Highlights
-
•
Glycolysis genes are strikingly upregulated by hypoxia in SGBS adipocytes.
-
•
The AP-1 family and KLF7 are involved in hypoxia-mediated gene regulation.
-
•
Hypoxia shifts metabolism to glycogen and glutathione synthesis.
-
•
Hypoxia affected metabolism in adipocytes might start a vicious circle of lipogenesis.
Keywords: Hypoxia, Adipocytes, SGBS, Gene ontology, HIF-1, Gene expression profiling
Abstract
To elucidate the complex impact of hypoxia on adipose tissue, resulting in biased metabolism, insulin resistance and finally diabetes we used mature adipocytes derived from a Simpson-Golabi-Behmel syndrome patient for microarray analysis. We found a significantly increased transcription rate of genes involved in glycolysis and a striking association between the pattern of upregulated genes and disease biomarkers for diabetes mellitus and insulin resistance. Although their upregulation turned out to be HIF-1α-dependent, we identified further transcription factors mainly AP-1 components to play also an important role in hypoxia response. Analyzing the regulatory network of mentioned transcription factors and glycolysis targets we revealed a clear hint for directing glycolysis to glutathione and glycogen synthesis. This metabolic switch in adipocytes enables the cell to prevent oxidative damage in the short term but might induce lipogenesis and establish systemic metabolic disorders in the long run.
1. Introduction
The prevalence of obesity is rising rapidly worldwide and obesity-associated disorders such as type 2 diabetes mellitus (T2DM) and metabolic syndrome constitute a serious threat to modern society. In obese subjects fat tissue expands faster than the vasculature that supplies it with oxygen. Thus, fat tissue in obesity is characterized by areas of low O2 pressure. As a consequence, hypertrophy and hyperplasia lead to a hypoxic condition in the adipocytes of obese subjects (Virtanen et al., 2002; Pasarica et al., 2009; Fleischmann et al., 2005; Kabon et al., 2004), although this picture has been challenged recently (Goossens et al., 2011; Hodson et al., 2013).
Inside cells, oxygen is sensed by the mitochondrial electron transfer chain and thus, reactive oxygen species (ROS) production is excessively elevated by hypoxia (Klimova and Chandel, 2008) As a result, HIF-1α, which is highly labile under normal oxygen conditions, is strongly stabilized by ROS, preventing its hydroxylation and proteasomal degradation (Fandrey et al., 2006; Klimova and Chandel, 2008).
The generation of ATP per molecule of glucose by anaerobic fermentation is much less efficient than oxidative phosphorylation under normal oxygen, producing only 2 molecules ATP per molecule of glucose instead of about 30 (Rich, 2003). To maintain their ATP levels under hypoxia, cells depend on an increased activity of glycolysis. This metabolic switch is regulated by the transcription factor HIF-1 as the most important mediator of the hypoxic signal (Seagroves et al., 2001), modulating the expression of genes involved in angiogenesis, cell proliferation, apoptosis, and energy metabolism (Rocha, 2007). In adipocytes, hypoxic treatment impacts the expression of over 500 genes and the expression of HIF-1 has been suggested to be directly responsible for the early response gene regulation. However, some early response genes are lacking specific HIF-1 binding sites, and their hypoxia-induced upregulation depends on different transcription factors (Geiger et al., 2011a). Though, little is known about the hypoxia-signaling network in adipocytes responsible for that metabolic switch.
In cancer cells there is a metabolic feature, known as the Warburg effect, which dramatically increases their glycolytic rate and triggers a switch from oxidative phosphorylation to fermentation. This metabolic switch to fermentation has been recently suggested to be more a consequence then a cause, to cope with their rapid proliferation as reduced mitochondrial activity might prevent apoptosis induction (Natter and Kohlwein, 2012). In adipose tissue, hypoxia also provokes an increase in glycolytic enzymes (Choi et al., 2009), apart from adipokine dysregulation, insulin resistance, and the initiation of the inflammatory cascade (Ye et al., 2007; Wang et al., 2007; Geiger et al., 2011b; Hosogai et al., 2007; Rausch et al., 2008 and reviewed by Trayhurn (2013)). It leads to impaired glucose tolerance (Halberg et al., 2009) and thus facilitates diabetes development. However the implications of these hypoxia-driven adaptations are still elusive. It is known that the hypoxic response is mediated also by transcription factors different from HIF-1α (Haxhiu et al., 1995; Cummins and Taylor, 2005; Erickson and Millhorn, 1994; Prabhakar et al., 1995; Chen et al., 2008) and their interplay finally triggering the metabolic switch is not yet understood. Thus we used Simpson-Golabi-Behmel syndrome (SGBS)-derived differentiated human adipocytes, analyzing the influence of reduced oxygen supply on gene expression with advanced computational methods. We propose a signal network which works at the initiation of the hypoxic response and might influence glucose metabolism with the primary aim to prevent oxidative damage.
2. Materials and methods
2.1. Cell culture and reagents
Human SGBS preadipocytes (Wabitsch et al., 2001) were cultivated and differentiated to mature adipocytes as described previously (Geiger et al., 2011a). Fully differentiated cells were exposed to hypoxia (of 1% O2, 5% CO2 and 94% N2), using a MIC-101 modular incubator chamber (Billups-Rothenberg, Inc., Del Mar, CA, USA), sealed and incubated at 37 °C. Adipocytes were cultured in the hypoxic environment for 3, 6 and 16 h while the control group was cultured under normoxic conditions (21% O2) prior to harvest. Reagents were obtained from Sigma–Aldrich (St. Louis, MO, USA) unless specified otherwise. For suppressing HIF-1α accumulation we used the HIF-1α inhibitor CAY10585 (Cayman Chemical) dissolved in DMSO and DMSO alone as a control.
2.2. Glycogen and glutathione determination
Glycogen and glutathione amounts were measured with the BioVision (CA, USA) glycogen (Cat. No. K646-100) and glutathione (Cat. No. K261-100) assay kits following the manufacturer’s protocol using a DTX 880 multimode detector (Beckman Coulter, Inc., CA, USA).
2.3. Microarray analysis
For obtaining the gene expression profile, fully differentiated SGBS adipocytes were incubated at 37 °C under hypoxic condition of 1% O2 and cultivated for 16 h, whereas the control group was cultured under normoxic conditions (21% O2). Hence, total RNA was prepared from control and treated SGBS cells, in three independently performed experiments, with the ‘RNeasy Lipid Tissue’ kit according to the manufacturer’s instructions (Qiagen, Hilden, Germany), followed by purification of the RNA with the ‘RNeasy Mini’ kit (Qiagen). RNA quantity and purity was determined by optical density measurements (OD260/280) and RNA integrity by using the 2100 Bioanalyzer (Agilent Technologies, Palo Alto, CA, USA). Only high quality RNA was further processed. Gene expression profiling analysis was performed by the Expression Profiling Unit at the Medical University Innsbruck using the the ‘Human Genome U133 2.0’ arrays (Affymetrix) as described previously (Geiger et al., 2011a).
Results from the statistical analysis to identify differentially expressed mRNAs are presented in suppl. 1A. A moderated t-test has been used to calculate raw p-values which then have been adjusted for multiple hypothesis testing using the method proposed by Benjamini and Hochberg (1995) for a strong control of the false discovery rate. Only moderate inter-sample variations were observed for the 757 identified differentially expressed probe sets as depicted in the heatmap of expression intensities (suppl. 1B).
Thus all probe sets were filtered according to their adjusted p-value (0.05) and separated in up (M > 1) and down (M < 1) regulated sets according to their differential expression M (=log2 fold change; suppl. 2). Fold change values (FC) of these probe sets range from 2 to 177 (up) and from 0.5 to 0.045 (down). From these data we generated different sets of genes assigning the probe sets to genes listed in the EntrezGene database. FC and p-value data derived from different probe sets were represented by the mean. As a result, we classified 332 genes as up regulated (FC > 2, p-value <0.05) and 210 as down regulated (FC < 0.5, p-value <0.05). As a control, we applied a FC = 1 ± 2.4 E-3 for the gene set and got 535 genes, which thus have been determined as unchanged (NC) and these were used as background gene set, whereas those also present in the up- or down-set have been excluded. A comparative overview over the top differentially expressed genes is given in the Supplementary section (suppl. 3).
2.4. Real-time PCR analyzes
RNA (1 μg) was reverse transcribed using the SuperScript III First-Strand Synthesis Kit (Invitrogen). Expression levels of genes, which were consistently differentially expressed in response to all applied time periods of hypoxia, were assessed by real-time PCR using the SYBR® Green PCR Master Mix (Applied Biosystems, Foster City, CA, USA) and fluorescence was detected with the LightCycler® 480 System (Roche Diagnostics GmbH, Mannheim, Germany). The primers were synthesized by Microsynth (Balgach, Switzerland), sequences are accessible in the Supplementary part (suppl. 4). A thermal profile of an initial 10 min melting step at 95 °C, followed by 40 cycles of 15 s at 95 °C and 1 min at 60 °C was used. A melting curve profile was processed after each run to confirm specific transcripts. The samples were normalized to the endogenous reference TBP values. The results are expressed as fold changes of cycle threshold value relative to controls using the 2−ΔΔCt method. Each value represents triplicate testing of three independent experiments. Statistical differences between the values were analyzed using the Jonckheere-Terpstra-Test.
2.5. Data processing and statistical analyzes
Microarray data analysis was performed in R (http://www.r-project.org) using packages from the affyPLM Bioconductor project (Gentleman et al., 2004). Raw data from three independent experiments were examined in basic quality control experiments, including raw data signal intensity distribution, and RNA degradation. More sophisticated quality assessments were performed, generating NUSE and pseudo-images plots as well as cluster analysis and PCA to group and visualize samples with a similar expression pattern. Only good quality arrays were further analyzed. GeneChip raw signals were preprocessed using the GC robust multi-array average (GCRMA) method (Wu et al., 2004), performing optical background substraction, background adjustment for non specific binding and quantile normalization. Quality control was repeated with preprocessed data to verify comparable intensity distribution. Thus differentially expressed probe sets were identified and significance was assessed using a moderated t-test. Raw p-values calculated by the moderated t-test were adjusted for multiple hypotheses testing using the method proposed by Benjamini and Hochberg (BHp; (Benjamini and Hochberg, 1995). Finally, resulting 54,675 probe sets were filtered according to their p-value (BHp) and M, the log differential expression ratio (M = log2 FC), as represented in suppl. 2 and separated in an upregulated (FC > 2; BHp < 0.05) downregulated (FC < 0.5; BHp < 0.05) and unchanged (FC = 1 ± 2 E-3) gene set. Further in silico analyzes were conducted using the software package ExPlain 3.0™ with the integrated Match™ tool and the linked TRANSFACPro® and BioKnowledge Library (BKL) as well as predefined position weight matrix (PWM) profiles provided by BIOBASE (http://www.biobase-international.com).
2.6. GO annotation and canonical pathway mapping
Gene ontology (GO) annotation allows a function analysis for identifying statistically relevant classification terms and investigating the biological properties of a given list of genes as described by Ashburner et al. (2000). Here we applied a commercial manual curated variant using the BKL database, which is integrated in the explain software package (BIOBASE). Canonical reaction pathways are a further kind of functional classification, which determines the representation of an input set of molecules to pathways using the transpath tool, which compares the input data to a knowledge base of signal transduction data (Krull et al., 2003).
2.7. F-Match
This program tool supports the finding of transcription factors which might control a certain genes set. Using a large amount of predefined Position Weight Matrices (PWM) it searches for matching binding sites for transcription factors in a query set (Yes set) and compares the frequencies of these sites with those in a control gene set (No set). To identify binding sites in the up or down regulated set of genes (Yes set), we used a background gene set (No set), consisting of 535 human genes, which were identified in the microarray not to change their expression (FC = 1 ± 2 E-3) during hypoxia and which were included neither in the up nor in the down regulated gene set. Using a predefined PWM profile consisting of 656 PWMs for human transcription factor binding sites, matching matrices in the query set were identified and considered overrepresented if the Yes/No ratio was >1. The significance of this value is measured by the p-value derived from a binomial distribution. An additional p-value, referred to as “matched promoter p-value” was used to assess the statistical significance of the number of promoters in the query set that have at least one predicted site compared to that of promoters in the background set.
2.8. GSEA
To identify an enrichment of genes assigned to a certain molecular classification group (in our case: transcription factors or diseases biomarkers), we used knowledge-based gene set enrichment analysis (GSEA; (Subramanian et al., 2005). This algorithm, which resembles a Kolmogorov–Smirnov statistic, is designed to detect such an enrichment of an input gene set and accounts for whether hit genes of a classification group tend to be located at the top of the sorted list at the bottom or distributed randomly. Thus the enrichment score ranges from 1 (highest) to -1 (lowest). Here, the full set of 709 identified genes was sorted according to the genes‘ FC values and was used to detect overrepresented transcription factor groups or disease biomarkers.
2.9. Network and key node analysis
The search for key nodes in ExPlain allows looking for common signaling molecules (key nodes) in the network vicinity of a gene set. It searches the network in a specified distance starting from each input molecule in order to find the most proximal molecule that is connected to a maximal number of input molecules. This is achieved by scoring each node that was visited on the path from any input molecule. Since the resulting score may be determined by a generally high level of connectivity of some molecule, the total number of connections reaching every node is also taken into account by the algorithm and penalized, in order to acquire a preference for molecules that are specific for the input genes. A more detailed description is given in (Stegmaier et al., 2011). The result of several key node analyzes was merged and visualized.
2.10. Composite module analysis
The composite module analysis (CMA) is an algorithm to build promoter models for a set of target promoters by characterizing promoters by different binding motifs, derived from a match search, and by rules for their arrangement as single motifs and pairs. These models are optimized with a genetic algorithm for maximal discrimination between target promoters and a control set (Kel et al., 2006). In our case, we applied this algorithm for promoters of 11 glycolysis and glycolysis-associated genes (ENO2, PFKP, PFKFB4, ALDOC, GPI, HK1, HK2, MPI, PFKL, PGK1, and TPI1) as well as for eight insulin pathway genes (CBL, CREB1, GRB10, GYS1, INSR, MAP2K1 (MEK1), MAPK7 (ERK5), and NEDD4L) using MATCH outputs as described above. We defined to combine two to five single matrices (no pairs allowed) in up to two modules of 200 bp size and in up to two independent groups for model A. Additionally, we defined an alternative model B consisting of two to five single matrices and two to five pairs (distance 3–30 bp) with module and group settings as in model A. We further defined the number of individual model solutions to be taken into account during each iteration (population size) to 1000 and stopped the calculation after 1000 iterations. Hence the obtained four composite models have been used to classify the set of all upregulated genes (with the set of 535 unchanged genes as background).
3. Results
3.1. Glycolysis genes are strikingly upregulated by hypoxia
To analyze the impact of hypoxia on adipocytes we incubated differentiated SGBS adipocytes at 37 °C under 1% O2 for 3, 6, and 16 h and extracted their RNA to be used for Affymetrix gene expression profiling. According to their differential expression, we obtained a set of 332 upregulated (FC > 2, p-value <0.05) and 210 downregulated (FC < 0.5, p-value < 0.05) genes (suppl. 3). From previous enrichment analysis, we are aware of a high representation of the biological process glycolysis within the set of upregulated genes (Geiger et al., 2011a). Repeating the functional classification with the set of upregulated genes, we conducted a gene ontology (GO) annotation with a manual curated database. As expected under hypoxic treatment of our cells, the GO term “response to hypoxia” comprising a set of 34 genes known to respond to hypoxia was the top ranked within the biological processes. Apart from that, we also identified “hexose metabolic process” with a strikingly high significance, which consists of genes mainly known from glycolysis (ALDOC, ENO2, GPI, HK, PFK, PGK, TPI), glycolysis-associated steps (GPT, MPI, PDK, PFKFB4), glycogen synthesis (GYS) or of those with a vague or ambiguous role in metabolism (FUT, STC2). Investigating the set of downregulated genes, GO term “metabolic process” was top ranked, confirming again the impact of hypoxia on expression of metabolic genes. Similarly, we mapped the set of upregulated genes to canonical reaction pathways. In accordance with the high ranking in the GO results we identified “glycolysis” as the most significant pathway, followed by “glucose → pyruvate”, consisting of identical genes. As top 3 pathway we got “insulin pathway”, comprising CBL, CREB1, MAPK7 (ERK5), GRB10, GYS1, INSR, MAP2K (MEK1), and NEDD4L. Hence, we also investigated the set of down regulated genes and identified “L-glutamate → 2-oxoglutarate” as the top ranking pathway (Table 1).
Table 1.
Functional classification by canonical pathway mapping. The genes of the upregulated (↑) and downregulated (↓) sets were classified according to their biological processes and gene ontology (GO) IDs by GO annotation, using manually curated GO groups. In accordance, the same gene sets were compared to annotated Transpath pathways. Enolase 2 is symbolized as ENO2, platelet-type 6-phosphofructokinase as PFKP, 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as PFKFB4, aldolase C as ALDOC, mannose-6-phosphate isomerase as MPI, phosphoglycerate kinase 1 as PGK1, liver-type 6-phosphofructokinase as PFKL, glucose-6-phosphate isomerase as GPI, triosephosphate isomerase as TPI, hexokinase as HK, glutamic pyruvate transaminase 2 as GPT2, pyruvate dehydrogenase kinase isoenzyme 1 as PDK1, fucosyltransferase 11 as FUT11, stanniocalcin 2 as STC2, pyruvate dehydrogenase kinase isozyme 3 as PDK3, Cas-Br-M ecotropic retroviral transforming sequence as CBL, cAMP responsive element binding protein 1 as CREB1 (CREB-A and CREB-B), growth factor receptor-bound protein 10 as GRB10, glycogen synthase as GYS1, insulin receptor as INSR, mitogen-activated protein kinase kinase 1 as MAP2K1 (=MEK1), mitogen-activated protein kinase kinase 7 as MAPK7 (=ERK5), neural precursor cell expressed developmentally downregulated 4-like as NEDD4L, and glutamate dehydrogenase as GLUD.
| Gene set | 1. GO ID//2. Pathway ID | 1. GO Term//2. Pathway term | Hits | p-Value |
|---|---|---|---|---|
| ↑ | GO:0001666 | Response to hypoxia | [34] | 6.74E−20 |
| ↑ | GO:0019318 | Hexose metabolic process | ALDOC, ENO2, FUT11, GP1, GPT2, GYS1, HKl, HK2, MPI, PDK1, PDK3, PFKFB4, PFKL, PFXP, PGK1, STC2, TPI1 | 2.28E−08 |
| ↑ | GO:0001568 | Blood vessel development | [27] | 4.07E−08 |
| ↓ | GO:0008152 | Metabolic process | [127] | 5.46E−05 |
| ↑ | CH000003595 | Glycolysis | PFKP, PFKL, ALDOC, GPI, HK2, HK1, MPI, PGK1, TPI1 | 1.65E−07 |
| ↑ | CH000003563 | Glucose → pyruvate | PFKL. PFKP, ALDOC, GP1, HK2, HK1, PGK1, TPI | 2.11E−O7 |
| ↑ | CH000000750 | Insulin pathway | CBL, CREB1, MAPK7, GRB10, GYS1. INSR, MAP2K1, NEDD4L | 3.71E−06 |
| ↓ | CH000003662 | L-glutamate → 2-oxoglutarate | GLUD1, GLUD2 | 8.95E−06 |
3.2. qPCR verifies upregulation of glycolytic genes ENO2, PFKP, PFKFB4, and ALDOC
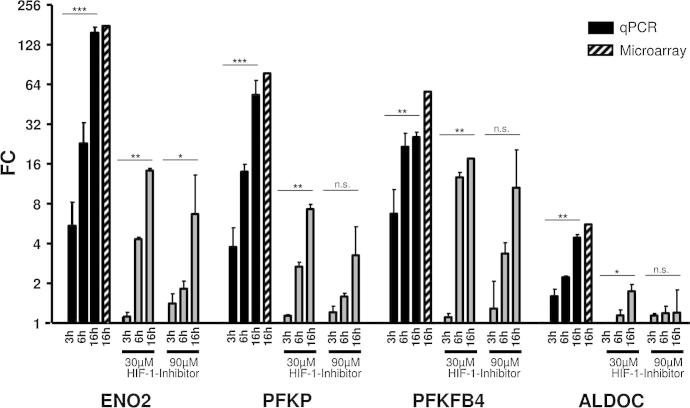
In view of the role of glucose metabolism we verified the results obtained by the microarray (suppl. 3). Hence we repeated the hypoxic cultivation of the SGBS and individually determined the transcriptional activation of the four most upregulated genes involved in or directly associated with glycolysis, enolase 2 (ENO2, FC: 177), platelet-type 6-phosphofructokinase (PFKP, FC: 77.6), 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 (PFKFB4; FC: 55.4), and aldolase C (ALDOC, FC: 5.57), by qPCR at three different time points (3, 6, 16 h). We found that fold change values for their transcriptional upregulation from three independent experiments were very similar to those from microarrays (Fig. 1, left panels) and in the case of PFKFB4 within the same range as analyzed previously by us (Geiger et al., 2011a).
Fig. 1.
Transcriptional activation of glycolysis genes ENO2, PFKP, PFKFB4, and ALDOC. Left panels (black): Quantitative real-time PCR analysis was performed analyzing mRNA levels of ENO2, PFKP, PFKFB4, ALDOC, and TBP as reference gene and compared to microarray data (hatched bars). RNA was prepared from mature SGBS adipocytes after 3, 6 and 16 h of cultivation under hypoxic conditions (1% O2). Results represent three independent experiments each performed in triplicate and are expressed as mean values ± SD. Middle and right panels (grey): SGBS adipocytes were treated additionally with 30 and 90 μM of CAY10585 (HIF-1 inhibitor). It should be noted that the scale on the y-axis is logarithmic. Results represent two independent experiments each performed in triplicate. All data are expressed as mean values ± SD. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
To clarify the activation of these four glycolysis-related genes we repeated the hypoxic cultivation of mature SGBS adipocytes in the presence of the HIF-1-Inhbitor CAY10585 which efficiently blocks HIF-1 accumulation in a concentration-dependent manner (suppl. 5) as demonstrated previously (Geiger et al., 2011b). As depicted in Fig. 1 (middle and right panels), the inhibitor clearly counteracted the hypoxia induced upregulation of all tested genes. In detail, applying 30 and 90 μM inhibitor extensively reduced induction of ENO2 from FC 157.2 to 14.3 (30 μM inhibitor) and 6.7 (90 μM inhibitor) representing a fold decline of 11 and 24 respectively after 16 h compared to cells not treated with the inhibitor. Similarly, PFKP expression declined 7 and 16-fold from FC 53.3 to 7.3 and 3.3 respectively. A less striking reduction was observed for PFKFB4 expression from 25.5 to 17.6 and 10.6 in keeping with a 1.4 and 2.4-fold decline respectively, and for ALDOC from 4.4 to 1.7 and 1.6, meaning a 2.5 and 2.7-fold decline.
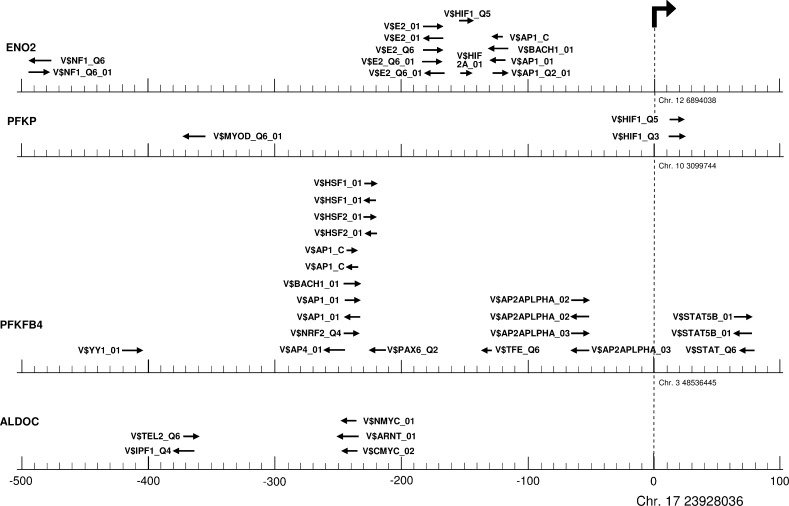
As this points out an important but not exclusive role of HIF-1α a for its activation, we set out to identify the transcription factors responsible for the massive transcriptional activation of ENO2 and other genes involved in glycolysis. Hence we applied the F-match search algorithm on each of the promoter sequence of ENO2, PFKP, PFKFB4 and ALDOC. By doing this, we used a predefined PWM profile consisting of 656 matrices for vertebrate transcription factors and matched them to each of the four genes. As background gene set we used 535 human genes, which had shown no hypoxia-mediated change in expression. The best-supported matrices for ENO2, PFKP, PFKFB4, and ALDOC promoter regions are shown at their respective binding sites in Fig. 2. As depicted, matrices representing HIF-1α and the AP-1 family of transcription factors are the best matches, binding to sequence parts −154 to −143, and −131 to −116 within the ENO2 core promoter region. Furthermore, activation of PFKP seems to depend on direct binding of HIF-1α too, whereas no direct sequence match of HIF-1α matrices have been determined for PFKFB4 and ALDOC. However, the ALDOC promoter region harbors ARNT and MYC binding sites, both containing ACGTG as core sequence, which is also part of HIF-1α matrices (suppl. 6), and thus does not preclude HIF-1α binding. In contrast, the PFKFB4 promoter seems to be bound predominately by AP-1, AP-2 and AP-4.
Fig. 2.
Binding sites for transcription factors within promoter regions of ENO2, PFKP, PFKFB4, and ALDOC. Significantly matching matrices (arrows) are shown within the proximal promoter of ENO2, PFKP, PFKFB4, and ALDOC, representing transcription factor binding sites. The result was filtered according to the Yes/No ratio (>2), its p-value (<0.01), and the matched promoter p-value (<0.05). For ALDOC only the top five of 26 matrices are displayed.
This result obtained by F-match search in the promoter sequences is in line with the data above supposing a minor effect of the HIF-1 inhibitor on PFKFB4 and ALDOC. This suggests rather an indirect activation by HIF-1α than a direct binding and a contribution of further transcription factors.
3.3. Transcription factors KLF7 and the AP-1 family are involved in hypoxia-mediated gene regulation
In view of the upregulation of these four glycolytic genes we were interested in the interplay of transcription factors responsible for global hypoxia response in adipocytes going beyond glycolysis or insulin-signaling as determined above. Thus we used the CMA software (Kel et al., 2006) which arranges and combines single binding motifs and pairs to generate promoter models for the set of glycolysis genes on the one hand and for insulin pathway genes on the other hand. Two different model presets were used for the calculation of each gene set promoter model (Table 2 and suppl. 7). Hence we used the obtained four models to classify again the set of all upregulated genes to identify genes, which are likely to be activated by these models, suggesting a common gene regulation. Genes with matching sequence scores greater than 85% from the top score for each model were used to build an intersection set of all four matrices. Table 2 displays that ENO2, the most upregulated gene within the microarray data, the transcription factor Kruppel-like factor 7 (KLF7), which is supposed to contribute to the pathogenesis of type 2 diabetes (Zobel et al., 2009; Kanazawa et al., 2005), and the vascular endothelial growth factor A (VEGFA), share promoter regions probably enabling their activation in analogy to glycolytic and insulin pathway genes.
Table 2.
Genes regulated by the promoter models for glycolysis and insulin pathway gene sets. Listed genes are part of the upregulated gene set and were classified according to their potential activation by the promoter models for glycolytic and insulin pathway genes. Respective sequence scores of genes’ promoters are given for the calculated models. The composite model analysis and classification were generated via the composite module analysis (CMA) algorithm. FC values and adjusted p-values (Benjamini and Hochberg) for differential expression obtained in microarray analysis are indicated.
| Gene symbol | BHp | FC | Model a (Glycolysis I) | Model b (Glycolysis II) | Model c (Insulin I) | Model d (Insulin I) |
|---|---|---|---|---|---|---|
| ENO2 | 6.12E−06 | 177.28 | 0.550 | 0.654 | 0.408 | 0.864 |
| KLF7 | 1.44E−02 | 11.72 | 0.582 | 0.585 | 0.424 | 0.871 |
| VEGFA | 1.52E−03 | 11.15 | 0.564 | 0.574 | 0.394 | 0.851 |
In addition to KLF7, further transcription factors might be involved in the comprehensive hypoxia-induced transcriptional regulation. To extract these information from the adipocyte expression profile we used knowledge-based gene set enrichment analysis (GSEA; (Subramanian et al., 2005). Thus the full set of 709 identified genes, comprising upregulated, downregulated as well as unchanged transcripts, was sorted according to the genes‘ FC values. Applying the algorithm for transcription factor classification, we detected AP-1(-like) components ATF3, Fosl2 and Jun with the highest enrichment score and lowest p-value, representing a very high ranking and expression respectively of these genes within the group of 709 genes (Table 3).
Table 3.
Transcription factor classification via enrichment analysis for the whole gene set. Result of gene set enrichment analysis (GSEA) for all genes with FC values ranging from 177 to 0.045, including those with unchanged expression values (FC: 2–0.5) as well. Functional groups referred to as transcription factor classes were identified to be overrepresented (+1 ⩾ “score” > 0) or underrepresented (−1 ⩽ “score” < 0) according to their FC-dependent ranking.
| Factor class description | Hits | Score | p-Value |
|---|---|---|---|
| AP-1 (-like) components | ATF3, FOSL2, JUN | 0.9037 | 9.66E−04 |
| Basic domains | n/a | 0.4760 | 2.90E−02 |
| Leucine zipper factors (bZIP) | n/a | 0.4165 | 9.48E−02 |
| Homeo domain only | n/a | −0.5699 | 4.85E−02 |
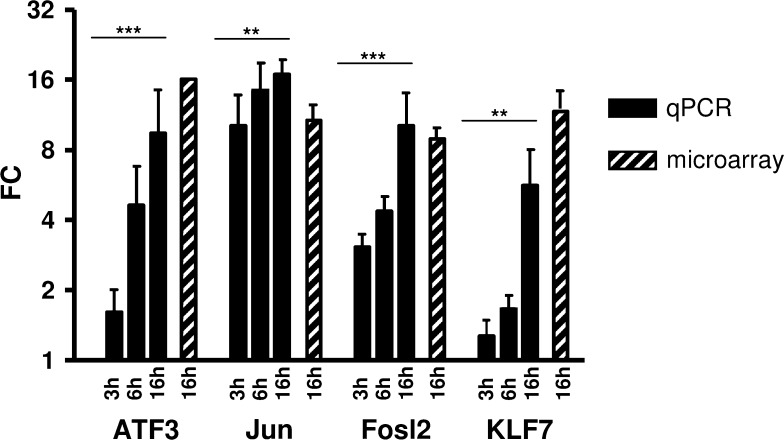
To prove the results of the GSEA, which is based on the microarray expression data, we individually assessed the expression of these transcription factors by qPCR. Obtained fold change values for the three AP-1 components and KLF7 of three independent experiments and at three different time points (3, 6, 16 h in hypoxic cultivation) proof that microarray and qPCR data were concordant (Fig. 3).
Fig. 3.
Transcriptional activation of ATF3, FOSL2, JUN, and KLF7. QPCR analysis of mRNA levels of the transcription factor genes ATF3, FOSL2, JUN, and KLF7 after 3, 6 and 16 h treatment under hypoxic conditions (1% O2) was performed and results were compared to microarray data. Three independent experiments were each performed in triplicate and are expressed as mean values ± SD. ∗p < 0.05; ∗∗p < 0.01; ∗∗∗p < 0.001.
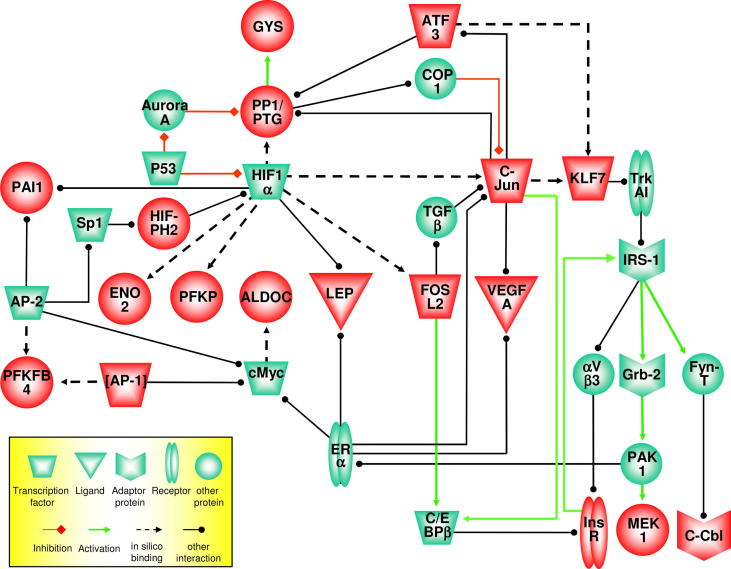
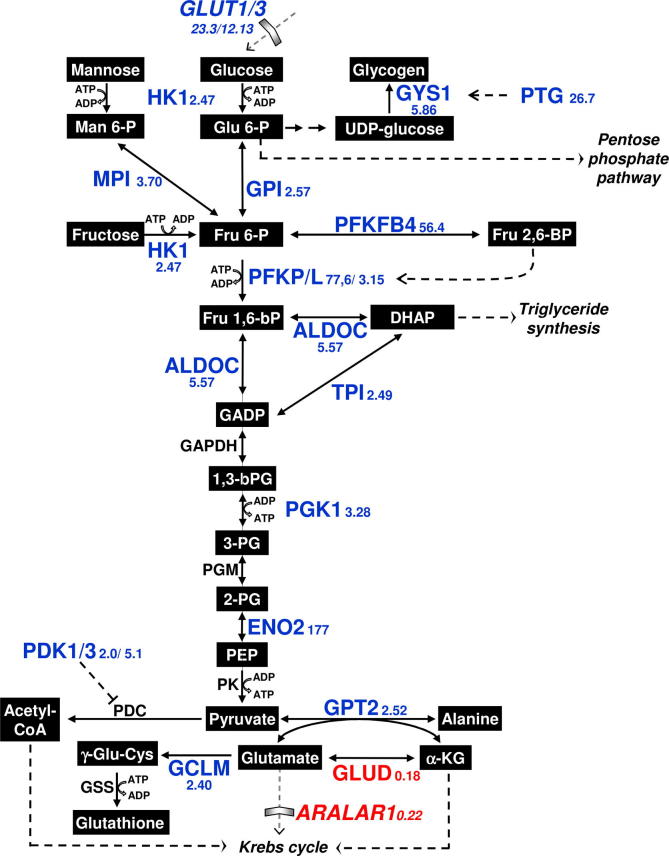
In view of the important role of hypoxia-induced transcription factors, the question arises whether the upregulation of ATF3, Jun, Fols2, and KLF7 can also be directly assigned to activation by HIF- transcription factor family members. Thus as described above for the genes involved in glycolysis, we applied the F-Match analysis on the promoter sequences of ATF3, Jun, Fosl2, and KLF7 and identified relevant HIF-1α binding sites in Fosl2 (position −441 to −428, with respect to start codon) and Jun (−299 to −291), as well as a ATF3/Jun-specific binding site in KLF7 (30–43). Thereupon, we tried to find molecular connections between (i) identified transcription factors ATF3, Jun, Fosl2, and KLF7, (ii) members of the glycolytic and insulin pathways, and (iii) HIF-1α. We used a “key node analysis” algorithm to look for key nodes in the signaling network vicinity of (i)–(iii) and thus got individual networks containing the respective genes connected by signal transduction reactions to relevant interaction partners. Combining these networks, or at least parts in case of the glycolytic and insulin pathways, with our F-Match binding data, we generated an overview about the molecular interplay (Fig. 4). According to our analyzes, HIF-1α is a central transcription factor directly involved in glycolysis (ENO2, and PFKP) lipid metabolism (leptin, LEP) and angiogenesis (VEGFA, PAI1) and in the activation of FOSL2 and JUN. Indirectly and via additional transcriptional activators, it impacts the insulin signaling pathway and further glycolytic genes.
Fig. 4.
Visualized network analysis. Based on information about signal transduction networks contained in the BKL database, key node search analyses for several gene sets have been performed. The obtained results have been combined and visualized. Highlighted (red) molecules have been upregulated as demonstrated by microarray data. Aurora A denotes aurora kinase A, aVb3 the vitronectin receptor, C/EBP the CCAAT-enhancer binding protein, COP1 the ring finger and WD repeat domain 2, C-Cbl the Cas-Br-M ectropic retroviral transforming sequence, ER estrogen receptor, Grb-2 growth factor receptor-bound protein, HIF-PH2 hypoxia-inducible factor prolyl hydroxylase 2, IRS-1 insulin receptor substrate 1, MEK1 the kinase MAP2K1, PAK p21 protein-activated kinase, TGF transforming growth factor, TrkA1 the neurotrophic tyrosine kinase receptor type 1. Further abbreviations are referred to in the article text. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
With respect to initial GO data suggesting an upregulation of glycogen synthesis, we performed an additional F-match search and identified several highly significant HIF-1α PWMs (−262 to −251 and −162 to −151, and −47 to −34) within the promoter sequence of protein targeting to glycogen (PTG), also known as glycogen synthase 1 (GYS1) activator protein phosphatase 1 regulatory inhibitor subunit 3C (PPP1R3C) and GYS1 (suppl. 8). Thus, HIF-1α seems to be involved in gene regulation for glycogen synthesis, too.
In addition to the activation network initiated by hypoxia, we then aimed to assess the complex impact and outcome on metabolism in SGBS adipocytes. Starting, with genes involved in hexose metabolism and in the insulin pathway, initially identified by GO and pathway analysis, we also added those players which have been downregulated e.g. glutamate dehydrogenase 1 and 2 (GLUD1 and 2; Table 1) and added further players which have been identified to be differentially expressed and involved in the regulation of glutamate level as glutamic pyruvate transaminase (GPT2) and glutamate cysteine ligase modifier subunit (GCLM) or have been identified from the CMA-analysis. As cellular metabolism closely relies on its respective transporter complexes, we once again analyzed the regulatory pattern for transporters of the solute carrier family (SLC) and were able to detect amongst them glucose and lactate transporters GLUT 1, GLUT 3 and MCT4 as the most upregulated members and the aspartate glutamate transmembrane transporter ARALAR1 as the most downregulation one (suppl. 9). The resulting overview about the hypoxia-induced gene regulation of transporters and enzymes as well as the assumed metabolic consequences (Fig. 5) finally indicates an impact of hypoxia on glycogen production as well as the generation of ROS antagonists.
Fig. 5.
Hypoxia-mediated regulation of genes involved in glucose metabolism. Genes which have been identified to be up-(in red) or down (in blue)-regulated due to hypoxia are given in bold letters with their fold change values. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
3.4. Hypoxia promotes glycogen and glutathione accumulation
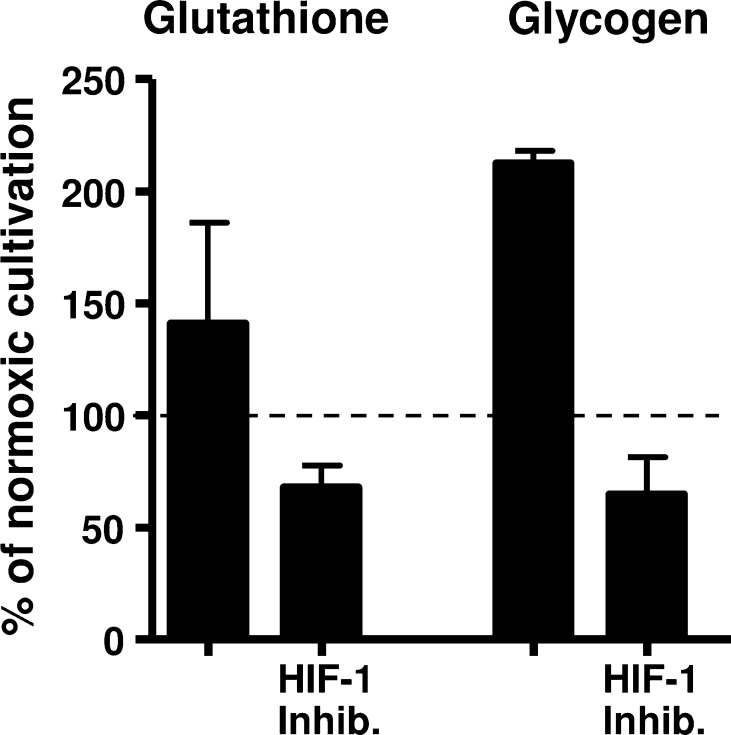
To test our assumption for elevated glycogen production under hypoxia, we again incubated differentiated SGBS adipocytes at 37 °C under 1% O2 for 16 h in the presence and absence of the HIF-1 inhibitor CAY10585 and measured their glycogen amount. As demonstrated in Fig. 6 glycogen amounts were clearly raised under hypoxic treatment. Similarly, we assessed the level of total glutathione in those adipocytes and also assessed increased accumulation after hypoxic treatment, whereas the use of HIF-1 inhibitor declined cellular accumulation of both molecules.
Fig. 6.
Effect of 16 h hypoxic cultivation on glutathione and glycogen accumulation in SGBS adipocytes. Fully differentiated SGBS adipocytes were incubated under hypoxic conditions in presence or absence of 90 μM of the HIF-1 inhibitor CAY10585 for 16 h and under normoxia as control and levels of glutathione and glucogen were examined subsequently. Experiments were performed in duplicate. Resulting amounts are given as mean percent (with standard error) of normoxic cultivation.
3.5. Adipocyte genes differentially regulated by hypoxia significantly correlate with diabetes and insulin resistance biomarkers
Finally, we were interested in how strongly the gene expression profile assessed for hypoxic SGBS adipocytes correlates with known disease biomarkers. Thus, we applied the GSEA again to the full set of genes (adjusted p-value <0.05) comprising up and down regulated ones as well as those with unchanged expression values and filtered the results according to very high significance (p-value <5 E-4). As calculated by the algorithm, diabetes mellitus strikingly matches the mentioned gene expression profile (Table 4), and the same is true for insulin resistance. Apart from that, there are also biomarkers associated with carcinomas and neoplasms. Taken together, there is a striking correlation between the gene expression pattern of hypoxia-treated adipocytes and matched biomarker for metabolic syndrome characteristics, namely diabetes mellitus and insulin resistance.
Table 4.
Disease classification via enrichment analysis for the whole gene set. Functional groups referred to as diseases have been estimated overrepresented (+1 ⩾ “score” > 0) or underrepresented (−1 ⩽ “score” < 0; not applied) within the set according to the FC-dependent ranking of respective biomarkers.
| Disease name | Biomarker associations | Score | p-Value | |
|---|---|---|---|---|
| 1 | Diabetes mellitus | Correlation | 0.9163 | 5.52E−05 |
| 2 | Carcinoma, ductal, breast | Correlation | 0.8480 | 9.11E−05 |
| 3 | Insulin resistance | Correlation | 0.7667 | 1.85E−04 |
| 4 | Carcinoma, non-small-cell lung | Correlation | 0.5054 | 3.41E−05 |
| 5 | Prostatic neoplasms | Correlation | 0.4567 | 4.06E−06 |
| 6 | Breast neoplasms | Correlation | 0.3550 | 1.60E−06 |
| 7 | Lung neoplasms | Correlation | 0.3194 | 2.19E−04 |
4. Discussion
4.1. Hypoxia extensively and intensively influences expression of genes involved in glucose metabolism
Here, we report a massive transcriptional induction of genes involved in hexose metabolism of adipocytes upon hypoxic cultivation. The most striking transcriptional induction in our data has been determined for ENO2, whose gene product converts phosphoglycerate to phophoenolpyruvate, with a fold change expression of 177. ENO2 has been known for a while to be inducible by hypoxia (Szturmowicz et al., 1998), and its upregulation has been determined in murine melanoma cells (Olbryt et al., 2006), human cord blood and bone marrow mesenchymal stem cells (Martin-Rendon et al., 2007), as well as in adipocytes (Mazzatti et al., 2012) but not to the extent as revealed by our data. In view of our binding site analysis and the striking transcriptional attenuation after HIF-1 inhibition we conclude that HIF-1α directly binds the ENO2 promoter and therefore is responsible for their mRNA upregulation in adipocytes. This also applies to PFKP and thus is in line with recent reports of HIF-1-dependency in tumor tissue (Guerin et al., 2012) and with our data using HIF-1 inhibitor. Of interest, PFKP, which is associated with increased BMI and obesity (Scuteri et al., 2007; Liu et al., 2008) has also been reported to be activated by KLF4 (Moon et al., 2011). In our settings, we also have determined a 4.5-fold increase in KLF4 expression, although the respective p-value of 0.057 just failed our significance cut off of 0.05. Similarly, PFKFB4 and ALDOC expression were upregulated under hypoxia. However, inhibiting HIF-1 was not sufficient to completely block ENO2 and PFKP upregulation, and this applied even more to PFKFB4 and ALDOC. Thus further transcription factors and pathways must be involved in their activation. This is in accordance with recent data obtained for tumorigenicity, which similarly disfavor the existence of one master regulator (Stelniec-Klotz et al., 2012). Our in silico data support an indirect activation mediated by the AP-1 family and further supported by AP-2, AP-4 or cMyc. However, a hypoxia-responsive element within the PFKFB4 promoter has previously been described leading to hypoxia-triggered upregulation in different cancer cells (Minchenko et al., 2004). Although we were unable to confirm such a HIF-1α-specific binding site with our settings, we cannot rule out that HIF-1α might cooperate with Arnt or further factors for a direct binding. Hence we were also able to demonstrate the link between HIF-1α, AP-1 and KLF7 and upregulated key players in the insulin pathway, lipid metabolism, angiogenesis and glycogen synthesis. Although the artificial interaction network as given in Fig. 4 is based on general interactions and not limited to reactions proven in adipocytes, it well reflects the possible impact of hypoxia and HIF-1α on the metabolic fate of the cell.
4.2. Hypoxia might induce NADPH and glutathione production for controlling ROS-levels
The mitochondrial electron transport chain consumes most of the cell’s oxygen and at the same time produces ROS. Under hypoxia, ROS production increases and thus HIF-1α is stabilized. HIF-1-dependent gene regulation leads to an adaption to the hypoxic state allowing the cell to survive (Klimova and Chandel, 2008). Here we report a potential mechanism how HIF-1α might reduce the ROS production, triggered by hypoxia.
GLUD catalyzes the reversible conversion of glutamate to α-ketoglutarate and thus provides energy via delivering α-ketoglutarate to the Krebs cycle. In β-cells, an overexpression of GLUD is known to result in an enhanced glutamate oxidation and insulin secretion (Carobbio et al., 2004), improving glucose tolerance in vivo (Han et al., 2012). In hypoxic SGBS adipocytes, we assessed on the one hand elevated glutathione levels and on the other hand impaired GLUD mRNA levels. The latter could contribute to abandoning the Krebs cycle and the oxidative phosphorylation, but also might increase glutamate levels for glutathione biosynthesis protecting cells from ROS-caused damage. Glutamic pyruvate transaminase (alanine aminotransferase)-2 catalyzes the reversible transamination between alanine and 2-oxoglutarate to form pyruvate and glutamate. Thus it plays an important role in gluconeogenesis and fatty acid metabolism (Yang et al., 2002). As overexpressed glutamic pyruvate transaminase in HeLa cells enables them to proliferate in glutamine-free media supplemented with alanine and α-ketoglutarate (Meng et al., 2010), these metabolic direction may also supply hypoxic adipocytes with pyruvate for glycogen synthesis and with glutamate for glutathione production. This is further supported by the upregulation of glutamate cysteine ligase (GCLM) which catalyzes the production of gamma-glutamylcysteine and thus enables glutathione generation.
PFKFB4 is a bifunctional enzyme comprising kinase as well as phosphatase activity, with the latter being slightly stronger (Sakakibara et al., 1997). It regulates the steady-state concentration of fructose 2,6-bisphosphate which is an activator of PFK-1 and therefore of glycolysis. Recently, PFKFB4 emerged as a promising new target with respect to cancer therapy, as its upregulation has been demonstrated to be important to control ROS levels in prostate cancer cells and thus to support tumor survival (Ros et al., 2012). Similarly this may also be true for adipocytes which control their redox balance that way. As a result, the enormous upregulation of PFKFB4 might decrease the 2,6-bisphosphate level, thereby promoting the generation of fructose 6-phosphate from fructose 1,6 bisphosphate. This would mean an increase of metabolic intermediates for the pentose phosphate pathway and the generation of NADPH. As NADPH maintains the cellular stores of reduced glutathione, PFKFB4 upregulation might prevent the accumulation of ROS in adipocytes as it has been described in cancer cells (Ros et al., 2012). In this context the upregulation of PDK-1 and -3 (FC: 2.0 and 5.1), which are also HIF-1-targets, as demonstrated in fibroblasts and cancer cells (Kim et al., 2006; Papandreou et al., 2006), seems to be consistent. Both enzymes are known to inhibit the conversion of pyruvate to acetyl CoA by phosphorylating pyruvate dehydrogenase. As a result, pyruvate influx to the Krebs cycle is inhibited and also the NADH delivery to the mitochondrial respiratory chain. This attenuates oxidative phosphorylation and excessive mitochondrial ROS production, and on the other hand, facilitates NAD+ regeneration via anaerobic fermentation of pyruvate to lactate. In tumor tissue, cells switch their metabolism from mitochondrial oxidation to a highly increased uptake of glucose and glycolysis followed by fermentation to lactate, known as the Warburg effect. Although LDHA expression has not been upregulated in our adipocytes, we have detected an increase of lactate and glucose transmembrane transporter gene expression (MCT1, MCT4, GLUT1, GLUT3), which has been reported before also by Wood et al. (2007) and Perez et al. (2010). Thus our data clearly support previous data demonstrating increased glucose uptake by adipocytes in response to hypoxia (Wood et al., 2007; Regazzetti et al., 2009).
In a previous microarray study also examining the impact of hypoxia on human adipocyte gene expression, Mazzatti et al. (2012) identified glucose uptake and gluconeogenesis among the top five pathways and cell death as top cell function. Whereas the latter function was not represented in our expression pattern enhanced glucose uptake and the utilization of pyruvate or lactate to generate glucose strikingly supports our observations.
4.3. Hypoxia induces glycogen accumulation
Apart from the upregulation of the mentioned glucose transporter genes, hypoxia also triggers the downregulation of the aspartate–glutamate carrier ARALAR1. It is a member of the aspartate–malate NADH shuttle system and transfers glutamate from the cytoplasmic compartment to the mitochondrial matrix. For pancreatic β-cells it is known that ARALAR1 overexpression increases mitochondrial glucose oxidation and insulin secretion and reduces lactate production and the glycogen content in β-cells (Rubi et al., 2004) and thereby is thought to augment insulin secretion (Bender et al., 2009). As ARALAR1 gene expression in adipocytes declines upon hypoxic cultivation, the amount of cytosolic glutamate might increase and facilitate glutathione synthesis as described above, but also glucose oxidation might become restricted. In addition this may also be a hint for glycogen synthesis activity. As proposed by Pescador et al., (2010), hypoxia is a driving force for glycogen synthesis and accumulated glycogen enables the cell to cope with future hypoxic challenges, preventing cell damage. Thus our data further support a coordinated response of glycogen metabolism upon hypoxia. In line with data from myoblasts (Pescador et al., 2010), we also observed upregulated mRNA levels of GYS1. In addition, we assessed a strikingly upregulation of PTG, which is known to bind the GYS1 gene product and mediate its activation (Fong et al., 2000), promoting glycogen accumulation in a HIF-1-dependent way (Shen et al., 2010). Although glycogen is present in adipocytes at very low levels, it may be possible that glycogen synthesis as a protection mechanism plays a rather important role in hypoxic adipocytes.
Glycogen and lipid synthesis are known to be linked in adipose tissue and conditions favoring glycogen synthesis also favor lipid synthesis. During the transition from fasted to fed state, glycogen stores are refilled prior to lipid stores, generating a glycogen spike. In their review Markan and her colleagues suppose that this glycogen spike indicates energy abundance and the suitability for filling lipid storage. Moreover, they have also proposed that glycogen stores in adipocytes may serve rather as an energy sensor triggering a metabolic switch than only as carbon and energy source (Markan et al., 2010). We hypothesize that this capacity of modulating metabolic pathways in adipocytes thus might also apply to hypoxic episodes in adipose tissue, pretending energy abundance and triggering lipogenesis. With respect to the whole body metabolism, hypoxia in obese adipose tissue might start a vicious circle, further exacerbating obesity, shifting metabolism, and finally contributing to disease manifestation. Nevertheless, it should be kept in mind that this study has been conducted in cells derived from a patient suffering from Simpson-Golabi-Behmel syndrome and thus data may not necessarily reflect the situation of adipocytes in healthy subjects. Likewise, 1% O2 as applied in this study should be considered just as a model for hypoxia as the real oxygen tension in fat tissue of obese and lean subjects is still a matter of debate (Lecoultre and Tam, 2012; Trayhurn, 2013).
Finally, the impact of an altered production of glycolytic enzymes might not only serve to maintain ATP production via anaerobic glycolysis. In adipocytes, it might serve also for controlling the level of ROS via glutathione production and for glycogen synthesis preventing cellular damage. However, metabolism shifted this way in hypoxic adipose tissue may be causative for metabolic disorders and disease manifestation as given for T2DM on the long way (Dang, 2012).
Acknowledgement
This study was supported by the Austrian Science Fund (FWF, Project 21057). Microarray analysis was performed by the Expression Profiling Unit at the Medical University Innsbruck and the authors thank Dr. Johannes Rainer for help with data processing.
Footnotes
This is an open-access article distributed under the terms of the Creative Commons Attribution-NonCommercial-ShareAlike License, which permits non-commercial use, distribution, and reproduction in any medium, provided the original author and source are credited.
Appendix A. Supplementary material
References
- Ashburner M., Ball C.A., Blake J.A., Botstein D., Butler H., Cherry J.M., Davis A.P., Dolinski K., Dwight S.S., Eppig J.T., Harris M.A., Hill D.P., Issel-Tarver L., Kasarskis A., Lewis S., Matese J.C., Richardson J.E., Ringwald M., Rubin G.M., Sherlock G. Gene ontology: tool for the unification of biology. The Gene Ontology Consortium. Nat. Genet. 2000;25:25–29. doi: 10.1038/75556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender K., Maechler P., McClenaghan N.H., Flatt P.R., Newsholme P. Overexpression of the malate-aspartate NADH shuttle member Aralar1 in the clonal beta-cell line BRIN-BD11 enhances amino-acid-stimulated insulin secretion and cell metabolism. Clin. Sci. (Lond) 2009;117:321–330. doi: 10.1042/CS20090126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamini Y., Hochberg Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. Journal of the Royal Statistical Society. Series B (Methological) 1995;57:289–300. [Google Scholar]
- Carobbio S., Ishihara H., Fernandez-Pascual S., Bartley C., Martin-Del-Rio R., Maechler P. Insulin secretion profiles are modified by overexpression of glutamate dehydrogenase in pancreatic islets. Diabetologia. 2004;47:266–276. doi: 10.1007/s00125-003-1306-2. [DOI] [PubMed] [Google Scholar]
- Chen S.C., Liu Y.C., Shyu K.G., Wang D.L. Acute hypoxia to endothelial cells induces activating transcription factor 3 (ATF3) expression that is mediated via nitric oxide. Atherosclerosis. 2008;201:281–288. doi: 10.1016/j.atherosclerosis.2008.02.014. [DOI] [PubMed] [Google Scholar]
- Choi S., Cho K., Kim J., Yea K., Park G., Lee J., Ryu S.H., Kim J., Kim Y.H. Comparative proteome analysis using amine-reactive isobaric tagging reagents coupled with 2D LC/MS/MS in 3T3-L1 adipocytes following hypoxia or normoxia. Biochem. Biophys. Res. Commun. 2009;383:135–140. doi: 10.1016/j.bbrc.2009.03.124. [DOI] [PubMed] [Google Scholar]
- Cummins E.P., Taylor C.T. Hypoxia-responsive transcription factors. Pflugers Arch. 2005;450:363–371. doi: 10.1007/s00424-005-1413-7. [DOI] [PubMed] [Google Scholar]
- Dang C.V. Links between metabolism and cancer. Genes Dev. 2012;26:877–890. doi: 10.1101/gad.189365.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson J.T., Millhorn D.E. Hypoxia and electrical stimulation of the carotid sinus nerve induce Fos-like immunoreactivity within catecholaminergic and serotoninergic neurons of the rat brainstem. J. Comp. Neurol. 1994;348:161–182. doi: 10.1002/cne.903480202. [DOI] [PubMed] [Google Scholar]
- Fandrey J., Gorr T.A., Gassmann M. Regulating cellular oxygen sensing by hydroxylation. Cardiovasc. Res. 2006;71:642–651. doi: 10.1016/j.cardiores.2006.05.005. [DOI] [PubMed] [Google Scholar]
- Fleischmann E., Kurz A., Niedermayr M., Schebesta K., Kimberger O., Sessler D.I., Kabon B., Prager G. Tissue oxygenation in obese and non-obese patients during laparoscopy. Obes. Surg. 2005;15:813–819. doi: 10.1381/0960892054222867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fong N.M., Jensen T.C., Shah A.S., Parekh N.N., Saltiel A.R., Brady M.J. Identification of binding sites on protein targeting to glycogen for enzymes of glycogen metabolism. J. Biol. Chem. 2000;275:35034–35039. doi: 10.1074/jbc.M005541200. [DOI] [PubMed] [Google Scholar]
- Geiger K., Leiherer A., Muendlein A., Stark N., Geller-Rhomberg S., Saely C.H., Wabitsch M., Fraunberger P., Drexel H. Identification of hypoxia-induced genes in human SGBS adipocytes by microarray analysis. PLoS One. 2011;6:e26465. doi: 10.1371/journal.pone.0026465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger K., Muendlein A., Stark N., Saely C.H., Wabitsch M., Fraunberger P., Drexel H. Hypoxia induces apelin expression in human adipocytes. Horm. Metab Res. 2011;43:380–385. doi: 10.1055/s-0031-1273767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentleman R.C., Carey V.J., Bates D.M., Bolstad B., Dettling M., Dudoit S., Ellis B., Gautier L., Ge Y., Gentry J., Hornik K., Hothorn T., Huber W., Iacus S., Irizarry R., Leisch F., Li C., Maechler M., Rossini A.J., Sawitzki G., Smith C., Smyth G., Tierney L., Yang J.Y., Zhang J. Bioconductor: open software development for computational biology and bioinformatics. Genome Biol. 2004;5:R80. doi: 10.1186/gb-2004-5-10-r80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens G.H., Bizzarri A., Venteclef N., Essers Y., Cleutjens J.P., Konings E., Jocken J.W., Cajlakovic M., Ribitsch V., Clement K., Blaak E.E. Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation. Circulation. 2011;124:67–76. doi: 10.1161/CIRCULATIONAHA.111.027813. [DOI] [PubMed] [Google Scholar]
- Guerin E., Raffelsberger W., Pencreach E., Maier A., Neuville A., Schneider A., Bachellier P., Rohr S., Petitprez A., Poch O., Moras D., Oudet P., Larsen A.K., Gaub M.P., Guenot D. In vivo topoisomerase I inhibition attenuates the expression of hypoxia-inducible factor 1alpha target genes and decreases tumor angiogenesis. Mol. Med. 2012;18:83–94. doi: 10.2119/molmed.2011.00120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halberg N., Khan T., Trujillo M.E., Wernstedt-Asterholm I., Attie A.D., Sherwani S., Wang Z.V., Landskroner-Eiger S., Dineen S., Magalang U.J., Brekken R.A., Scherer P.E. Hypoxia-inducible factor 1alpha induces fibrosis and insulin resistance in white adipose tissue. Mol. Cell Biol. 2009;29:4467–4483. doi: 10.1128/MCB.00192-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han S.J., Choi S.E., Yi S.A., Lee S.J., Kim H.J., Kim D.J., Lee H.C., Lee K.W., Kang Y. Beta-cell-protective effect of 2-aminobicyclo-(2,2,1)-heptane-2-carboxylic acid as a glutamate dehydrogenase activator in db/db mice. J. Endocrinol. 2012;212:307–315. doi: 10.1530/JOE-11-0340. [DOI] [PubMed] [Google Scholar]
- Haxhiu M.A., Strohl K.P., Cherniack N.S. The N-methyl-D-aspartate receptor pathway is involved in hypoxia-induced c-Fos protein expression in the rat nucleus of the solitary tract. J. Auton. Nerv. Syst. 1995;55:65–68. doi: 10.1016/0165-1838(95)00029-w. [DOI] [PubMed] [Google Scholar]
- Hodson L., Humphreys S.M., Karpe F., Frayn K.N. Metabolic signatures of human adipose tissue hypoxia in obesity. Diabetes. 2013;62:1417–1425. doi: 10.2337/db12-1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosogai N., Fukuhara A., Oshima K., Miyata Y., Tanaka S., Segawa K., Furukawa S., Tochino Y., Komuro R., Matsuda M., Shimomura I. Adipose tissue hypoxia in obesity and its impact on adipocytokine dysregulation. Diabetes. 2007;56:901–911. doi: 10.2337/db06-0911. [DOI] [PubMed] [Google Scholar]
- Kabon B., Nagele A., Reddy D., Eagon C., Fleshman J.W., Sessler D.I., Kurz A. Obesity decreases perioperative tissue oxygenation. Anesthesiology. 2004;100:274–280. doi: 10.1097/00000542-200402000-00015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanazawa A., Kawamura Y., Sekine A., Iida A., Tsunoda T., Kashiwagi A., Tanaka Y., Babazono T., Matsuda M., Kawai K., Iiizumi T., Fujioka T., Imanishi M., Kaku K., Iwamoto Y., Kawamori R., Kikkawa R., Nakamura Y., Maeda S. Single nucleotide polymorphisms in the gene encoding Kruppel-like factor 7 are associated with type 2 diabetes. Diabetologia. 2005;48:1315–1322. doi: 10.1007/s00125-005-1797-0. [DOI] [PubMed] [Google Scholar]
- Kel A., Konovalova T., Waleev T., Cheremushkin E., Kel-Margoulis O., Wingender E. Composite module analyst: a fitness-based tool for identification of transcription factor binding site combinations. Bioinformatics. 2006;22:1190–1197. doi: 10.1093/bioinformatics/btl041. [DOI] [PubMed] [Google Scholar]
- Kim J.W., Tchernyshyov I., Semenza G.L., Dang C.V. HIF-1-mediated expression of pyruvate dehydrogenase kinase: a metabolic switch required for cellular adaptation to hypoxia. Cell Metab. 2006;3:177–185. doi: 10.1016/j.cmet.2006.02.002. [DOI] [PubMed] [Google Scholar]
- Klimova T., Chandel N.S. Mitochondrial complex III regulates hypoxic activation of HIF. Cell Death. Differ. 2008;15:660–666. doi: 10.1038/sj.cdd.4402307. [DOI] [PubMed] [Google Scholar]
- Krull M., Voss N., Choi C., Pistor S., Potapov A., Wingender E. TRANSPATH: an integrated database on signal transduction and a tool for array analysis. Nucl. Acids Res. 2003;31:97–100. doi: 10.1093/nar/gkg089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lecoultre V., Tam C.S. Letter by Lecoultre and Tam regarding article, “Increased adipose tissue oxygen tension in obese compared with lean men is accompanied by insulin resistance, impaired adipose tissue capillarization, and inflammation”. Circulation. 2012;125:e315. doi: 10.1161/CIRCULATIONAHA.111.050286. [DOI] [PubMed] [Google Scholar]
- Liu Y.J., Liu X.G., Wang L., Dina C., Yan H., Liu J.F., Levy S., Papasian C.J., Drees B.M., Hamilton J.J., Meyre D., Delplanque J., Pei Y.F., Zhang L., Recker R.R., Froguel P., Deng H.W. Genome-wide association scans identified CTNNBL1 as a novel gene for obesity. Hum. Mol. Genet. 2008;17:1803–1813. doi: 10.1093/hmg/ddn072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markan K.R., Jurczak M.J., Brady M.J. Stranger in a strange land: roles of glycogen turnover in adipose tissue metabolism. Mol. Cell Endocrinol. 2010;318:54–60. doi: 10.1016/j.mce.2009.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Rendon E., Hale S.J., Ryan D., Baban D., Forde S.P., Roubelakis M., Sweeney D., Moukayed M., Harris A.L., Davies K., Watt S.M. Transcriptional profiling of human cord blood CD133+ and cultured bone marrow mesenchymal stem cells in response to hypoxia. Stem Cells. 2007;25:1003–1012. doi: 10.1634/stemcells.2006-0398. [DOI] [PubMed] [Google Scholar]
- Mazzatti D., Lim F.L., O’Hara A., Wood I.S., Trayhurn P. A microarray analysis of the hypoxia-induced modulation of gene expression in human adipocytes. Arch. Physiol. Biochem. 2012;118:112–120. doi: 10.3109/13813455.2012.654611. [DOI] [PubMed] [Google Scholar]
- Meng M., Chen S., Lao T., Liang D., Sang N. Nitrogen anabolism underlies the importance of glutaminolysis in proliferating cells. Cell Cycle. 2010;9:3921–3932. doi: 10.4161/cc.9.19.13139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minchenko O., Opentanova I., Minchenko D., Ogura T., Esumi H. Hypoxia induces transcription of 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase-4 gene via hypoxia-inducible factor-1alpha activation. FEBS Lett. 2004;576:14–20. doi: 10.1016/j.febslet.2004.08.053. [DOI] [PubMed] [Google Scholar]
- Moon J.S., Kim H.E., Koh E., Park S.H., Jin W.J., Park B.W., Park S.W., Kim K.S. Kruppel-like factor 4 (KLF4) activates the transcription of the gene for the platelet isoform of phosphofructokinase (PFKP) in breast cancer. J. Biol. Chem. 2011;286:23808–23816. doi: 10.1074/jbc.M111.236737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Natter K., Kohlwein S.D. Yeast and cancer cells - common principles in lipid metabolism. Biochim. Biophys. Acta. 2012 doi: 10.1016/j.bbalip.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olbryt M., Jarzab M., Jazowiecka-Rakus J., Simek K., Szala S., Sochanik A. Gene expression profile of B 16(F10) murine melanoma cells exposed to hypoxic conditions in vitro. Gene Exp. 2006;13:191–203. doi: 10.3727/000000006783991818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papandreou I., Cairns R.A., Fontana L., Lim A.L., Denko N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- Pasarica M., Sereda O.R., Redman L.M., Albarado D.C., Hymel D.T., Roan L.E., Rood J.C., Burk D.H., Smith S.R. Reduced adipose tissue oxygenation in human obesity: evidence for rarefaction, macrophage chemotaxis, and inflammation without an angiogenic response. Diabetes. 2009;58:718–725. doi: 10.2337/db08-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez d.H., Wood I.S., Trayhurn P. Hypoxia stimulates lactate release and modulates monocarboxylate transporter (MCT1, MCT2, and MCT4) expression in human adipocytes. Pflugers Arch. 2010;459:509–518. doi: 10.1007/s00424-009-0750-3. [DOI] [PubMed] [Google Scholar]
- Pescador N., Villar D., Cifuentes D., Garcia-Rocha M., Ortiz-Barahona A., Vazquez S., Ordonez A., Cuevas Y., Saez-Morales D., Garcia-Bermejo M.L., Landazuri M.O., Guinovart J., del Peso L. Hypoxia promotes glycogen accumulation through hypoxia inducible factor (HIF)-mediated induction of glycogen synthase 1. PLoS One. 2010;5:e9644. doi: 10.1371/journal.pone.0009644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prabhakar N.R., Shenoy B.C., Simonson M.S., Cherniack N.S. Cell selective induction and transcriptional activation of immediate early genes by hypoxia. Brain Res. 1995;697:266–270. doi: 10.1016/0006-8993(95)00994-2. [DOI] [PubMed] [Google Scholar]
- Rausch M.E., Weisberg S., Vardhana P., Tortoriello D.V. Obesity in C57BL/6J mice is characterized by adipose tissue hypoxia and cytotoxic T-cell infiltration. Int. J. Obes. (Lond) 2008;32:451–463. doi: 10.1038/sj.ijo.0803744. [DOI] [PubMed] [Google Scholar]
- Regazzetti C., Peraldi P., Gremeaux T., Najem-Lendom R., Ben Sahra I., Cormont M., Bost F., Marchand-Brustel Y., Tanti J.F., Giorgetti-Peraldi S. Hypoxia decreases insulin signaling pathways in adipocytes. Diabetes. 2009;58:95–103. doi: 10.2337/db08-0457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich P.R. The molecular machinery of Keilin’s respiratory chain. Biochem. Soc. Trans. 2003;31:1095–1105. doi: 10.1042/bst0311095. [DOI] [PubMed] [Google Scholar]
- Rocha S. Gene regulation under low oxygen: holding your breath for transcription. Trends Biochem. Sci. 2007;32:389–397. doi: 10.1016/j.tibs.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Ros S., Santos C.R., Moco S., Baenke F., Kelly G., Howell M., Zamboni N., Schulze A. Functional metabolic screen identifies 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 4 as an important regulator of prostate cancer cell survival. Cancer Discov. 2012;2:328–343. doi: 10.1158/2159-8290.CD-11-0234. [DOI] [PubMed] [Google Scholar]
- Rubi B., del Arco A., Bartley C., Satrustegui J., Maechler P. The malate-aspartate NADH shuttle member Aralar1 determines glucose metabolic fate, mitochondrial activity, and insulin secretion in beta cells. J. Biol. Chem. 2004;279:55659–55666. doi: 10.1074/jbc.M409303200. [DOI] [PubMed] [Google Scholar]
- Sakakibara R., Uemura M., Hirata T., Okamura N., Kato M. Human placental fructose-6-phosphate,2-kinase/fructose-2,6-bisphosphatase: its isozymic form, expression and characterization. Biosci. Biotechnol. Biochem. 1997;61:1949–1952. doi: 10.1271/bbb.61.1949. [DOI] [PubMed] [Google Scholar]
- Scuteri A., Sanna S., Chen W.M., Uda M., Albai G., Strait J., Najjar S., Nagaraja R., Orru M., Usala G., Dei M., Lai S., Maschio A., Busonero F., Mulas A., Ehret G.B., Fink A.A., Weder A.B., Cooper R.S., Galan P., Chakravarti A., Schlessinger D., Cao A., Lakatta E., Abecasis G.R. Genome-wide association scan shows genetic variants in the FTO gene are associated with obesity-related traits. PLoS Genet. 2007;3:e115. doi: 10.1371/journal.pgen.0030115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seagroves T.N., Ryan H.E., Lu H., Wouters B.G., Knapp M., Thibault P., Laderoute K., Johnson R.S. Transcription factor HIF-1 is a necessary mediator of the pasteur effect in mammalian cells. Mol. Cell Biol. 2001;21:3436–3444. doi: 10.1128/MCB.21.10.3436-3444.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen G.M., Zhang F.L., Liu X.L., Zhang J.W. Hypoxia-inducible factor 1-mediated regulation of PPP1R3C promotes glycogen accumulation in human MCF-7 cells under hypoxia. FEBS Lett. 2010;584:4366–4372. doi: 10.1016/j.febslet.2010.09.040. [DOI] [PubMed] [Google Scholar]
- Stegmaier P., Voss N., Meier T., Kel A., Wingender E., Borlak J. Advanced computational biology methods identify molecular switches for malignancy in an EGF mouse model of liver cancer. PLoS One. 2011;6:e17738. doi: 10.1371/journal.pone.0017738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stelniec-Klotz I., Legewie S., Tchernitsa O., Witzel F., Klinger B., Sers C., Herzel H., Bluthgen N., Schafer R. Reverse engineering a hierarchical regulatory network downstream of oncogenic KRAS. Mol. Syst. Biol. 2012;8:601. doi: 10.1038/msb.2012.32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subramanian A., Tamayo P., Mootha V.K., Mukherjee S., Ebert B.L., Gillette M.A., Paulovich A., Pomeroy S.L., Golub T.R., Lander E.S., Mesirov J.P. Gene set enrichment analysis: a knowledge-based approach for interpreting genome-wide expression profiles. Proc. Natl. Acad. Sci. USA. 2005;102:15545–15550. doi: 10.1073/pnas.0506580102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szturmowicz M., Burakowski J., Tomkowski W., Sakowicz A., Filipecki S. Neuron-specific enolase in non-neoplastic lung diseases, a marker of hypoxemia? Int. J. Biol. Markers. 1998;13:150–153. doi: 10.1177/172460089801300305. [DOI] [PubMed] [Google Scholar]
- Trayhurn P. Hypoxia and adipose tissue function and dysfunction in obesity. Physiol Rev. 2013;93:1–21. doi: 10.1152/physrev.00017.2012. [DOI] [PubMed] [Google Scholar]
- Virtanen K.A., Lonnroth P., Parkkola R., Peltoniemi P., Asola M., Viljanen T., Tolvanen T., Knuuti J., Ronnemaa T., Huupponen R., Nuutila P. Glucose uptake and perfusion in subcutaneous and visceral adipose tissue during insulin stimulation in nonobese and obese humans. J. Clin. Endocrinol. Metab. 2002;87:3902–3910. doi: 10.1210/jcem.87.8.8761. [DOI] [PubMed] [Google Scholar]
- Wabitsch M., Brenner R.E., Melzner I., Braun M., Moller P., Heinze E., Debatin K.M., Hauner H. Characterization of a human preadipocyte cell strain with high capacity for adipose differentiation. Int. J. Obes. Relat Metab Disord. 2001;25:8–15. doi: 10.1038/sj.ijo.0801520. [DOI] [PubMed] [Google Scholar]
- Wang B., Wood I.S., Trayhurn P. Dysregulation of the expression and secretion of inflammation-related adipokines by hypoxia in human adipocytes. Pflugers Arch. 2007;455:479–492. doi: 10.1007/s00424-007-0301-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood I.S., Wang B., Lorente-Cebrian S., Trayhurn P. Hypoxia increases expression of selective facilitative glucose transporters (GLUT) and 2-deoxy-D-glucose uptake in human adipocytes. Biochem. Biophys. Res. Commun. 2007;361:468–473. doi: 10.1016/j.bbrc.2007.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, Z., Irizarry, R., Martinez Murillo, F. L. F., and Spencer, F., 2004. A model based background adjustment for oligonucleotide expression arrays. Johns Hopkins University, Dept. of Biostatistics Working Papers.
- Yang R.Z., Blaileanu G., Hansen B.C., Shuldiner A.R., Gong D.W. CDNA cloning, genomic structure, chromosomal mapping, and functional expression of a novel human alanine aminotransferase. Genomics. 2002;79:445–450. doi: 10.1006/geno.2002.6722. [DOI] [PubMed] [Google Scholar]
- Ye J., Gao Z., Yin J., He Q. Hypoxia is a potential risk factor for chronic inflammation and adiponectin reduction in adipose tissue of ob/ob and dietary obese mice. Am. J. Physiol Endocrinol. Metab. 2007;293:E1118–E1128. doi: 10.1152/ajpendo.00435.2007. [DOI] [PubMed] [Google Scholar]
- Zobel D.P., Andreasen C.H., Burgdorf K.S., Andersson E.A., Sandbaek A., Lauritzen T., Borch-Johnsen K., Jorgensen T., Maeda S., Nakamura Y., Eiberg H., Pedersen O., Hansen T. Variation in the gene encoding Kruppel-like factor 7 influences body fat: studies of 14 818 Danes. Eur. J. Endocrinol. 2009;160:603–609. doi: 10.1530/EJE-08-0688. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.