Abstract
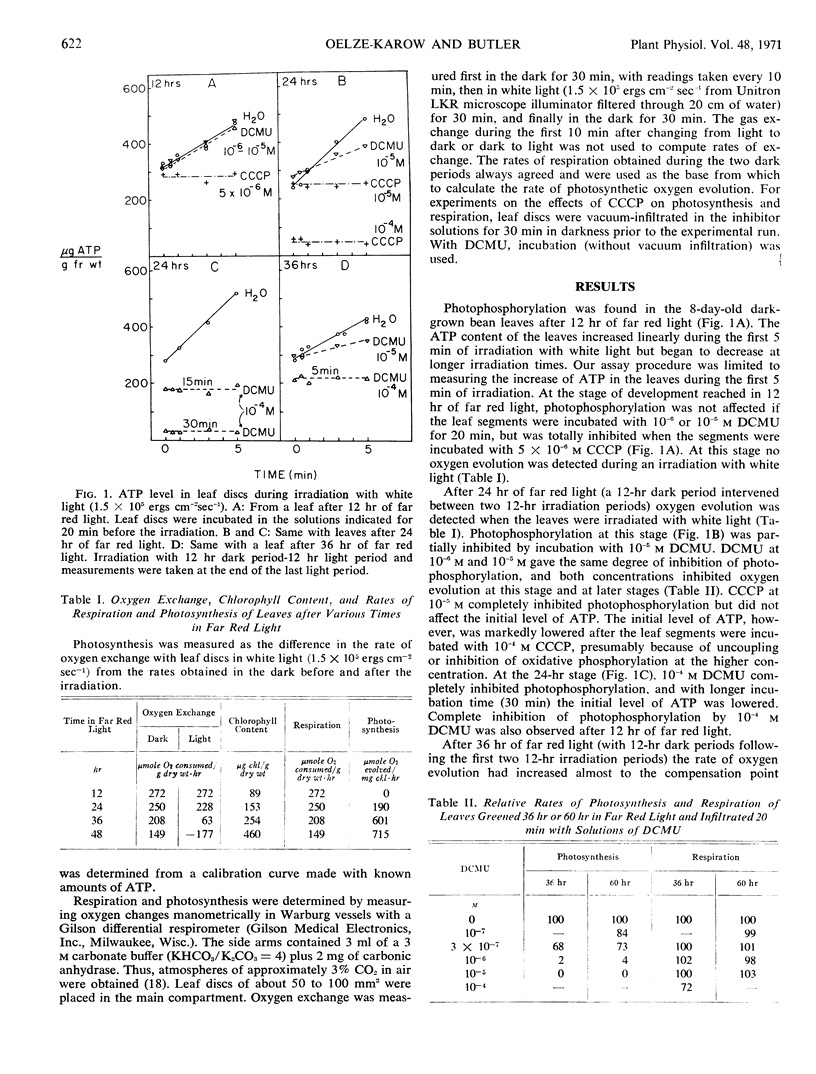
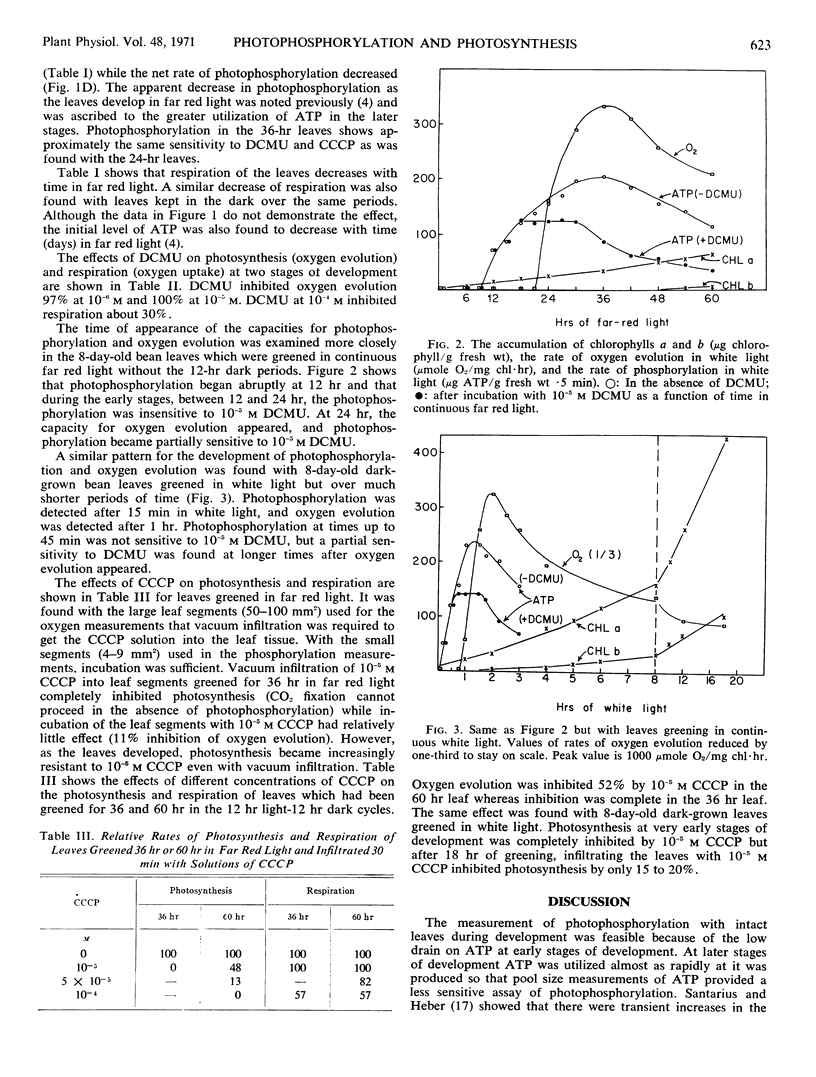
Photophosphorylation and oxygen evolution were measured in 8-day-old dark-grown bean leaves (Phaseolus vulgaris) after various times of greening in far red light and in white light. The sequence of development was the same for both greening regimes, but the processes were much more rapid in white light. The capacity for photophosphorylation, as assayed by the firefly luciferase assay, appeared after 12 hours in far red light. At this stage and for times up to 24 hours, photophosphorylation was not inhibited by 10−5m 3-(3,4-dichlorophenyl)-1,1-dimethylurea. At 24 hours, the capacity for oxygen evolution appeared and photophosphorylation became partially inhibited by 3-(3,4-dichlorophenyl)-1,1-dimethylurea at concentrations which inhibited oxygen evolution. In white light photophosphorylation appeared after 15 minutes, and oxygen evolution at one hour. Photophosphorylation became partially sensitive to 3-(3,4-dichlorophenyl)-1,1-dimethylurea when oxygen evolution appeared. Carbonylcyanide m-chlorophenyl-hydrazone inhibited photophosphorylation and photosynthesis at low concentrations, 10−5m, with immature leaves, but the leaves developed resistance to carbonylcyanide m-chlorophenyl-hydrazone as they greened.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop D. G., Andersen K. S., Smillie R. M. Incomplete membrane-bound photosynthetic electron transfer pathway in agranal chloroplasts. Biochem Biophys Res Commun. 1971 Jan 8;42(1):74–81. doi: 10.1016/0006-291x(71)90364-0. [DOI] [PubMed] [Google Scholar]
- De Greef J., Butler W. L., Roth T. F. Greening of etiolated bean leaves in far red light. Plant Physiol. 1971 Apr;47(4):457–464. doi: 10.1104/pp.47.4.457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good N. E. Inhibitors of the Hill reaction. Plant Physiol. 1961 Nov;36(6):788–803. doi: 10.1104/pp.36.6.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh S., San Pietro A. Ascorbate-supported NADP photoreduction by heated Euglena chloroplasts. Arch Biochem Biophys. 1967 Oct;122(1):144–152. doi: 10.1016/0003-9861(67)90133-6. [DOI] [PubMed] [Google Scholar]
- Santarius K. A., Heber U. Changes in the intracellular levels of ATP, ADP, AMP and P1 and regulatory function of the adenylate system in leaf cells during photosynthesis. Biochim Biophys Acta. 1965 May 25;102(1):39–54. doi: 10.1016/0926-6585(65)90201-3. [DOI] [PubMed] [Google Scholar]
- WARBURG O., KRIPPAHL G., GATTUNG H. W. [The 1-vessel method for measurement of the quantum requirement in photosynthesis]. Z Naturforsch B. 1960 Jun;15B:370–372. [PubMed] [Google Scholar]
- Woo K. C., Anderson J. M., Boardman N. K., Downton W. J., Osmond C. B., Thorne S. W. Deficient Photosystem II in Agranal Bundle Sheath Chloroplasts of C(4) Plants. Proc Natl Acad Sci U S A. 1970 Sep;67(1):18–25. doi: 10.1073/pnas.67.1.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Inhibition of chloroplasts by UV-irradiation and heat-treatment. Plant Physiol. 1968 Dec;43(12):2037–2040. doi: 10.1104/pp.43.12.2037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita T., Butler W. L. Photoreduction and photophosphorylation with tris-washed chloroplasts. Plant Physiol. 1968 Dec;43(12):1978–1986. doi: 10.1104/pp.43.12.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]


