Abstract
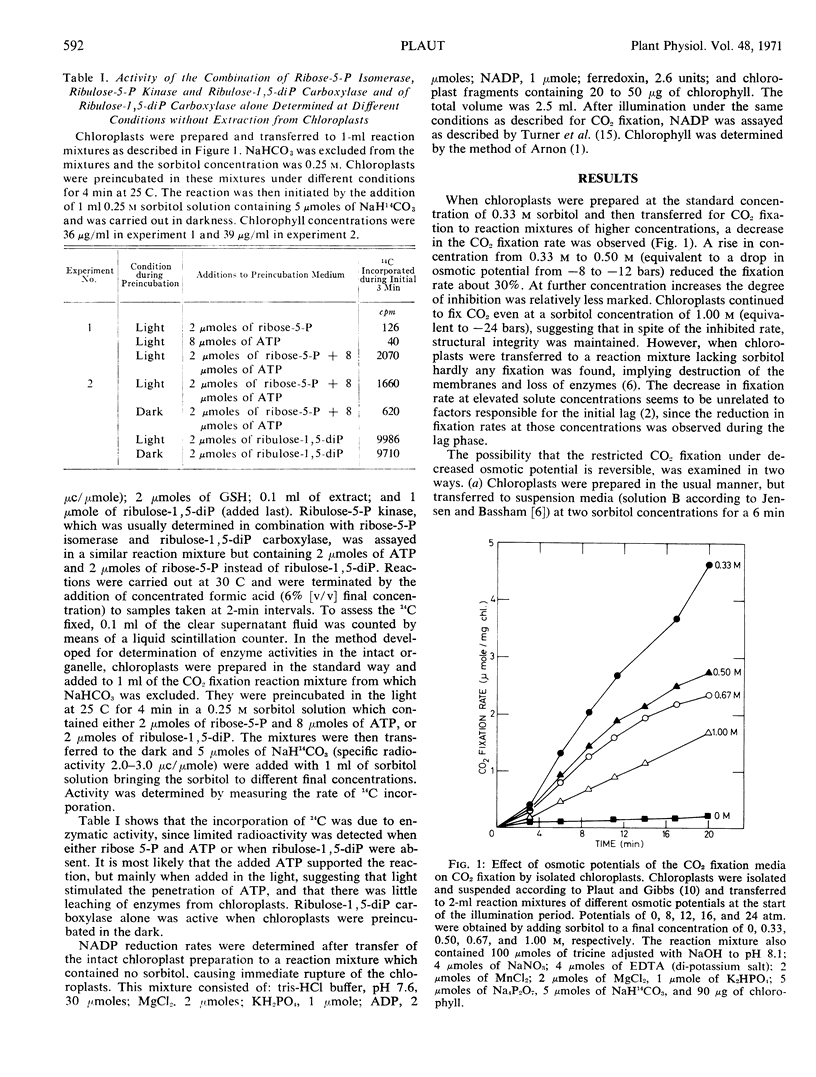
Reduced osmotic potentials inhibited the rate of CO2 fixation by isolated intact spinach (Spinacia oleracea) chloroplasts. This inhibition was observed immediately after transfer of chloroplasts from a solution containing 0.33 m sorbitol to higher sorbitol concentrations, and the depressed rate remained constant. The inhibited CO2 fixation could not be attributed to a decreased rate of photosynthetic electron transport, since NADP reduction was unaffected by subjecting the chloroplasts to low potentials. It could also not result from restricted permeability to CO2, as CO2 concentrations had no effect on the relative inhibition induced by the lowered potential.
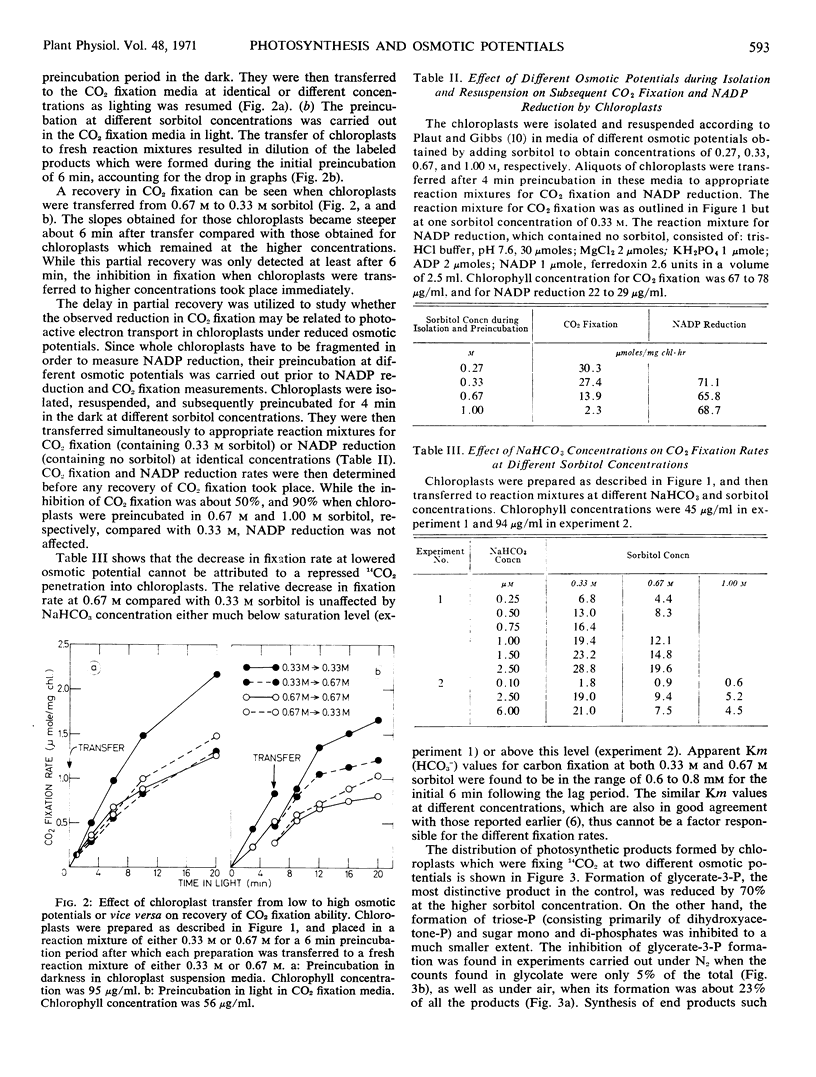
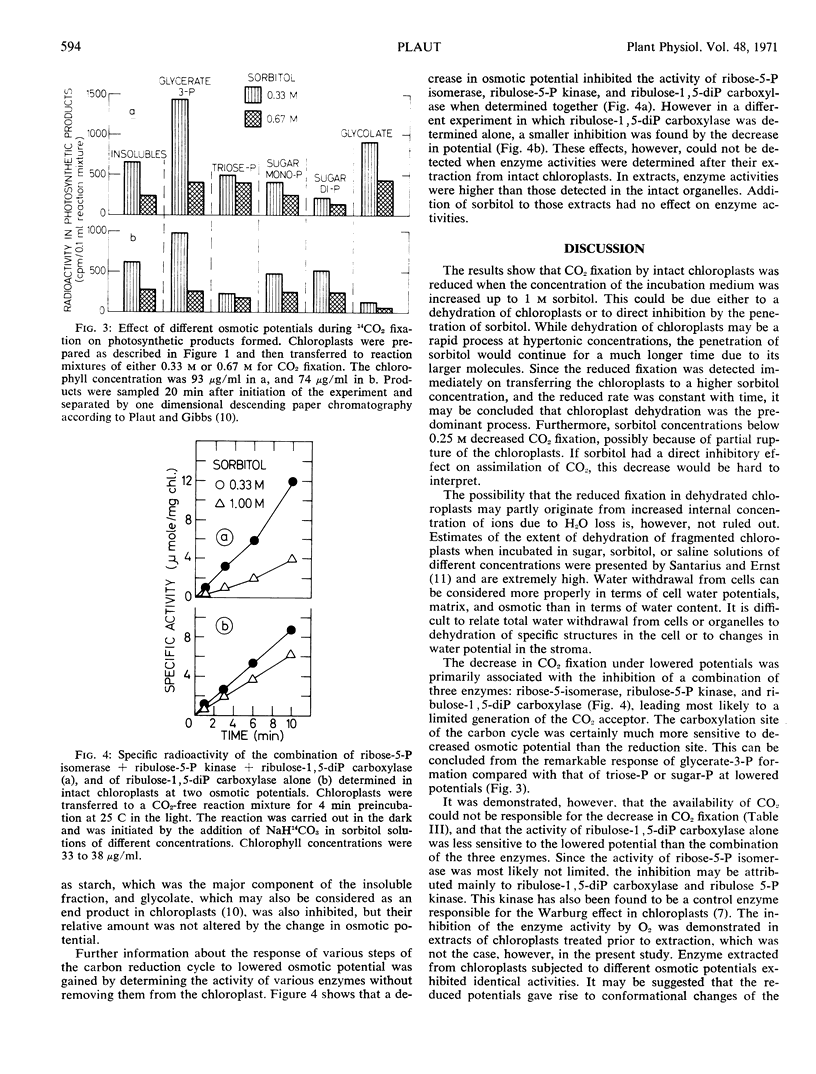
A procedure was developed for the determination of several enzymes of the photosynthetic carbon reduction cycle in the intact chloroplast without their being extracted. The activities of the combined three enzymes: ribose-5-phosphate isomerase, ribulose-5-phosphate kinase, and ribulose-1,5-diphosphate carboxylase and of ribulose-1,5-diphosphate carboxylase alone were found to be inhibited at low osmotic potentials. Analysis of the photosynthetic products showed that the formation of glycerate-3-phosphate was inhibited to a greater extent than the formation of all other products.
CO2 fixation was partly resumed when chloroplasts were returned from a 0.67 m sorbitol to a 0.33 m sorbitol solution, regardless whether the transfer occurred in the light or in the dark.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnon D. I. COPPER ENZYMES IN ISOLATED CHLOROPLASTS. POLYPHENOLOXIDASE IN BETA VULGARIS. Plant Physiol. 1949 Jan;24(1):1–15. doi: 10.1104/pp.24.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer J. S., Bowen B. L. Inhibition of oxygen evolution in chloroplasts isolated from leaves with low water potentials. Plant Physiol. 1970 May;45(5):612–615. doi: 10.1104/pp.45.5.612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry K. E. Some factors affecting the Hill reaction activity in cotton chloroplasts. Plant Physiol. 1970 Apr;45(4):465–469. doi: 10.1104/pp.45.4.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- JAKOBY W. B., BRUMMOND D. O., OCHOA S. Formation of 3-phosphoglyceric acid by carbon dioxide fixation with spinach leaf enzymes. J Biol Chem. 1956 Feb;218(2):811–822. [PubMed] [Google Scholar]
- Jensen R. G., Bassham J. A. Photosynthesis by isolated chloroplasts. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1095–1101. doi: 10.1073/pnas.56.4.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- TURNER J. F., BLACK C. C., GIBBS M. Studies on photosynthetic processes. I. The effect of light intensity on triphosphopyridine nucleotide reduction, adenosine triphosphate formation, and carbon dioxide assimilation in spinach chloroplasts. J Biol Chem. 1962 Feb;237:577–579. [PubMed] [Google Scholar]
- Walker D. A., Baldry C. W., Cockburn W. Photosynthesis by isolated chloroplasts, simultaneous measurement of carbon assimilation and oxygen evolution. Plant Physiol. 1968 Sep;43(9):1419–1422. doi: 10.1104/pp.43.9.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]


