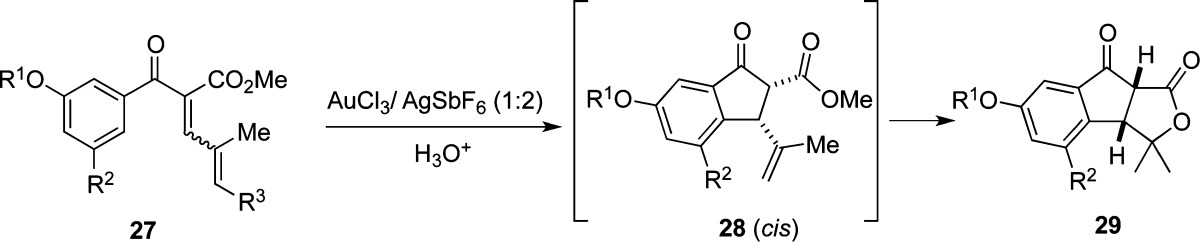
Exposure of aryl enone 27a to the [Au] catalyst for 5 h at room temperature exclusively generates tricyclic lactone 29a, via a novel Nazarov cyclization/lactonization cascade (Table 3, entry 1).20 Aryl vinyl enone 14 undergoes a related, but less efficient, cyclization/lactonization (Table 2, entry 6). Similarly, cyclization of precursors 27b–d produces lactones 29a–c, respectively, but requires heating in a mixture of dichloromethane/toluene and a longer reaction time (entries 2–4).
Table 3. Nazarov/Lactonization Cascade Reactions of Aryl Vinyl Enones with Heterogeneous Gold Catalysta.
| no. | dienone | R1 | R2 | R3 | timeb (h) | temp (°C) | solvent | additive | product | yieldc (%) |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 27a | TIPS | Br | TBS | 5 | rt | CH2Cl2 | none | 29a | 79 |
| 2 | 27b | TIPS | Br | TMS | 20 | 60 | CH2Cl2/PhMe (1:1) | none | 29a | 82 |
| 3 | 27c | Me | OMe | TBS | 24 | 60 | CH2Cl2/PhMe (1:1) | none | 29b | 83 |
| 4 | 27d | Me | H | TMS | 24 | 60 | CH2Cl2/PhMe (1:1) | none | 29c | 88 |
| 5 | 27c | Me | OMe | TBS | 24 | 60 | CH2Cl2/PhMe (1:1) | Proton-sponged | – | NR |
| 6 | 27c | Me | OMe | TBS | 24 | 60 | CH2Cl2/PhMe (1:1) | 3 Å mol sieves | 28b | 80 |
| 7 | 27c | Me | OMe | TBS | 24 | 60 | CH2Cl2/PhMe (1:1) | HOTf (1 equiv)e | 27e (R3=H) | 92 |
Reaction conditions (unless otherwise specified): 1 mol % of AuCl3 was used; catalyst was premixed prior to reaction; reactions were protected from light.
Reactions monitored using thin layer chromatography.
Isolated yields.
100 mol %.
Reaction was conducted without the AuCl3/AgSbF6 catalyst. TBS = tert-butyldimethylsilyl, TIPS = triisopropylsilyl, TMS = trimethylsilyl, Proton-sponge = 1,8-bis(dimethylamino)naphthalene. NR = no reaction, HOTf = triflic acid.
Probing the reaction further, when enone 27c is added to a solution containing the heterogeneous gold catalyst in the presence of Proton-sponge, no reaction is observed (entry 5). On the other hand, enone 27c cyclizes to give 28b, but not lactone 29b, when molecular sieves are added to the reaction (entry 6). Furthermore, treating 27c with HOTf effects protodesilylation without cyclization (entry 7). From these experiments, it can be deduced that protic acid must effect protodesilylation of 27 before cyclization can occur and that water is necessary for the lactonization (conversion of 28 to 29).
Aryl enone 27a can be cyclized with stoichiometric amounts of AlCl3, allowing isolation of intermediate 28a (Scheme 1).8b,21 Subsequently, heating cyclopentanone 28a with [Au] at 60 °C furnishes tricycle 29a in 80% yield. However, heating 28a to 60 °C in wet 1,2-dichloroethane for 16 h did not produce lactone 29a. This finding, along with the result in Table 3, entry 6, indicates that both water and [Au] are required for lactone formation. The isolation of product 16 (Table 2, entry 6) is additional evidence that [Au] promotes lactone formation, although the mechanism for formation of 16 is not yet clear to us.
Scheme 1. Stepwise Nazarov Electrocyclization/Lactonization.
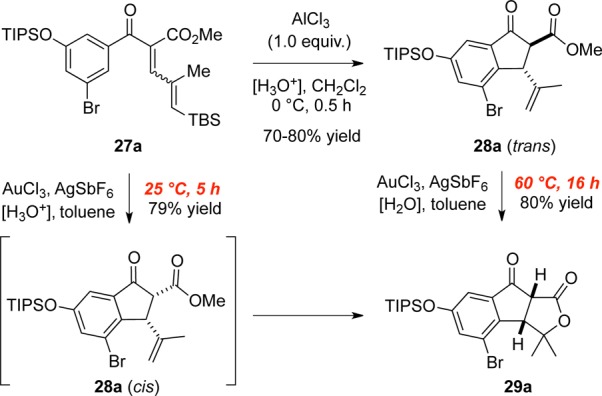
There is a significant rate difference between the one-step cyclization/lactonization and the stepwise version (Scheme 1). We propose that the one-pot sequence occurs via the kinetically favored 28a (cis),22 which is rapidly converted to 29a. However, in the stepwise reaction sequence, elevated temperature and extended reaction times are required for the isomerization of 28a (trans) to 28a (cis) which has the geometry necessary for lactone formation.
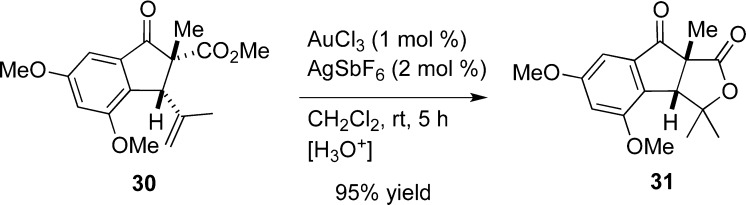
To test this hypothesis, 28b was methylated at the α position to lock the ester and olefin groups of cyclopentanone 30 in the cis conformation (Scheme 2). Indeed, the lactonization of 30 occurs at room temperature to give 31 in near-quantitative yield.
Scheme 2. Room Temperature Conversion of Nazarov Product to Lactone.
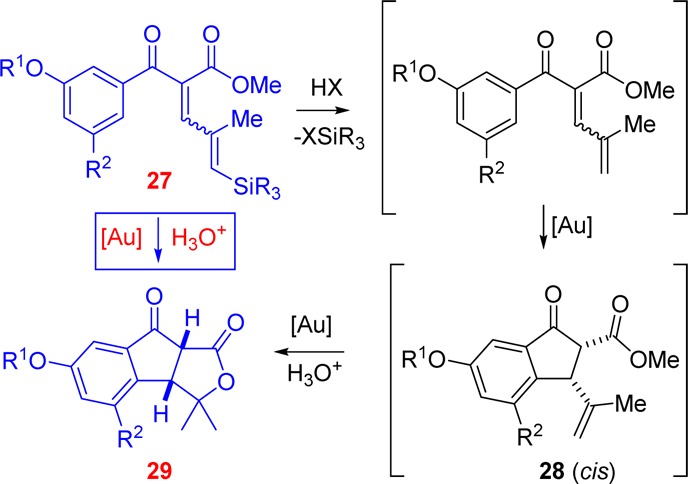
A tentative mechanism for the multistep transformation of the Nazarov cyclization/lactonization cascade is presented in Scheme 3. The serendipitous discovery of the cascade is due to the adventitious presence of water and acid in the reaction medium. Thus, the sequence begins with protodesilylation, promoted by trace amounts of acid in the reaction mixture. The Nazarov cyclization step only occurs upon exposure to the gold catalyst: protic acid alone does not promote cyclization, and cyclization was also efficient when water was excluded from the reaction using molecular sieves. Lactone formation, however, requires the presence of both the [Au] catalyst and water.
Scheme 3. Proposed Mechanism for the Nazarov Cyclization/Lactonization Cascade.
In conclusion, we report an electrophilic heterogeneous gold catalyst that promotes cationic reactions of relatively unreactive polarized dienones. The reactivity of the gold catalyst is comparable to the unusually reactive Ir(III) tricationic complex,6 but the gold catalyst is easily prepared and can be recycled, and only 1 mol % of AuCl3 is used as the catalyst precursor. Aryl and heteroaryl enones with diverse functionalities are readily cyclized, and other cationic reaction chemistry is also observed, including [1,2]-rearrangements and an unexpected lactonization.
Acknowledgments
We thank the National Science Foundation (Grants CHE-0556225 and CHE-0849892) and the NIH (NIGMS Grant R01 GM079364) for funding this work. We are grateful to Dr. Alice Bergmann (SUNY Buffalo) and Dr. Furong Sun (UIUC) for HRMS analysis.
Supporting Information Available
Experimental details, preparation, and characterization of new compounds. This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- For recent reviews of the Nazarov cyclization, see:; a Grant T. N.; Rieder C. J.; West F. G. Chem. Commun. 2009, 5676. [DOI] [PubMed] [Google Scholar]; b W. W. Nakanishi W. W.; West F. G. Curr. Opin. Drug Discovery Dev. 2009, 12, 732. [PubMed] [Google Scholar]; c Vaidya T.; Eisenberg R.; Frontier A. J. ChemCatChem 2011, 3, 1531. [Google Scholar]; d Shimada N.; Stewart C.; Tius M. A. Tetrahedron 2011, 67, 5851. [DOI] [PMC free article] [PubMed] [Google Scholar]; e Spencer W. T. III; Vaidya T.; Frontier A. J. Eur. J. Org. Chem. 2013, 18, 3621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordier P.; Aubert C.; Malacria M.; Lacote E.; Gandon V. Angew. Chem., Int. Ed. 2009, 48, 8757. [DOI] [PubMed] [Google Scholar]
- Wender P. A.; Stemmler R. T.; Sirois L. E. J. Am. Chem. Soc. 2010, 132, 2532. [DOI] [PubMed] [Google Scholar]
- a Grant T. N.; West F. G. J. Am. Chem. Soc. 2006, 128, 9348. [DOI] [PubMed] [Google Scholar]; b Grant T. N.; West F. G. Org. Lett. 2007, 9, 3789. [DOI] [PubMed] [Google Scholar]; c Bonderoff S. A.; Grant T. N.; West F. G.; Tremblay M. Org. Lett. 2013, 15, 2888. [DOI] [PubMed] [Google Scholar]
- a Lin C.-C.; Teng T.-M.; Odedra A.; Liu R.-S. J. Am. Chem. Soc. 2007, 129, 3798. [DOI] [PubMed] [Google Scholar]; b Lin C.-C.; Teng T.-M.; Tsai C.-C.; Liao H.-Y.; Liu R.-S. J. Am. Chem. Soc. 2008, 130, 16417. [DOI] [PubMed] [Google Scholar]
- Jin T.; Yamamoto Y. Org. Lett. 2008, 10, 3137. [DOI] [PubMed] [Google Scholar]
- Krafft M. E.; Vidhani D. V.; Cran J. W.; Manoharan M. Chem. Commun. 2011, 47, 6707. [DOI] [PubMed] [Google Scholar]
- Vaidya T.; Atesin A. C.; Herrick I. R.; Frontier A. J.; Eisenberg R. Angew. Chem., Int. Ed. 2010, 49, 3363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya T.; Manbeck G. F.; Chen S.; Frontier A. J.; Eisenberg R. J. Am. Chem. Soc. 2011, 133, 3300. [DOI] [PubMed] [Google Scholar]
- Reetz M. T.; Sommer K. Eur. J. Org. Chem. 2003, 3485. [Google Scholar]
- a Yao X.; Li C.-J. J. Am. Chem. Soc. 2004, 126, 6884. [DOI] [PubMed] [Google Scholar]; b Luo Y.; Li C.-J. Chem. Commun. 2004, 1930. [DOI] [PubMed] [Google Scholar]; c Nguyen R.-V.; Yao X.-Q.; Bohle D. S.; Li C.-J. Org. Lett. 2005, 7, 673. [DOI] [PubMed] [Google Scholar]; d Nguyen R.-V.; Yao X.; Li C.-J. Org. Lett. 2006, 8, 2397. [DOI] [PubMed] [Google Scholar]
- a Shi Z.; He C. J. Org. Chem. 2004, 69, 3669. [DOI] [PubMed] [Google Scholar]; b Li Z.; Shi Z.; He C. J. Organomet. Chem. 2004, 690, 5049. [Google Scholar]; c Shi Z.; He C. J. Am. Chem. Soc. 2004, 126, 5964. [DOI] [PubMed] [Google Scholar]; d Shi Z.; He C. J. Am. Chem. Soc. 2004, 126, 13596. [DOI] [PubMed] [Google Scholar]
- Youn S. W. J. Org. Chem. 2006, 71, 2521. [DOI] [PubMed] [Google Scholar]
- Jin T.; Yamamoto Y. Org. Lett. 2007, 9, 5259. [DOI] [PubMed] [Google Scholar]
- Sun X.; Sun W.; Fan R.; Wu J. Adv. Synth. Catal. 2007, 349, 2151. [Google Scholar]
- For reviews on organic reactions catalyzed by gold, see:; a Boorman T. C.; Larrosa I. Chem. Soc. Rev. 2011, 40, 1910. [DOI] [PubMed] [Google Scholar]; b Fürstner A. Chem. Soc. Rev. 2009, 38, 3208. [DOI] [PubMed] [Google Scholar]; c Arcadi A. Chem. Rev. 2008, 108, 3266. [DOI] [PubMed] [Google Scholar]; d Hashmi A. S. K.; Rudolph M. Chem. Soc. Rev. 2008, 37, 1766. [DOI] [PubMed] [Google Scholar]; e Skouta R.; Li C.-J. Tetrahedron 2008, 64, 4917. [Google Scholar]; f Li Z.; Brouwer C.; He C. Chem. Rev. 2008, 108, 3239. [DOI] [PubMed] [Google Scholar]; g Hashmi A. S. K. Chem. Rev. 2007, 107, 3180. [DOI] [PubMed] [Google Scholar]
- He W.; Herrick I. R.; Atesin T. A.; Caruana P. A.; Kellenberger C. A.; Frontier A. J. J. Am. Chem. Soc. 2008, 130, 1003. [DOI] [PubMed] [Google Scholar]
- Malona J. A.; Colbourne J. M.; Frontier A. J. Org. Lett. 2006, 8, 5661. [DOI] [PubMed] [Google Scholar]
- These reactions can also be promoted by tricationic Ir(III), with similar efficiency (see ref (9)).
- In order to reveal the product identity, silyl ether 29a was deprotected with TBAF (tetrabutylammonium fluoride) to obtain the phenol product.
- Marcus A. P.; Lee A. S.; Davis R. L; Tantillo D. J.; Sarpong R. Angew. Chem., Int. Ed. 2008, 47, 6379. [DOI] [PubMed] [Google Scholar]
- Janka M.; He W.; Frontier A. J.; Flaschenriem C.; Eisenberg R. Tetrahedron 2005, 61, 6193. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.