Abstract

A controllable and inexpensive electrochemical nitric oxide (NO) release system is demonstrated to improve hemocompatibility and reduce bacterial biofilm formation on biomedical devices. Nitric oxide is produced from the electrochemical reduction of nitrite using a copper(II)-tri(2-pyridylmethyl)amine (Cu(II)TPMA) complex as a mediator, and the temporal profile of NO release can be modulated readily by applying different cathodic potentials. Single lumen and dual lumen silicone rubber catheters are employed as initial model biomedical devices incorporating this novel NO release approach. The modified catheters can release a steady, physiologically-relevant flux of NO for more than 7 days. Both single and dual lumen catheters with continuous NO release exhibit greatly reduced thrombus formation on their surfaces after short-term 7-h intravascular placement in rabbit veins (p < 0.02, n = 6). Three day in vitro antimicrobial experiments, in which the catheters are “turned on” for only 3 h of NO release each day, exhibit more than a 100-fold decrease in the amount of surface attached live bacteria (n = 5). These results suggest that this electrochemical NO generation system could provide a robust and highly effective new approach to improving the thromboresistance and antimicrobial properties of intravascular catheters and potentially other biomedical devices.
Keywords: nitric oxide, electrochemical reduction of nitrite, antimicrobial catheters, thromboresistant catheters, modulated NO release, anti-biofilm
Biomedical devices are central to everyday medical care, and intravascular catheters in particular play an indispensable role in monitoring patients by providing access to the blood and enabling infusion of drugs and nutrients. However, there are two major complications associated with the use of such devices; microbial infections and thrombosis/clotting. An estimated 80 000 catheter-related bloodstream infections (CR-BSIs) occur in patients within intensive care units (ICUs) in the United States each year, causing as many as 28 000 deaths and $2.3 billion in additional medical costs.1 Thrombosis (clotting) is another major issue associated with catheter use, and this problem is only partly circumvented by intermittent or continuous heparin infusion. Additionally, use of heparin poses a risk of systemic anticoagulation as well as heparin sensitivity in certain patients (including heparin induced thrombocytopenia (HIT)). Further, use of heparin does not prevent platelet activation and adhesion, the primary events in a foreign body induced coagulation process.2
Nitric oxide is an endogenously produced molecule (endothelial cells produce NO at a flux of 1.0–4.0 × 10–10 mol min–1 cm–2)3 that exhibits exceptional therapeutic potential, including killing bacteria4 and preventing thrombosis.5 Its short lifetime in blood (seconds) is both beneficial and challenging, as the short-lived radical NO will only have a localized effect.6 Thus, appropriate storage and delivery methods must be carefully considered, as NO should be released precisely to a given area where it is needed. Strategies of doping NO donors such as diazeniumdiolates and S-nitrosothiols within polymer matrices have created new materials that exhibit improved biocompatibility in various in vivo blood contacting device/applications.7,8 However, such NO donors are fragile and can lose NO during storage as a result of increased temperature or exposure to moisture or light. This increases the cost required for shipping and storage, limiting their utility in commercial biomedical products.
Nitric oxide generation via electrochemical reduction of nitrite could provide a cheap and controllable alternative method. However, direct nitrite reduction on metal electrodes is complicated and the products can vary from NO, N2O, N2, NH2OH, to NH3, depending on pH, nitrite concentration, potential applied, and nature of the metal electrode itself.9,10 To achieve high selectivity towards NO generation, catalysts are necessary. Iron meso-tetrakis(4-N-methylpyridiniumyl) porphyrin has been shown to produce NO in a two-step electrolysis method in a flow system.11 Recently, we demonstrated that Cuo electrodes can be used to generate NO from nitrite, via a pulsed applied potential sequence.12 The sequence involved an anodic pulse to generate Cu(I) species and a subsequent cathodic pulse to clean the electrode.
Herein, we demonstrate a simpler and much more attractive method to generate NO from a reservoir of nitrite ions using constant potential electrochemistry and apply the technique to fabricate antithrombotic/antimicrobial catheters. In nature, Cu-containing nitrite reductases (E.C. 1.7.99.3) found in bacteria convert nitrite to NO via a 1 electron reduction.13 Many Cu(II) complexes have been studied to mimic the active site of this enzyme,14,15 as well as detect NO via fluorescence,16 and several Cu(I) model systems have been shown to mediate nitrite reduction to NO.17−19 In addition, Cu(II)-tri(2-pyridylmethyl)amine (Cu(II)TPMA) has been reported to catalyze the electrochemical reduction of nitrite to produce primarily N2O.20 In the present work, we demonstrate that conditions can be tuned for this Cu(II)TPMA species to electrochemically generate predominately NO.
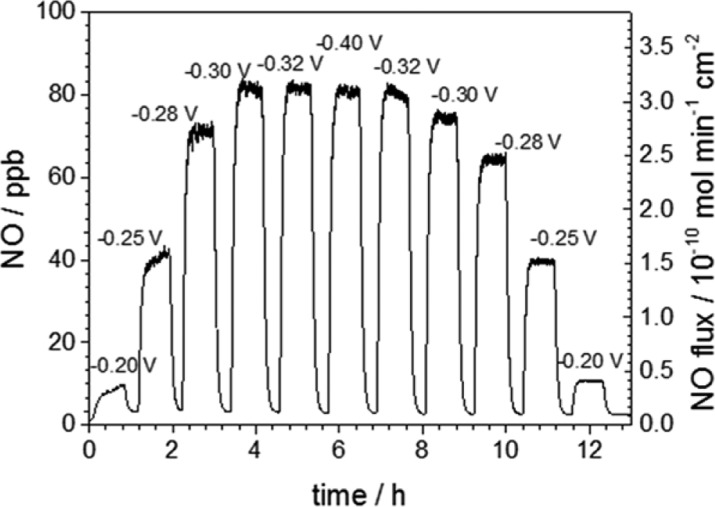
Figure 1 shows the structure of Cu(II)TPMA (inset) and the resulting cyclic voltammetry (CV) of Cu(II)TPMA on a gold (Au) electrode in the presence of different levels of nitrite in solution. The reversible peaks in the absence of nitrite correspond to a one electron reduction from Cu(II) to Cu(I) within the complex, and the characteristic catalytic peak in the presence of nitrite indicates that the nitrite is reduced by the Cu(I) species. The CVs observed are similar on platinum (Pt) and glassy carbon electrodes (Figure S1 in the Supporting Information). To detect the NO product, a bulk electrolysis experiment was performed by applying cathodic potentials in a cell that is connected to a chemiluminescence nitric oxide analyzer (NOA) (see experimental details in Supporting Information). We use pH 7.2 3-(N-morpholino)propanesulfonic acid (MOPS) buffer, because at a pH lower than 6, nitrite disproportionation occurs, producing background NO and NO2. At pH higher than 8, we observed that the activity of the catalyst decreases significantly, and nitric oxide is not detected. Figure 2 demonstrates that a low, medium and high flux of NO release can be modulated by applying −0.2, −0.3, and −0.4 V, respectively, to the working electrode (vs. 3 M Ag/AgCl reference electrode).
Figure 1.
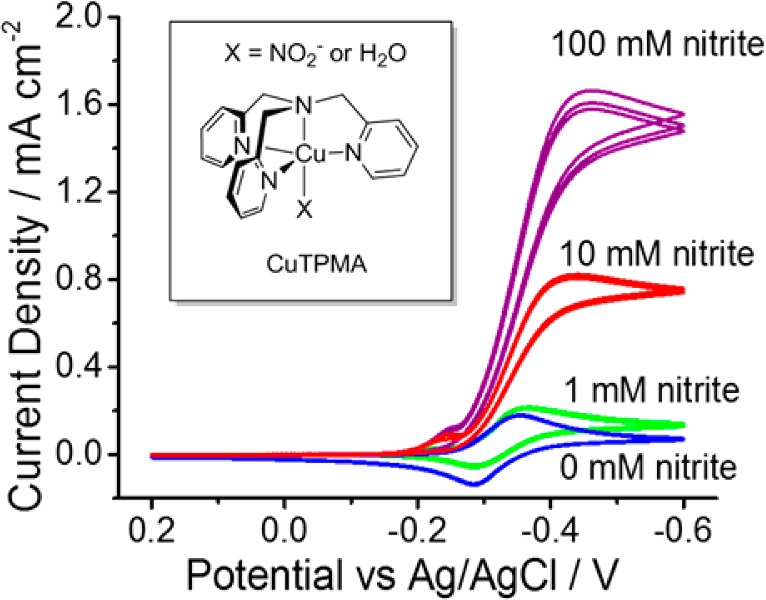
Cyclic voltammogram of 1 mM Cu(II)TPMA in 0.1 M MOPS buffer (pH 7.2) on a 0.0314 cm2 gold disc electrode with different levels of nitrite in solution saturated with N2. Scan rate is 50 mV/s. Inset: structure of Cu(II)TPMA.
Figure 2.
Modulation of NO generation in bulk solution by applying −0.2, −0.3, and −0.4 V (vs. 3 M Cl– Ag/AgCl) on a 0.071 cm2 GC electrode. The solution contains 2 mM Cu(II)TPMA, 100 mM nitrite, and 0.1 M MOPS buffer (pH 7.2).
To clarify the difference in product composition with an earlier paper20 where N2O was found to be the dominant product, bulk electrolysis experiments were performed. In these experiments, different levels of nitrite were present while applying a constant potential for 3 h, followed by careful analysis of the N2O content by gas phase IR (see the Supporting Information section). It was found that N2O is produced (from the reaction of CuTPMA with NO) but can be suppressed to a relatively low level (< 6%) when higher concentrations of nitrite are employed (see Figure S2 and Table S1 in the Supporting Information). Note this low amount of N2O is quite safe because up to 70% N2O by volume is used routinely as an inhalation anesthetic in dentistry.21 Previous studies have shown that Cu(I) complexes can disproportionate NO to generate N2O and NO2.22,23 We believe that the excess nitrite used in our experiments competitively binds to the Cu(I/II) center of the TMPA complex (after reduction of nitrite to NO), and prevents NO binding to the Cu(I/II) center, thereby suppressing N2O generation. Note that NO only weakly binds to Cu(I) complexes.23
As proof-of-concept, such electrochemical NO release chemistry was applied to catheters as model biomedical devices to assess the antithrombotic and antimicrobial efficacy of this new NO release strategy. Nitric oxide releasing catheters (cross sectional geometries shown in Figure S3a in the Supporting Information) were fabricated by filling the lumen of silicone tubing with a solution containing 2 mM Cu(II)TPMA, 0.4 M nitrite, 0.2 M NaCl, and 0.5 M MOPS buffer (pH 7.2). Teflon coated Pt and Ag/AgCl wires with 0.039 cm2 (1 cm long) and 0.079 cm2 (2 cm long) surface areas exposed, respectively, were used as the electrodes to conduct electrochemistry within the lumen (see Figure 3).
Figure 3.
Schematics of (a) single and (b) dual lumen electrochemically modulated NO releasing catheter configurations examined in this work.
Finite element analysis modeling shows that NO flux out of the catheter mostly concentrates around the silicone surface near the working electrode. Hence, the flux is calculated based on the area of the device within a 3 cm long region encompassing the 1 cm exposed electrode, where >99.8% NO resides (see Figure S4 in the Supporting Information). Similarly, in the catheter configuration, the NO flux can be modulated by applying different voltage, and the observed flux can vary from 0.4 to 3.0 × 10–10 mol min–1 cm–2 (Figure 4). Current efficiencies observed are up to 81% towards NO production and decrease as the applied potential is made more negative (see Table S2 in the Supporting Information). To test the long-term stability of NO release, such devices were turned “on” and NO release monitored by chemiluminescence for an extended time period. We found that physiologically relevant NO fluxes (> 1.0 × 10–10 mol min-1cm-2) can be emitted from the catheter for more than 7 days (see Figure S5 in the Supporting Information).
Figure 4.
Modulation of NO flux from a single lumen catheter with 0.0798 cm2 Pt wire. The solution contains 4 mM Cu(II)TPMA, 0.4 M NaNO2, 0.2 M NaCl, and 0.5 M MOPS (pH 7.2). Flux calculated based on the 3 cm silicone surface area near the Pt wire. Potentials are vs. 0.2 M Cl– Ag/AgCl.
To test the efficacy of this new NO release concept, we conducted 7 h in vivo testing by placing the two single lumen catheters described above in jugular veins of anesthetized rabbits with one of the catheters “turned on” (flux ∼2.0 × 10–10 mol min–1 cm–2) and the other “turned-off” (not linked to potentiostat; control). The degree of surface thrombus formation was assessed after removal using ImageJ 1.47 software24 to determine the amount of clot covering the surface. The NO releasing catheters consistently exhibited reduced thrombosis (p < 0.05, n = 3), with an average of 88 ± 14% reduction in thrombus area when compared with the control catheters (Figure 5a and SL 1–3 in Figure 5c). This reduction in thrombus is not due to any significant temperature change for the active NO releasing catheters wired to the potentiostats owing to current flow. Indeed, based on simple calculations from Joule’s law, the temperature change within the small volume of inner nitrite reservoir solution due to μA levels of current flow would be ≪1 °C over a 24 h period.
Figure 5.

Antithrombotic effect of e-chem NO releasing catheters in veins of six rabbits for 7 h. Representative pictures of (a) single and (b) dual lumen catheters after removal from the vein; (c) thrombosis coverage percentage on the catheters (single lumen, SL 1–3; dual lumen, DL 1–3).
In potential clinical practice, any e-chem NO releasing catheter would require at least one open lumen to sample blood or infuse agents and a second, closed-off lumen dedicated to NO release. Therefore, it is important to demonstrate that the new e-chem NO release methods could be adapted to such a configuration. This was accomplished using a dual lumen catheter (cross sectional geometry shown in Figure S3b in the Supporting Information). Although such a catheter’s asymmetry could cause an uneven distribution of NO at the outer and inner surfaces of the lumens, the silicone rubber catheter material has a very high solubility and mobility for lipophilic NO,25,26 that provides a reservoir for the e-chem generated NO and improves the distribution of the gas. Indeed, such effect is confirmed by finite element analysis simulations, as similar NO concentrations are found near the surfaces of the two respective lumens after 2 h of NO generation (see Figure S6 in the Supporting Information). In vivo thrombosis experiments with the dual lumen configuration also showed significant reduction of thrombosis on NO releasing catheters (p < 0.05, n = 3) with a 83 ± 12% reduction of the thrombosis area compared with corresponding controls in the same animals (Figure 5b and DL 1–3 in Figure 5c).
To assess the antimicrobial/antibiofilm activity of the new e-chem NO releasing catheters, the total adhered viable bacteria on their surfaces after exposure to a flowing stream of medium containing the bacteria for 3 d was determined. The dual lumen catheters were tested in a drip flow bioreactor system, which mimics the catheter environment in vivo. The catheter and its peripheral environment (channel surface) were first inoculated with high number of bacteria (∼1 × 108 CFU/channel) and then flushed continuously with fresh high nutrient medium (1/10 Lauria Broth) to remove any unattached cells and allow biofilm development on surfaces. Then, E. coli biofilms were grown on the catheter with continuous medium flowing (100 mL/h) for 3 days, and the NO release was “turned on” for only 3 h each day (at flux of 0.6 × 10–10 mol min–1 cm–2). Even with this low flux of NO release and with this release turned on only periodically, bacterial plate counts showed a >1000-fold decrease of viable bacteria on the channel surfaces in which the NO releasing catheters (n = 5) were placed and a >100-fold decrease in viable bacteria on the catheter surfaces (Figures 6a, b) when compared to controls. The reduction of biofilm formation on the channel walls was so significant that it could easily be observed visually (Figure 6c). It should be noted that the biofilm data presented here are viable bacterial counts on the entire surface of the channel.
Figure 6.
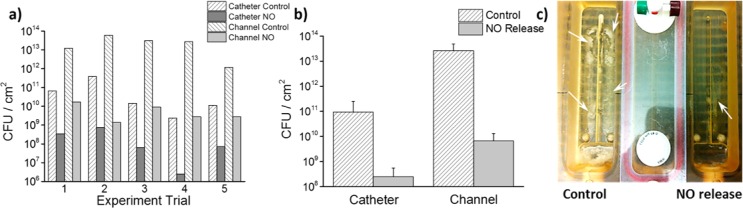
E. coli biofilm developed on dual lumen catheters in a drip flow reactor for 3 days with NO turned on for 3 h each day. (a, b) Plate count of the number of viable bacteria attached to the catheter surface and channel surface. (c) Picture shows the dramatic reduction of biofilm (indicated by arrow) formed on the channel with NO releasing catheter.
One potential concern is the competing reaction of oxygen with reduced Cu(I)TPMA. However, the CV of 1 mM Cu(II)TPMA with nitrite looks similar in air and in N2 (see Figure S7 in the Supporting Information), suggesting oxygen reduction has little effect under such conditions. The rabbit and biofilm experiments described above all were conducted in the presence of oxygen, and the data obtained in these experiments suggest that physiological levels of oxygen do not significantly suppress NO production by competing for the Cu(I)TPMA sites.
In the long term, the potential leaching of Cu is also important to consider. Literature suggests that Cu(II) ions cannot transport through silicone rubber to any significant degree27 and this was confirmed by conducting a 7-d Cu leaching test (see experimental details in the Supporting Information). The soaking solution contains no Cu above the trace background levels found initially within the PBS buffer (see Table S4 in the Supporting Information).
In summary, electrochemically controlled NO releasing catheters have been developed using Cu(II)TPMA mediated nitrite reduction, and these devices exhibit significant thromboresistance and antiseptic activity. Longer term (i.e., 3–7 days) in vivo experiments to test thromboresistance in freely moving animals are now being planned with such catheters using miniaturized battery powered potentiostats to control the NO release fluxes of the devices. The NO flux control in real-time via such chemistry should also enable development of excellent NO(g) sources with different temporal release pattern for other biomedical applications, including a new generation of infection-resistant urinary catheters and wound healing patches, as well as NO inhalation therapy equipment for critically ill patients.
Acknowledgments
This research was supported by NIH (EB-000783). We thank Dr. Dipankar Koley for his useful discussion. We also thank Sheng Zheng for his discussions regarding the finite element modeling of NO fluxes from the dual lumen catheters.
Supporting Information Available
Experiment details on bulk electrolysis, gas phase IR nitrous oxide quantification, catheter fabrication, copper leaching test, and numeric simulations with supplementary figures and tables. This material is available free of charge via the Internet at http://pubs.acs.org.”
The authors declare no competing financial interest.
Funding Statement
National Institutes of Health, United States
Supplementary Material
References
- O’Grady N. P.; Alexander M.; Burns L. A.; Dellinger E. P.; Garland J.; Heard S. O.; Lipsett P. A.; Masur H.; Mermel L. A.; Pearson M. L.; Raad I. I.; Randolph A. G.; Rupp M. E.; Saint S. Committee the Healthcare Infection Control Practices Advisory. Guidelines for the Prevention of Intravascular Catheter-related Infections. Clin. Infect. Dis. 2011, 52, e162–e193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geary R. L.; Koyama N.; Wang T. W.; Vergel S.; Clowes A. W. Failure of Heparin to Inhibit Intimal Hyperplasia in Injured Baboon Arteries: The Role of Heparin-Sensitive and -Insensitive Pathways in the Stimulation of Smooth Muscle Cell Migration and Proliferation. Circulation 1995, 91, 2972–2981. [DOI] [PubMed] [Google Scholar]
- Vaughn M. W.; Kuo L.; Liao J. C. Estimation of Nitric Oxide Production and Reaction Rates in Tissue by Use of a Mathematical Model. Am. J. Physiol.: Heart Circ. Physiol. 1998, 274, H2163–H2176. [DOI] [PubMed] [Google Scholar]
- Nichols S. P.; Schoenfisch M. H. Nitric Oxide Flux-Dependent Bacterial Adhesion and Viability at Fibrinogen-Coated Surfaces. Biomater. Sci. 2013, 1, 1151–1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loscalzo J. Nitric Oxide Insufficiency, Platelet Activation, and Arterial Thrombosis. Circ. Res. 2001, 88, 756–762. [DOI] [PubMed] [Google Scholar]
- Pacher P.; Beckman J.; Liaudet L. Nitric Oxide and Peroxynitrite in Health and Disease. Physiol. Rev. 2007, 87, 315–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frost M. C.; Rudich S. M.; Zhang H.; Maraschio M. A.; Meyerhoff M. E. In Vivo Biocompatibility and Analytical Performance of Intravascular Amperometric Oxygen Sensors Prepared with Improved Nitric Oxide-Releasing Silicone Rubber Coating. Anal. Chem. 2002, 74, 5942–5947. [DOI] [PubMed] [Google Scholar]
- Major T. C.; Brant D. O.; Reynolds M. M.; Bartlett R. H.; Meyerhoff M. E.; Handa H.; Annich G. M. The Attenuation of Platelet and Monocyte Activation in a Rabbit Model of Extracorporeal Circulation by a Nitric Oxide Releasing Polymer. Biomaterials 2010, 31, 2736–2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duca M.; Kavvadia V.; Rodriguez P.; Lai S. C. S.; Hoogenboom T.; Koper M. T. M. New Insights into the Mechanism of Nitrite Reduction on a Platinum Electrode. J. Electroanal. Chem. 2010, 649, 59–68. [Google Scholar]
- Duca M.; van der Klugt B.; Koper M. T. M. Electrocatalytic Reduction of Nitrite on Transition and Coinage Metals. Electrochim. Acta 2012, 68, 32–43. [Google Scholar]
- Chi Y.; Chen J.; Aoki K. Electrochemical Generation of Free Nitric Oxide from Nitrite Catalyzed by Iron Meso-Tetrakis(4-N-Methylpyridiniumyl)Porphyrin. Inorg. Chem. 2004, 43, 8437–8446. [DOI] [PubMed] [Google Scholar]
- Höfler L.; Koley D.; Wu J.; Xi C.; Meyerhoff M. E. Electromodulated Release of Nitric Oxide through Polymer Material from Reservoir of Inorganic Nitrite Salt. RSC Adv. 2012, 2, 6765–6767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adman E. T.; Murphy M. E. P.. Copper Nitrite Reductase. In Handbook of Metalloproteins; Messerschmidt A., Huber R., Poulos T., Wieghardt K., Eds.;Wiley: New York, 2001; pp 1381–1382. [Google Scholar]
- Merkle A. C.; Lehnert N. Binding and Activation of Nitrite and Nitric Oxide by Copper Nitrite Reductase and Corresponding Model Complexes. Dalton Trans. 2012, 41, 3355–3368. [DOI] [PubMed] [Google Scholar]
- Casella L.; Carugo O.; Gullotti M.; Doldi S.; Frassoni M. Synthesis, Structure, and Reactivity of Model Complexes of Copper Nitrite Reductase. Inorg. Chem. 1996, 35, 1101–1113. [DOI] [PubMed] [Google Scholar]
- Tan L.; Wan A.; Li H. Fluorescent Chitosan Complex Nanosphere Diazeniumdiolates as Donors and Sensitive Real-time Probes of Nitric Oxide. Analyst 2013, 138, 879–886. [DOI] [PubMed] [Google Scholar]
- Halfen J. A.; Mahapatra S.; Wilkinson E. C.; Gengenbach A. J.; Young V. G.; Que L.; Tolman W. B. Synthetic Modeling of Nitrite Binding and Activation by Reduced Copper Proteins. Characterization of Copper(I)–Nitrite Complexes That Evolve Nitric Oxide. J. Am. Chem. Soc. 1996, 118, 763–776. [Google Scholar]
- Kujime M.; Izumi C.; Tomura M.; Hada M.; Fujii H. Effect of a Tridentate Ligand on the Structure, Electronic Structure, and Reactivity of the Copper(I) Nitrite Complex: Role of the Conserved Three-Histidine Ligand Environment of the Type-2 Copper Site in Copper-Containing Nitrite Reductases. J. Am. Chem. Soc. 2008, 130, 6088–6098. [DOI] [PubMed] [Google Scholar]
- Kumar M.; Dixon N. A.; Merkle A. C.; Zeller M.; Lehnert N.; Papish E. T. Hydrotris(triazolyl)borate Complexes as Functional Models for Cu Nitrite Reductase: The Electronic Influence of Distal Nitrogens. Inorg. Chem. 2012, 51, 7004–7006. [DOI] [PubMed] [Google Scholar]
- Komeda N.; Nagao H.; Kushi Y.; Adachi G.; Suzuki M.; Uehara A.; Tanaka K. Molecular Structure of Nitro- and Nitrito-Copper Complexes as Reaction Intermediates in Electrochemical Reduction of Nitrite to Dinitrogen Oxide. Bull. Chem. Soc. Jpn. 1995, 68, 581–589. [Google Scholar]
- Kanagasundaram S. A.; Lane L. J.; Cavalletto B. P.; Keneally J. P.; Cooper M. G. Efficacy and Safety of Nitrous Oxide in Alleviating Pain and Anxiety During Painful Procedures. Arch. Dis. Child. 2001, 84, 492–495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero C. E.; Carrier S. M.; Tolman W. B. Reductive Disproportionation of NO Mediated by Copper Complexes: Modeling N2O Generation by Copper Proteins and Heterogeneous Catalysts. Angew. Chem., Int. Ed. 1994, 33, 895–897. [Google Scholar]
- Fujisawa K.; Tateda A.; Miyashita Y.; Okamoto K.-i.; Paulat F.; Praneeth V. K. K.; Merkle A.; Lehnert N. Structural and Spectroscopic Characterization of Mononuclear Copper(I) Nitrosyl Complexes: End-on Versus Side-on Coordination of NO to Copper(I). J. Am. Chem. Soc. 2008, 130, 1205–1213. [DOI] [PubMed] [Google Scholar]
- Rasband W. S.Image J; U.S. National Institutes of Health: Bethesda, MD, 1997–2012; http://imagej.nih.gov/ij/. [Google Scholar]
- Mowery K. A.; Meyerhoff M. E. The Transport of Nitric Oxide through Various Polymeric Matrices. Polymer 1999, 40, 6203–6207. [Google Scholar]
- Abraham M. H.; Gola J. M. R.; Cometto-Muniz J. E.; Cain W. S. The Solvation Properties of Nitric Oxide. J. Chem. Soc., Perkin Trans. 2 2000, 10, 2067–2070. [Google Scholar]
- Donaldson N.; Baviskar P.; Cunningham J.; Wilson D. The Permeability of Silicone Rubber to Metal Compounds: Relevance to Implanted Devices. J. Biomed. Mater. Res., Part A 2012, 100A, 588–598. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.