Abstract
The large-conductance Ca2+- and voltage-activated K+ (MaxiK, BK, BKCa, Slo1, KCa1.1) channel role in cell signalling is becoming apparent as we learn how the channel interacts with a multiplicity of proteins not only at the plasma membrane but in intracellular organelles including the endoplasmic reticulum, nucleus and mitochondria. In this review, we focus on the interactions of MaxiK channels with seven transmembrane G-protein coupled receptors, and discuss information suggesting that the channel big C-terminus may act as nucleus of signalling molecules including kinases relevant for cell death and survival. Increasing evidence indicates that the channel is able to associate with a variety of receptors including β-adrenergic receptors, G-protein coupled estrogen receptors, acetylcholine receptors, thromboxane A2 receptors and angiotensin II receptors, which highlights the varied functions that the channel has (or may have) not only in regulating contraction/relaxation of muscle cells or neurotransmission in the brain but also in cell metabolism, proliferation, migration and gene expression. In line with this view, MaxiK channels have been implicated in obesity and in brain, prostate, and mammary cancers. A better understanding of the molecular mechanisms underlying or triggered by MaxiK channel abnormalities like overexpression in certain cancers may lead to new therapeutics to prevent devastating diseases.
Keywords: BK channel, BKCa channel, Slo1, KCa1.1, macromolecular complexes, protein-protein interactions, human pathology
MaxiK channel distinctive characteristics
MaxiK channels, also known as BK/BKCa/Slo1/KCa1.1 channels, are encoded by the KCNMA1 gene and characterized by a large conductance to potassium, their sensitivity to voltage and Ca2+ and ubiquitous expression. In addition to responding to changes in Ca2+ and voltage, MaxiK channels also sense gases, lipids, and associate with a multiplicity of plasma membrane and intracellular proteins being linkers of membrane potential, cell metabolism, and cell signalling [21, 41, 70].
MaxiK channel essential structure consists of four α-subunits, each formed by 7 transmembrane segments and a large C-terminus that constitutes about two thirds of the protein [46] (Fig. 1). This tetrameric structure can be complemented with regulatory subunits. In mammals, the auxiliary subunits, β1-β4 and the recently discovered, γ1-γ4, can greatly modify channel performance including its response to pharmacological agents, kinetics, and Ca2+/voltage sensitivities. For example, β4 makes channels resistant to iberiotoxin blockade [47], γ1 (also named LRRC26) makes channels resistant to mallotoxin activation [3], β2 produces MaxiK channels that inactivate with time [84], β3b produces channels with very fast inactivation producing currents that appear to activate fast and rectify [81, 89], β1 increases Ca2+ /voltage sensitivity when free Ca2+ facing the inside of the channel is beyond 1 μM [45], while γ1-γ4 produce channels with increased voltage sensitivity even without Ca2+ [91, 92]. Functional diversity of MaxiK channels is also conferred by alternative splicing of both α- and β-subunit mRNAs and by posttranslational modifications like phosphorylation and lipidation [1, 76, 99].
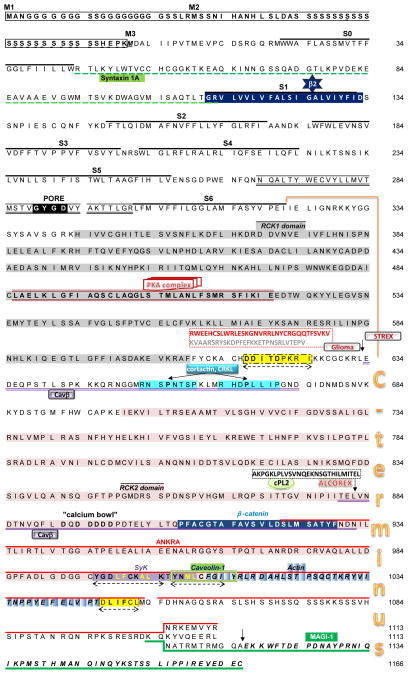
Figure 1. MaxiK α-subunit sequence: interacting regions and protein partners.
Amino acid sequence ending with QEERL corresponds to “insertless” hSlo; numbering starts at the third Methionine (M3) (NCBI # U11058). Highlighted are 3 possible start sites M1–M3 [83], the 7 transmembrane domains S0–S6, the pore region, the whole C-terminus, the regulator of K+ conductance (RCK) domains 1 (RCK1; gray) and 2 (RCK2; pink), the calcium bowl, export signals (dashed double arrows, yellow), and examples of regions reported to date to be involved in direct or indirect protein interactions. For a complete list of interacting proteins and references see Table 1. For clarity, interacting sequences in Table 2 that include large portions of the protein were omitted in this figure. Arrows mark the position of the glioma-specific 33 aa splice insert (red) upstream a 29 aa splice insert (gray), of the 59 aa splice insert encoded by the STREX exon, of the 27 aa splice insert known as ALCOREX [57], and of the 50 aa C-terminal exon named after the last three amino acids, DEC. Three C-terminal isoforms are shown; the sequences of the DEC containing isoform correspond to the mouse brain isoform, mbr5 [66]. For the crystal structure of the C-terminus, see [96].
In spite of its ubiquitous expression, the genetic ablation of MaxiK α-subunit in mice was not lethal indicating that animals developed compensatory mechanisms to substitute for MaxiK potential vital function in organs where it is normally expressed and/or that MaxiK is not essential to sustain life but rather it may serve to fine tune numerous body functions. In line with this view, more and more reports show MaxiK multiple physiological roles and involvement in a wide variety of diseases. In this respect, the lack of the α-subunit produces body deficiencies that, although not fatal, on the long run, decrease the quality of life. For example, mice lacking the α-subunit suffer from incontinence, erectile dysfunction, hypertension, altered circadian rhythm, and age-dependent hearing deficiencies [48, 49, 62, 63, 86].
MaxiK cellular compartmentalization
Immuno-mapping or electrophysiological methods indicate that in most cells MaxiK channels are expressed at the plasma membrane with adult cardiomyocytes being an interesting exception. In addition to its plasma membrane targeting, MaxiK channels can also localize to intracellular organelles like the endoplasmic reticulum, nucleus and mitochondria (Fig. 2) [67, 68, 70, 90]. The latter opens the tantalizing possibility that MaxiK channels might play a role in regulating mitochondria function and consequently cell death.
Figure 2. MaxiK α-subunit expression at the plasma membrane in smooth muscle cells and in cardiac mitochondria.
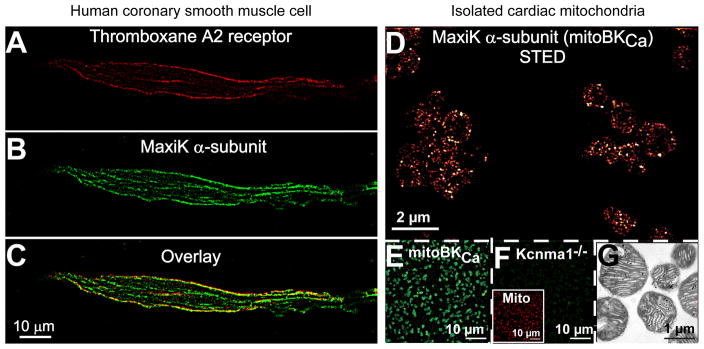
A–C. Freshly dissociated human coronary arterial myocyte double-labeled with anti-MaxiK monoclonal (A) and anti-thromboxane A2 receptor polyclonal (B) antibodies. The overlay (C) shows co-localization of both proteins at the plasma membrane. D. Super-resolution fluorescence images of Percoll-purified cardiac mitochondria [69] labeled for endogenous MaxiK α-subunit (mitoBKCa) with anti-MaxiK polyclonal antibody as described in [68]. Note distinct punctae of mitoBKCa clusters. Images were acquired with a custom-made stimulation emission depletion (STED) microscope and pseudocolored for presentation. E–F. Low resolution confocal fluorescence images of purified mitochondria showing the specificity of the anti-MaxiK antibody (green) with only background signals in mitochondria from the knockout animal (Kcnma1−/−). The inset in F shows the same field double labeled with mitotracker (red). G. Electron micrograph of the purified mitochondria preparation. Images were acquired at 0.0575 μm/pixel in (A–B, E–F) and at 0.0035 μm/pixel in (D), and were median filtered to reduce non-specific background as described earlier [36].
What are the intrinsic MaxiK signals targeting the channel to different cellular compartments or organelles? This is an active line of research. Currently it is known that there are several signals within the channel backbone that serve as different checkpoints to deliver the protein to the plasma membrane; three of them are located after the Ca2+-bowl in the regulator of conductance for K+ (RCK) 2 domain and another is located in the linker of RCK1 and RCK2 domains (Fig. 1, double arrows). The former contains dihydrophobic motifs (YGDLFCKALK; YNMLCFGI, which is also a caveolin-1 interaction site; and DLIFCL) known to act as endoplasmic reticulum export signals [2, 5, 31, 88]; while the latter contains an acidic cluster DDITDPKRI important for trafficking of other potassium channels [11, 44]
In addition to intrinsic signals in the constitutive MaxiK protein, splice variation of the α-subunit can add or delete signal sequences modifying the channel localization by facilitating its retention/targeting to intracellular organelles like the endoplasmic reticulum [11, 26, 43, 97, 98] and mitochondria [68]. A current challenge is to correlate the expression of specific splice variants to functional effects in native systems. In this respect, a 33 aa insert in transmembrane domain 1 containing CVLF trafficking signal that retains MaxiK in the endoplasmic reticulum of HEK293T cells [97, 98] is increased in rat aging corpora decreasing the channel surface expression [14]; this property may contribute to aging-related changes in male sexual activity. Another example is a 27 aa encoding exon, named ALCOREX (Fig. 1), which produces channels in HEK293 cells that develop a transient sensitivity to alcohol that is higher and more persistent than the one observed for the insertless channel. Importantly, MaxiK channel activation by alcohol decays in ~10 min which correlates with decreased ALCOREX expression in hypothalamo-neurohypophysial system explants exposed to alcohol [57]. This 27 aa insert also confers MaxiK channels its ability to be activated by arachidonic acid in growth hormone secreting neurons, GH3 cells [34].
Interestingly, intron containing cytoplasmic mRNAs can also contribute to differences in channel expression levels and electrical activity in hippocampal neurons. This regulatory mechanism seems to provide a way for specific and local expression of MaxiK variants containing the 59 aa splice insert, STREX (Fig. 1) [7, 8]. Another regulatory mechanism for splice variant expression is via microRNA-9 (miR9), which can be upregulated by exposure to alcohol, immunodeficiency virus and methamphetamines in the brain [57, 73].
Membrane receptors and MaxiK channels
Biologically active substances like hormones, peptides, and lipids bind to membrane receptors triggering the activation of signalling molecules, many of which are kinases and phosphatases, to regulate multiple cellular events like contraction/relaxation, cell proliferation, migration and gene expression.
MaxiK channels are known to be functionally coupled to a variety of plasma membrane receptors like β-adrenergic receptors, ACh receptors, thromboxane A2 receptors, angiotensin II receptors, and to the G-protein coupled estrogen receptor 1. The functional coupling between MaxiK channels and membrane receptors usually uses intermediary proteins as G-proteins and protein kinases that serve to transduce the signals received by the receptor to induce changes in channel activity which can be inhibitory or excitatory depending on the receptor being stimulated or the hormonal status of the tissue. Recently, we uncovered a new transduction mechanism that utilizes direct protein-protein interactions between the Thromboxane A2 receptor and the MaxiK channel that is independent of G-protein activation and results in channel inhibition (“trans-inhibition”) [35].
The coupling between membrane receptors and MaxiK channels has been mostly studied in smooth muscle where muscle relaxants like β-adrenergic agents and estrogen induce MaxiK channel activation and constrictors like Acetylcholine, Thromboxane A2, and Angiotensin II result in channel inhibition.
β2-adrenergic receptor
Early studies using bilayers and smooth muscle membranes showed that, after biochemical reconstitution, MaxiK channels can remain associated with β-adrenergic receptors in complex with G-proteins pointing to stable and multi-protein interactions [64, 80]. Indeed, association of β2-adrenergic receptors and MaxiK channels has been demonstrated in brain where they form a large macromolecular complex that includes protein kinase A (PKA), cytosolic A-kinase-anchoring protein (AKAP79/150), and the L-type Ca2+ channel [38].
Signalling mechanisms triggered by β2-adrenergic stimulation coupled to MaxiK activation have been studied in smooth muscle reconstituted in lipid bilayers and native cells, and involve a membrane-delimited action of the α-subunit of Gs (Gsα) on MaxiK channels as well as protein kinase A mediated phosphorylation of the channel protein [27, 28, 51, 64].
At the molecular level, the target site for PKA-mediated phosphorylation and activation of MaxiK channels is located in the RCK2 domain (866RQPS*869, numbers are as in GenBank U11058) [51]. All four MaxiK α-subunits need to be phosphorylated at this site for the channel to be activated [76]. Evidence for a direct action of PKA on the channel protein, is its ability to associate with MaxiK channels in the brain and the finding of the corresponding MaxiK phospho-peptide by proteomic analysis of immunopurified MaxiK also from the brain [38, 93]. Whether Gsα interacts directly or forms a complex with MaxiK channels during channel activation is yet to be determined.
Interestingly in non-pregnant myometrium, PKA can also cause inhibition of the MaxiK channel [56] but the triggering membrane receptor/mechanism of this response is unknown. The molecular correlate of the PKA-inhibited MaxiK channel is a channel isoform that contains the STREX insert, which introduces an additional site for PKA dependent phosphorylation [77]. Only a single STREX containing MaxiK α-subunit is required for PKA-dependent inhibition of channel activity but the constitutive S869 site must be dephosphorylated. Importantly, STREX is more abundant in non-pregnant myometrium and decreases with estrogen levels or pregnancy [102] explaining why, in non-pregnant myometrium, the majority of MaxiK channels are inhibited by PKA whereas during mid-pregnancy they are activated [56] probably contributing to the maintenance of uterine quiescence for a successful pregnancy.
G-protein coupled estrogen receptor 1
The G-protein coupled estrogen receptor 1 (GPER1) is a recently discovered seven transmembrane receptor that is activated by estrogen. GPER1 plays a role in protecting the myocardium from ischemic insult by the activation of salvage kinase pathways and prevention of mitochondrial dysfunction [10, 15, 52]. Using its agonist G1, it has been shown that in coronary smooth muscle, GPER1 stimulation results in an increased MaxiK channel activity. This increased channel activity correlates with relaxation of precontracted vessels by G1, which can be prevented by iberiotoxin, a MaxiK channel blocker [95]. Whether GPER1 and MaxiK channels interact closely with each other and/or the identification of signalling pathways used by GPER1 to modulate MaxiK channel activity are still open questions.
Muscarinic acetylcholine receptor M2
Initial evidence for acetylcholine receptor Gi-mediated inhibitory coupling to MaxiK channels was given in ileum and tracheal myocytes [13, 29]. However, a positive regulation has also been observed in canine tracheal myocytes [82] perhaps due to experimental conditions lacking GTP for proper coupling with G proteins. Although PKC dependent inhibition of MaxiK channels is well known, its role as an inhibitory pathway induced by muscarinic M2 receptors was only addressed recently. In HEK cells as well as in tracheal myocytes, M2-mediated inhibition of MaxiK channels involves two mechanisms; i) a membrane delimited Gβγ mediated inhibition, and ii) phospholipase C/PKC activation. Purified transducin Gβγ-mediated inhibition of MaxiK channel activity occurs independently of activation of PLCβ isozymes, intracellular Ca2+ concentration, or expression of MaxiK β1-subunit. Further, Gβγ can associate with MaxiK channels as it is possible to coimmunoprecipitate all proteins in HEK cells expressing the recombinant proteins [100]. PKC phosphorylation of MaxiK channel α-subunit occurs in the C-terminus at consensus PKC sites 642S*PKKK646 and 1097KS*R1099 with only one channel subunit requiring phosphorylation for channel inhibition [101]. Phosphorylation at 642*S but not 1098*S was detected by proteomic analysis due to missing sequence information in this region [93].
Thromboxane A2 and Angiotensin II receptors
Thromboxane A2 prostanoid receptor (TPR) and Angiotensin II type 1 receptor (AT1R) are G-protein coupled receptors (GPCR) that play important roles in the development of vascular diseases such as heart angina, hypertension and stroke due to the potent vasoconstrictor effects of their agonists, Thromboxane A2 (TXA2) and Angiotensin II (Ang II). In models of chronic vascular disease (e.g. hypertension, aortic regurgitation, atherosclerosis), TPR and AT1R gene ablation ameliorate disease symptoms (i.e. reduces blood pressure, cardiac hypertrophy, age-related progression of atherosclerosis) underscoring TPR and AT1R role in the pathogenesis of vascular disease and end-organ injury [17–19]. The functional interaction between these receptors and MaxiK channels was first made evident in experiments performed in lipid bilayers using coronary smooth muscle membrane vesicles, where thromboxane A2 as well as angiotensin II produced the inhibition of channel activity [65, 79]. The inhibition of MaxiK channel activity by angiotensin II presumably via AT1R was later observed in coronary myocytes [42]. Interestingly, in lipid bilayers the inhibition of channel activity occurred without the addition of GTP suggesting the involvement of a G-protein independent mechanism(s) [65, 79]. In line with this view and as mentioned earlier, we recently demonstrated that in fact TPR trans-inhibits MaxiK channels independent of G-protein activation and via a mechanism that likely involves direct protein-protein interaction(s) between the receptor and the α-subunit of the channel. The MaxiK-TPR association utilizes the voltage-sensing-conduction cassette of the channel α-subunit and the first intracellular loop and C-terminus of the receptor [35]. Figure 2A–C shows the colocalization of TPR and MaxiK channel α-subunit at the cell surface of human coronary myocytes.
The MaxiK channel-TPR association also includes the channel β1 regulatory subunit. Interestingly, the β1-subunit can independently associate with the receptor and the channel α-subunit leading to the interesting hypothesis that β1-subunit could alter TPR function. Supporting this view, we found that β1 gene ablation produces blood vessels with twice the sensitivity to thromboxane A2, i.e. aortic strips showed an EC50 to thromboxane A2 agonist, U46619, of 18 nM in the wild type animals and an EC50 of 9 nM in the β1 null mice [36]. Whether the β1-subunit also associates with other G-protein coupled receptors, modifying their vasoconstricting/vasorelaxing potencies remains open to research. Also, a detailed study of how angiotensin II modifies MaxiK channel activity and whether AT1R is in close contact with the channel are topics that need scrutiny.
MaxiK and cell signalling
The coupling of MaxiK channels with 7 transmembrane receptors described above necessarily link MaxiK channels with cell signalling events, like activation of PKA and PKC. Moreover, direct protein-protein interactions have been found between the channel and focal adhesion kinase (FAK), and cytosolic phospholipase A2 (cPLA2), as well as regions in MaxiK channel necessary for association with PKA complexes and spleen tyrosine kinase (SyK) (Fig. 1 and Table 1). This information in combination with recent proteomic data indicates that aside from its role in K+ conduction, MaxiK channel may provide surface contact for a variety of proteins. In this view, MaxiK channel could act as “coordinator” or “linker” of signalling events.
Table 1.
MaxiK α subunit domains and protein complexes
| Interacting proteins | MaxiKα interaction/association motif | Method | Co-IP or co-labeling | Ref |
|---|---|---|---|---|
| Transmembrane proteins | ||||
| Direct Interactions | ||||
| β2-subunit | S1 transmembrane domain (aa 112GRVL--YFID133, #U11058) | prokaryote two-hybrid system | in vitro | [50] |
| Thromboxane A2 receptor | voltage-sensing-conduction cassette including N-terminus (aa 1MDAL---YVPE321, #U11058) | FRET | coronary smooth muscle | [35] |
| Intracellular proteins | ||||
| Direct interactions | ||||
| FAK | Whole C-terminus (388IELI---EERL1178, #U13913) | yeast two-hybrid system | osteosarcoma cells (MG63) | [58] |
| Microtubule-associated protein1A | partial C-terminus (aa 746–1144*) | yeast two-hybrid system | brain, Purkinje cells | [55] |
| β-catenin | “S10” hydrophobic segment (941PFAC---TYF962, numbers as in [61]) | yeast two-hybrid system | chicken hair cells | [33] |
| Cereblon | Partial C-terminus (encompassing RCK1 and part of RCK2 domains upstream the Ca2+-bowl; aa 394–955 of rSlo*) | yeast two-hybrid system | brain, hippocampal neurons | [23] |
| ANKRA | C-terminal end (downstream the Ca2+ bowl, aa 1019SLM--MVYR1210, #AF135265.1) | yeast two-hybrid system | brain | [37] |
| Actin | FGIYRLRDAHLSTPSQCTKRYVITNPPYEFELVPT | purified proteins | chick ciliary ganglion | [103] |
| cPLA2 | AKPGKLPLVSVNQEKNSGTHILMITEL (in 27 aa splice insert or ALCOREX) | mammalian two-hybrid system | GH3 cells | [34] |
| Rab11b | region excluding N- and C-terminus | yeast two-hybrid system | chick cochlea | [72] |
| Cortactin, CRKL | 656P and 667P in RxxPxxxP proline rich motifs | overlay assay | brain | [74] |
| MAGI-1 | C-terminus DEC variant (1111–1171*); sequence in Fig. 2 is as in [43] | yeast two-hybrid system | podocyte cell line | [60] |
| Cavβ1 | C-terminus fragments including Ca2+ bowl (884T–N936) or non-canonical SH3 binding domain (E637–D677)* | yeast two-hybrid system and purified proteins | chick ciliary ganglion neurons | [104] |
| Direct/Indirect? | ||||
| Nephrin | VEDEC variant* | GST pull-down assays | podocyte cell line | [25] |
| Syntaxin 1A | S0–S1 loop/C-terminus (336YSAVSG----VEDEC1166; numbers as in Fig. 2) | co-IP | brain | [12] |
| Caveolin-1 | Caveolin binding motif, YNMLCFGIY | co-IP | aorta | [2] |
| Tubulin | RCK2 to C-terminal end (679MDS---QEERL1113, #U11058) | pull-down with purified protein | astrocytes | [54] |
| PKA complex (PKA indirect) | leucine zipper 1, LAELKLGFIAQSCLAQGLSTMLANLFSMRSFIKIE | co-IP | brain | [75] |
| SyK | ITAM, YGDLFCKALKTYNML | co-IP | osteosarcoma cells(MG63) | [59] |
aa, amino acid; ANKRA, Ankyrin-repeat family A protein; co-IP, co-immunoprecipitation; cPLA2, cytosolic phospholipase A2; CRKL, Crk-like protein; FAK, focal adhesion kinase; ITAM, immunoreceptor tyrosine-based activation motif; MAGI-1, membrane-associated guanylate kinase with inverted orientation protein-1; PKA, protein kinase A; SH3, Src homology 3; ?, in these cases a direct or indirect interaction has not been demonstrated; #, NCBI accession number; *, NCBI accession number not given (amino acid numbers are as given in publications and may not coincide with template used in Fig. 2)
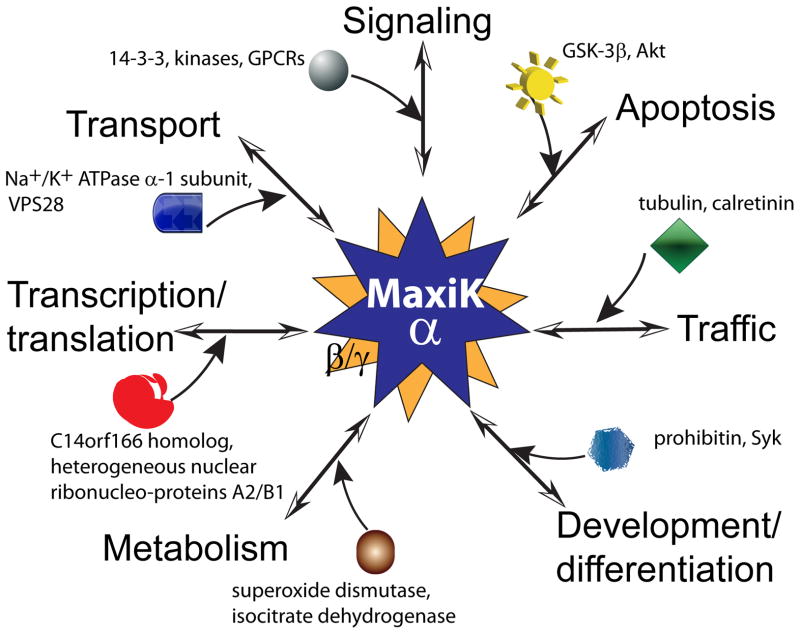
Back in 2006, our review listed near 20 proteins that had been recognized as MaxiK α-subunit partners including plasma membrane and cytosolic proteins [41]. Today this list has grown to the hundreds thanks to the establishment of the proteomics technology which has revealed that MaxiK channel is in complex with proteins not only at the plasma membrane or cytosol but also with proteins in organelles like the endoplasmic reticulum, nucleus and mitochondria [24]. Interestingly, this approach has revealed the association of MaxiK channels with kinases relevant for cell death and survival, like Akt, glycogen synthase kinase-3β (GSK-3β) and phosphoinositide-dependent kinase-1 (PDK1) opening the intriguing possibility that MaxiK channels are also operators of cell life and dead. In addition, proteins that associate with MaxiK channels also include proteins linked to other cellular processes like metabolism, development, traffic, transport and apoptosis (Figure 3) [71].
Figure 3. MaxiK channel potential functions uncovered by associated protein partners.
Proteomic analyses have revealed that MaxiK channels not only associate with β and γ regulatory subunits but also with a large variety of proteins involved in diverse cellular functions [24, 71]. Note that proteomics cannot differentiate between direct (binding to the channel) or indirect interactors. We hypothesize that MaxiK α subunit association with some proteins may be relatively steady like with regulatory α and γ subunits, while association with other partners (symbols) could be dynamic (incoming arrows); in both cases, interactions may be tissue-specific and distinct depending on gender, age and health-status among others. Examples of MaxiK associated proteins known to be involved in: 1) signaling are 14-3-3 proteins, visinin-like protein 1 [71], G-protein coupled receptors [35]and kinases like PKA complex [75], and Akt (protein kinase B); 2) traffic are tubulin [54], calretinin [71], the small GTPase Rab11b [72], actin [103]; 3) development/differentiation are prohibitin, Thy-1 membrane glycoprotein precursor [71], Syk [59]; in transcription/translation are protein C14orf166 homolog and heterogeneous nuclear ribonucleo-proteins A2/B1 [72]; 4) metabolism are mitochondrial precursors of fumarate hydratase and superoxide dismutase [Mn], isocitrate dehydrogenase [71]; 5) apoptosis are glycogen synthase kinase-3β (GSK-3β) and Akt [71]; and 6) transport are Na+/K+ ATPase alpha-1 subunit, vacuolar protein sorting associated protein 28 homolog (VPS28) [71].
Many protein partners of MaxiK α-subunit have been detected/confirmed by coimmunoprecipitation, including Akt, GSK-3β, PDK1, Src, β2-adrenergic receptors, transient receptor potential canonical 1 (TRPC1), and its modulatory β1- and γ1-subunits among others [30, 41, 71, 91]. In these cases, a direct protein-protein interaction has not been established and, thus indirect interactions cannot be strictly ruled out. Table 1 summarizes those proteins where direct protein-protein interactions have been reported or where MaxiK regions critical for protein associations have been identified. Although many of these interactions may be conserved in different cell types, a big challenge is to determine where, how and when MaxiK channels interact with specific proteins for cell-specific functions and how failures in these interactions may lead to disease.
New views in MaxiK channel physiology and human disease
The majority of studies have been directed to elucidate the physiological role of MaxiK channels expressed at the plasma membrane. However, MaxiK channels are also expressed in intracellular organelles like the mitochondria (Fig. 2D–G), where their physiological role is beginning to be understood [24, 70]. These advances together with the linkage of MaxiK channel defects to human disease are highlighted below.
Role in mitochondria
In cardiac mitochondria, pharmacological evidence indicates that MaxiK channel opening improves mitochondrial respiratory function and protects the heart from ischemic insult [4, 90]; in mitochondria from an astrocytoma cell line, their electrical activity is coupled to the respiratory chain [6], and in pulmonary artery smooth muscle, 11,12-epoxyecidosatrienoic acid induced vasoconstriction and mitochondrial depolarization has been linked to mitochondrial MaxiK (mitoBKCa) and its association with its β1-subunit [40].
The molecular correlate of mitoBKCa was unknown until recently. As expected from its electrophysiological properties, we found that mitoBKCa is encoded by the same gene (Kcnma1) encoding its plasma membrane counterpart and is formed by α-subunits of about 140 kDa. A C-terminal spliced exon (named after the 3 last aa DEC) (Fig. 1) is required for the channel mitochondrial targeting. Using the MaxiK α-subunit knockout animal, we also confirmed that opening of these channels with NS1619 protects the heart from ischemic insult. Mechanisms underlying this protection are enhanced performance of mitochondria and cardiac neurons [68, 87]. These findings together with the fact that MaxiK channels associate with the salvage kinase Akt and with GSK3β [71] an integrator of signals whose phosphorylation prevents mitochondrial permeability transition are strong arguments in favor of a role of MaxiK channels in cardioprotection and support a possible role in cell survival.
Role in human disease
During the last decade, genetic studies in humans have revealed mutations and genomic amplification of MaxiK channel gene leading to a variety of diseases involving the brain, metabolism, and cell proliferation/migration.
Brain disease. Paroxysmal movement disorder and generalized epilepsy was the first disorder correlated to a mutation in MaxiK channels. The mutation within the RCK1 domain neutralizes a negatively charged residue (D369-G). Out of 13 individuals affected with the disease, all carried the neutralizing mutation, which produces channels with higher sensitivity to Ca2+ in heterologous expression. This feature would accelerate the repolarization of action potentials and explain an increase in neuronal firing rate in the disease [16]. MaxiK channel dysfunction has also been associated with autism and mental retardation. These maladies have been related, in one case, to a chromosomal translocation event that resulted in silencing of one copy of the gene and thus, reduced MaxiK expression; while in another case a conserved mutation (A73-V) was found in the first intracellular loop [32].
Metabolic disease. Genome-wide association analyses identified the MaxiK channel gene as linked to human obesity in 5 from 6 case-control cohorts (total of 4214 obese vs. 5417 lean individuals). Moreover, MaxiK transcript expression was increased in adipose tissue and isolated fat cells from obese individuals [22]. These findings open a new line of research to understand the metabolic pathways linked to MaxiK expression and function.
Cardiovascular disease. Severe hypertension has been linked to a polymorphism in an intronic sequence of MaxiK channel gene, and a haplotype with an additional polymorphism (in the fourth exon, C864T) has been linked to increased risk of myocardial infarction in addition to systolic and general hypertension. However, the C864T haplotype corresponds to a synonymous single nucleotide polymorphism Phe229Phe, and the polymorphism in the intronic sequence did not generate a MaxiK channel isoform. Although there seems to be no functional effects, these mutations may serve as genetic markers of increased risk for cardiovascular disease [78]. In line with the view that the MaxiK channel itself is not modified in human hypertension, a recent report from Chinese patients with hypertension show that this was the case; instead in this population, MaxiK channel regulatory β1-subunit was the one that was reduced causing the expected decrease in the channel Ca2+ sensitivity and voltage-dependence of activation [94].
Cancer. MaxiK channel overexpression has been correlated with the malignancy of human gliomas; accordingly, its inhibition reduced glioma cell growth. A key factor in the development of glioma seems to be a distinctive 34 aa splice variant insert (Fig. 1, arrow, sequence in red), which to our knowledge has not been detected in healthy cells. This 34 aa insert is in tandem with a previously reported 29 aa spliced exon (Fig. 2, arrow, sequence in gray) producing channels with higher Ca2+ sensitivity [39, 85]. The glioma MaxiK isoform (gBK) is expressed at the cell membrane, mitochondria, Golgi, and endoplasmic reticulum and is also found in other types of tumor cell lines derived from duodenal, colon, hepatocellular and pancreatic cancers. Importantly, this molecular information has provided the tools to generate gBK peptide-specific cytotoxic T lymphocytes and kill cells expressing gBK [20].
Amplification of MaxiK channel gene is another mechanism that has been correlated with cancers; specifically with prostate and breast cancers. Obviously, it is important to elucidate the mechanisms triggered by this genomic amplification as breast cancers associated with MaxiK gene amplification are those of high tumor grade, high cell proliferation and poor prognosis [9, 53]. Moreover, it would be relevant to determine the molecular composition of MaxiK in breast cancer cells and to determine whether it contains the gBK isoform.
Concluding Remarks
From the stand point of cell signalling, it is intriguing the multitude and variety of molecules that can form complexes with MaxiK channels underscoring the necessity to understand the dynamics of these connections and their physiological consequences. One highlight in this regard, is the interaction of MaxiK channels and G-protein coupled receptors with opposite functions in smooth muscle, relaxation and constriction. When do these interactions take place and are they affected in disease or during aging are few of the questions that would be important to address in the future. At the molecular level, the soluble C-terminus of the channel α-subunit represents two thirds of the protein, and thus, may provide a significant surface contact for direct protein-protein interactions. Future studies need to identify which of the hundreds of proteins found by proteomics to associate with MaxiK channel α-subunit, may serve as scaffolds directly binding to the channel and thus, facilitating the association with other proteins and/or relay of signals to other signalling clusters.
Finally, the increasing evidence about the role of MaxiK channels in human disease including obesity and cancer offer opportunities to understand the mechanisms of human disease and design new strategies for their potential cure.
Acknowledgments
This work was supported by NIH grants, HL107418 and HL096740 (LT, ES), and HL088640 (ES), and by the American Heart Association National Scientist Development Award 11SDG7230059 (HS). Kcnma1−/− was kindly provided by Dr. Andrea Meredith (University of Maryland School of Medicine).
References
- 1.Alioua A, Li M, Wu Y, Stefani E, Toro L. Unconventional myristoylation of large-conductance Ca(2)-activated K channel (Slo1) via serine/threonine residues regulates channel surface expression. Proc Natl Acad Sci U S A. 2011;108:10744–10749. doi: 10.1073/pnas.1008863108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alioua A, Lu R, Kumar Y, Eghbali M, Kundu P, Toro L, Stefani E. Slo1 caveolin-binding motif, a mechanism of caveolin-1-Slo1 interaction regulating Slo1 surface expression. J Biol Chem. 2008;283:4808–4817. doi: 10.1074/jbc.M709802200. [DOI] [PubMed] [Google Scholar]
- 3.Almassy J, Begenisich T. The LRRC26 protein selectively alters the efficacy of BK channel activators. Mol Pharmacol. 2012;81:21–30. doi: 10.1124/mol.111.075234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aon MA, Cortassa S, Wei AC, Grunnet M, O’Rourke B. Energetic performance is improved by specific activation of K+ fluxes through K(Ca) channels in heart mitochondria. Biochim Biophys Acta. 2010;1797:71–80. doi: 10.1016/j.bbabio.2009.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Barlowe C. Signals for COPII-dependent export from the ER: what’s the ticket out? Trends Cell Biol. 2003;13:295–300. doi: 10.1016/s0962-8924(03)00082-5. [DOI] [PubMed] [Google Scholar]
- 6.Bednarczyk P, Wieckowski MR, Broszkiewicz M, Skowronek K, Siemen D, Szewczyk A. Putative Structural and Functional Coupling of the Mitochondrial BK Channel to the Respiratory Chain. PLoS One. 2013;8:e68125. doi: 10.1371/journal.pone.0068125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bell TJ, Miyashiro KY, Sul JY, Buckley PT, Lee MT, McCullough R, Jochems J, Kim J, Cantor CR, Parsons TD, Eberwine JH. Intron retention facilitates splice variant diversity in calcium-activated big potassium channel populations. Proc Natl Acad Sci U S A. 2010;107:21152–21157. doi: 10.1073/pnas.1015264107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bell TJ, Miyashiro KY, Sul JY, McCullough R, Buckley PT, Jochems J, Meaney DF, Haydon P, Cantor C, Parsons TD, Eberwine J. Cytoplasmic BK(Ca) channel intron-containing mRNAs contribute to the intrinsic excitability of hippocampal neurons. Proc Natl Acad Sci U S A. 2008;105:1901–1906. doi: 10.1073/pnas.0711796105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bloch M, Ousingsawat J, Simon R, Schraml P, Gasser TC, Mihatsch MJ, Kunzelmann K, Bubendorf L. KCNMA1 gene amplification promotes tumor cell proliferation in human prostate cancer. Oncogene. 2007;26:2525–2534. doi: 10.1038/sj.onc.1210036. [DOI] [PubMed] [Google Scholar]
- 10.Bopassa JC, Eghbali M, Toro L, Stefani E. A novel estrogen receptor GPER inhibits mitochondria permeability transition pore opening and protects the heart against ischemia-reperfusion injury. Am J Physiol Heart Circ Physiol. 2010;298:H16–H23. doi: 10.1152/ajpheart.00588.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen L, Jeffries O, Rowe IC, Liang Z, Knaus HG, Ruth P, Shipston MJ. Membrane trafficking of large conductance calcium-activated potassium channels is regulated by alternative splicing of a transplantable, acidic trafficking motif in the RCK1–RCK2 linker. J Biol Chem. 2010;285:23265–23275. doi: 10.1074/jbc.M110.139758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cibulsky SM, Fei H, Levitan IB. Syntaxin-1A binds to and modulates the Slo calcium-activated potassium channel via an interaction that excludes syntaxin binding to calcium channels. J Neurophysiol. 2005;93:1393–1405. doi: 10.1152/jn.00789.2004. [DOI] [PubMed] [Google Scholar]
- 13.Cole WC, Sanders KM. G proteins mediate suppression of Ca2+-activated K current by acetylcholine in smooth muscle cells. Am J Physiol. 1989;257:C596–C600. doi: 10.1152/ajpcell.1989.257.3.C596. [DOI] [PubMed] [Google Scholar]
- 14.Davies KP, Stanevsky Y, Tar MT, Chang JS, Chance MR, Melman A. Ageing causes cytoplasmic retention of MaxiK channels in rat corporal smooth muscle cells. Int J Impot Res. 2007;19:371–377. doi: 10.1038/sj.ijir.3901541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deschamps AM, Murphy E. Activation of a novel estrogen receptor, GPER, is cardioprotective in male and female rats. Am J Physiol Heart Circ Physiol. 2009;297:H1806–H1813. doi: 10.1152/ajpheart.00283.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du W, Bautista JF, Yang H, Diez-Sampedro A, You SA, Wang L, Kotagal P, Luders HO, Shi J, Cui J, Richerson GB, Wang QK. Calcium-sensitive potassium channelopathy in human epilepsy and paroxysmal movement disorder. Nat Genet. 2005;37:733–738. doi: 10.1038/ng1585. [DOI] [PubMed] [Google Scholar]
- 17.Eto H, Miyata M, Shirasawa T, Akasaki Y, Hamada N, Nagaki A, Orihara K, Biro S, Tei C. The long-term effect of angiotensin II type 1a receptor deficiency on hypercholesterolemia-induced atherosclerosis. Hypertens Res. 2008;31:1631–1642. doi: 10.1291/hypres.31.1631. [DOI] [PubMed] [Google Scholar]
- 18.Francois H, Athirakul K, Mao L, Rockman H, Coffman TM. Role for thromboxane receptors in angiotensin-II-induced hypertension. Hypertension. 2004;43:364–369. doi: 10.1161/01.HYP.0000112225.27560.24. [DOI] [PubMed] [Google Scholar]
- 19.Francois H, Makhanova N, Ruiz P, Ellison J, Mao L, Rockman HA, Coffman TM. A role for the thromboxane receptor in L-NAME hypertension. Am J Physiol Renal Physiol. 2008;295:F1096–F1102. doi: 10.1152/ajprenal.00369.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ge L, Hoa NT, Cornforth AN, Bota DA, Mai A, Kim DI, Chiou SK, Hickey MJ, Kruse CA, Jadus MR. Glioma big potassium channel expression in human cancers and possible T cell epitopes for their immunotherapy. J Immunol. 2012;189:2625–2634. doi: 10.4049/jimmunol.1102965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hou S, Heinemann SH, Hoshi T. Modulation of BKCa channel gating by endogenous signaling molecules. Physiology (Bethesda) 2009;24:26–35. doi: 10.1152/physiol.00032.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jiao H, Arner P, Hoffstedt J, Brodin D, Dubern B, Czernichow S, van’t HF, Axelsson T, Pedersen O, Hansen T, Sorensen TI, Hebebrand J, Kere J, hlman-Wright K, Hamsten A, Clement K, Dahlman I. Genome wide association study identifies KCNMA1 contributing to human obesity. BMC Med Genomics. 2011;4:51. doi: 10.1186/1755-8794-4-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jo S, Lee KH, Song S, Jung YK, Park CS. Identification and functional characterization of cereblon as a binding protein for large-conductance calcium-activated potassium channel in rat brain. J Neurochem. 2005;94:1212–1224. doi: 10.1111/j.1471-4159.2005.03344.x. [DOI] [PubMed] [Google Scholar]
- 24.Kathiresan T, Harvey M, Orchard S, Sakai Y, Sokolowski B. A protein interaction network for the large conductance Ca(2+)-activated K(+) channel in the mouse cochlea. Mol Cell Proteomics. 2009;8:1972–1987. doi: 10.1074/mcp.M800495-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kim EY, Choi KJ, Dryer SE. Nephrin binds to the COOH terminus of a large-conductance Ca2+-activated K+ channel isoform and regulates its expression on the cell surface. Am J Physiol Renal Physiol. 2008;295:F235–F246. doi: 10.1152/ajprenal.00140.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kim EY, Ridgway LD, Zou S, Chiu YH, Dryer SE. Alternatively spliced C-terminal domains regulate the surface expression of large conductance calcium-activated potassium channels. Neuroscience. 2007;146:1652–1661. doi: 10.1016/j.neuroscience.2007.03.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kume H, Graziano MP, Kotlikoff MI. Stimulatory and inhibitory regulation of calcium-activated potassium channels by guanine nucleotide-binding proteins. Proc Natl Acad Sci USA. 1992;89:11051–11055. doi: 10.1073/pnas.89.22.11051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kume H, Hall IP, Washabau RJ, Takagi K, Kotlikoff MI. β-Adrenergic agonists regulate KCa channels in airway smooth muscle by cAMP-dependent and -independent mechanisms. J Clin Invest. 1994;93:371–379. doi: 10.1172/JCI116969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kume H, Kotlikoff MI. Muscarinic inhibition of single KCa channels in smooth muscle cells by a pertussis-sensitive G protein. Am J Physiol. 1991;261:C1204–C1209. doi: 10.1152/ajpcell.1991.261.6.C1204. [DOI] [PubMed] [Google Scholar]
- 30.Kwan HY, Shen B, Ma X, Kwok YC, Huang Y, Man YB, Yu S, Yao X. TRPC1 associates with BK(Ca) channel to form a signal complex in vascular smooth muscle cells. Circ Res. 2009;104:670–678. doi: 10.1161/CIRCRESAHA.108.188748. [DOI] [PubMed] [Google Scholar]
- 31.Kwon SH, Guggino WB. Multiple sequences in the C terminus of MaxiK channels are involved in expression, movement to the cell surface, and apical localization. Proc Natl Acad Sci U S A. 2004;101:15237–15242. doi: 10.1073/pnas.0404877101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laumonnier F, Roger S, Guerin P, Molinari F, M’rad R, Cahard D, Belhadj A, Halayem M, Persico AM, Elia M, Romano V, Holbert S, Andres C, Chaabouni H, Colleaux L, Constant J, Le Guennec JY, Briault S. Association of a functional deficit of the BKCa channel, a synaptic regulator of neuronal excitability, with autism and mental retardation. Am J Psychiatry. 2006;163:1622–1629. doi: 10.1176/ajp.2006.163.9.1622. [DOI] [PubMed] [Google Scholar]
- 33.Lesage F, Hibino H, Hudspeth AJ. Association of beta-catenin with the alpha-subunit of neuronal large-conductance Ca2+-activated K+ channels. Proc Natl Acad Sci U S A. 2004;101:671–675. doi: 10.1073/pnas.0307681100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li J, Al-Khalili O, Ramosevac S, Eaton DC, Denson DD. Protein-protein interaction between cPLA2 and splice variants of alpha-subunit of BK channels. Am J Physiol Cell Physiol. 2010;298:C251–C262. doi: 10.1152/ajpcell.00221.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Li M, Tanaka Y, Alioua A, Wu Y, Lu R, Kundu P, Sanchez-Pastor E, Marijic J, Stefani E, Toro L. Thromboxane A2 receptor and MaxiK-channel intimate interaction supports channel trans-inhibition independent of G-protein activation. Proc Natl Acad Sci U S A. 2010;107:19096–19101. doi: 10.1073/pnas.1002685107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li M, Zhang Z, Koh H, Lu R, Jiang Z, Alioua A, Garcia-Valdes J, Stefani E, Toro L. The beta1-Subunit of the MaxiK Channel Associates with the Thromboxane A2 Receptor and Reduces Thromboxane A2 Functional Effects. J Biol Chem. 2013;288:3668–3677. doi: 10.1074/jbc.M112.426585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lim HH, Park CS. Identification and functional characterization of ankyrin-repeat family protein ANKRA as a protein interacting with BKCa channel. Mol Biol Cell. 2005;16:1013–1025. doi: 10.1091/mbc.E04-06-0537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu G, Shi J, Yang L, Cao L, Park SM, Cui J, Marx SO. Assembly of a Ca(2+)-dependent BK channel signaling complex by binding to beta2 adrenergic receptor. EMBO J. 2004;23:2196–2205. doi: 10.1038/sj.emboj.7600228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu X, Chang Y, Reinhart PH, Sontheimer H, Chang Y. Cloning and characterization of glioma BK, a novel BK channel isoform highly expressed in human glioma cells. J Neurosci. 2002;22:1840–1849. doi: 10.1523/JNEUROSCI.22-05-01840.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Loot AE, Moneke I, Keseru B, Oelze M, Syzonenko T, Daiber A, Fleming I. 11,12-EET stimulates the association of BK channel alpha and beta(1) subunits in mitochondria to induce pulmonary vasoconstriction. PLoS One. 2012;7:e46065. doi: 10.1371/journal.pone.0046065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lu R, Alioua A, Kumar Y, Eghbali M, Stefani E, Toro L. MaxiK channel partners: physiological impact. J Physiol. 2006;570:65–72. doi: 10.1113/jphysiol.2005.098913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lu T, Zhang DM, Wang XL, He T, Wang RX, Chai Q, Katusic ZS, Lee HC. Regulation of coronary arterial BK channels by caveolae-mediated angiotensin II signaling in diabetes mellitus. Circ Res. 2010;106:1164–1173. doi: 10.1161/CIRCRESAHA.109.209767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma D, Nakata T, Zhang G, Hoshi T, Li M, Shikano S. Differential trafficking of carboxyl isoforms of Ca2+-gated (Slo1) potassium channels. FEBS Lett. 2007;581:1000–1008. doi: 10.1016/j.febslet.2007.01.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ma D, Zerangue N, Lin YF, Collins A, Yu M, Jan YN, Jan LY. Role of ER export signals in controlling surface potassium channel numbers. Science. 2001;291:316–319. doi: 10.1126/science.291.5502.316. [DOI] [PubMed] [Google Scholar]
- 45.Meera P, Wallner M, Jiang Z, Toro L. A calcium switch for the functional coupling between α (hslo) and β subunits (KV,Caβ) of maxi K channels. FEBS Lett. 1996;382:84–88. doi: 10.1016/0014-5793(96)00151-2. [DOI] [PubMed] [Google Scholar]
- 46.Meera P, Wallner M, Song M, Toro L. Large conductance voltage- and calcium-dependent K+ channel, a distinct member of voltage-dependent ion channels with seven N-terminal transmembrane segments (S0–S6), an extracellular N terminus, and an intracellular (S9–S10) C terminus. Proc Natl Acad Sci U S A. 1997;94:14066–14071. doi: 10.1073/pnas.94.25.14066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meera P, Wallner M, Toro L. A neuronal beta subunit (KCNMB4) makes the large conductance, voltage- and Ca2+-activated K+ channel resistant to charybdotoxin and iberiotoxin. Proc Natl Acad Sci U S A. 2000;97:5562–5567. doi: 10.1073/pnas.100118597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Meredith AL, Thorneloe KS, Werner ME, Nelson MT, Aldrich RW. Overactive bladder and incontinence in the absence of the BK large conductance Ca2+-activated K+ channel. J Biol Chem. 2004;279:36746–36752. doi: 10.1074/jbc.M405621200. [DOI] [PubMed] [Google Scholar]
- 49.Meredith AL, Wiler SW, Miller BH, Takahashi JS, Fodor AA, Ruby NF, Aldrich RW. BK calcium-activated potassium channels regulate circadian behavioral rhythms and pacemaker output. Nat Neurosci. 2006;9:1041–1049. doi: 10.1038/nn1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morera FJ, Alioua A, Kundu P, Salazar M, Gonzalez C, Martinez AD, Stefani E, Toro L, Latorre R. The first transmembrane domain (TM1) of beta2-subunit binds to the transmembrane domain S1 of alpha-subunit in BK potassium channels. FEBS Lett. 2012;586:2287–2293. doi: 10.1016/j.febslet.2012.05.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nara M, Dhulipala PD, Wang YX, Kotlikoff MI. Reconstitution of beta-adrenergic modulation of large conductance, calcium-activated potassium (maxi-K) channels in Xenopus oocytes. Identification of the camp-dependent protein kinase phosphorylation site. J Biol Chem. 1998;273:14920–14924. doi: 10.1074/jbc.273.24.14920. [DOI] [PubMed] [Google Scholar]
- 52.Nilsson BO, Olde B, Leeb-Lundberg LM. G protein-coupled oestrogen receptor 1 (GPER1)/GPR30: a new player in cardiovascular and metabolic oestrogenic signalling. Br J Pharmacol. 2011;163:1131–1139. doi: 10.1111/j.1476-5381.2011.01235.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Oeggerli M, Tian Y, Ruiz C, Wijker B, Sauter G, Obermann E, Guth U, Zlobec I, Sausbier M, Kunzelmann K, Bubendorf L. Role of KCNMA1 in breast cancer. PLoS One. 2012;7:e41664. doi: 10.1371/journal.pone.0041664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ou JW, Kumar Y, Alioua A, Sailer C, Stefani E, Toro L. Ca2+- and thromboxane-dependent distribution of MaxiK channels in cultured astrocytes: from microtubules to the plasma membrane. Glia. 2009;57:1280–1295. doi: 10.1002/glia.20847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Park SM, Liu G, Kubal A, Fury M, Cao L, Marx SO. Direct interaction between BKCa potassium channel and microtubule-associated protein 1A. FEBS Lett. 2004;570:143–148. doi: 10.1016/j.febslet.2004.06.037. [DOI] [PubMed] [Google Scholar]
- 56.Pérez G, Toro L. Differential modulation of large-conductance KCa channels by PKA in pregnant and nonpregnant myometrium. Am J Physiol Cell Physiol. 1994;266:C1459–C1463. doi: 10.1152/ajpcell.1994.266.5.C1459. [DOI] [PubMed] [Google Scholar]
- 57.Pietrzykowski AZ, Friesen RM, Martin GE, Puig SI, Nowak CL, Wynne PM, Siegelmann HT, Treistman SN. Posttranscriptional regulation of BK channel splice variant stability by miR-9 underlies neuroadaptation to alcohol. Neuron. 2008;59:274–287. doi: 10.1016/j.neuron.2008.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Rezzonico R, Cayatte C, Bourget-Ponzio I, Romey G, Belhacene N, Loubat A, Rocchi S, Van Obberghen E, Girault JA, Rossi B, Schmid-Antomarchi H. Focal adhesion kinase pp125FAK interacts with the large conductance calcium-activated hSlo potassium channel in human osteoblasts: potential role in mechanotransduction. J Bone Miner Res. 2003;18:1863–1871. doi: 10.1359/jbmr.2003.18.10.1863. [DOI] [PubMed] [Google Scholar]
- 59.Rezzonico R, Schmid-Alliana A, Romey G, Bourget-Ponzio I, Breuil V, Breittmayer V, Tartare-Deckert S, Rossi B, Schmid-Antomarchi H. Prostaglandin E2 induces interaction between hSlo potassium channel and Syk tyrosine kinase in osteosarcoma cells. J Bone Miner Res. 2002;17:869–878. doi: 10.1359/jbmr.2002.17.5.869. [DOI] [PubMed] [Google Scholar]
- 60.Ridgway LD, Kim EY, Dryer SE. MAGI-1 interacts with Slo1 channel proteins and suppresses Slo1 expression on the cell surface. Am J Physiol Cell Physiol. 2009;297:C55–C65. doi: 10.1152/ajpcell.00073.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rosenblatt KP, Sun ZP, Heller S, Hudspeth AJ. Distribution of Ca2+-activated K+ channel isoforms along the tonotopic gradient of the chicken’s cochlea. Neuron. 1997;19:1061–1075. doi: 10.1016/s0896-6273(00)80397-9. [DOI] [PubMed] [Google Scholar]
- 62.Ruttiger L, Sausbier M, Zimmermann U, Winter H, Braig C, Engel J, Knirsch M, Arntz C, Langer P, Hirt B, Muller M, Kopschall I, Pfister M, Munkner S, Rohbock K, Pfaff I, Rusch A, Ruth P, Knipper M. Deletion of the Ca2+-activated potassium (BK) alpha-subunit but not the BKbeta1-subunit leads to progressive hearing loss. Proc Natl Acad Sci U S A. 2004;101:12922–12927. doi: 10.1073/pnas.0402660101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Sausbier M, Arntz C, Bucurenciu I, Zhao H, Zhou XB, Sausbier U, Feil S, Kamm S, Essin K, Sailer CA, Abdullah U, Krippeit-Drews P, Feil R, Hofmann F, Knaus HG, Kenyon C, Shipston MJ, Storm JF, Neuhuber W, Korth M, Schubert R, Gollasch M, Ruth P. Elevated blood pressure linked to primary hyperaldosteronism and impaired vasodilation in BK channel-deficient mice. Circulation. 2005;112:60–68. doi: 10.1161/01.CIR.0000156448.74296.FE. [DOI] [PubMed] [Google Scholar]
- 64.Scornik FS, Codina J, Birnbaumer L, Toro L. Modulation of coronary smooth muscle KCa channels by Gsα independent of phosphorylation by protein kinase A. Am J Physiol. 1993;265:H1460–5. doi: 10.1152/ajpheart.1993.265.4.H1460. [DOI] [PubMed] [Google Scholar]
- 65.Scornik FS, Toro L. U46619, a thromboxane A2 agonist, inhibits KCa channel activity from pig coronary artery. Am J Physiol. 1992;262:C708–C713. doi: 10.1152/ajpcell.1992.262.3.C708. [DOI] [PubMed] [Google Scholar]
- 66.Shikano K, Berkowitz BA. Endothelium-derived relaxing factor is a selective relaxant of vascular smooth muscle. J Pharmacol Exp Ther. 1987;243:55–60. [PubMed] [Google Scholar]
- 67.Siemen D, Loupatatzis C, Borecky J, Gulbins E, Lang F. Ca2+-Activated K Channel of the BK-Type in the Inner Mitochondrial Membrane of a Human Glioma Cell Line. Biochim Biophys Res Comm. 1999;257:549–554. doi: 10.1006/bbrc.1999.0496. [DOI] [PubMed] [Google Scholar]
- 68.Singh H, Lu R, Bopassa JC, Meredith AL, Stefani E, Toro L. mitoBKCa is encoded by the Kcnma1 gene, and a splicing sequence defines its mitochondrial location. Proc Natl Acad Sci U S A. 2013;110:10836–10841. doi: 10.1073/pnas.1302028110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh H, Lu R, Rodriguez PF, Wu Y, Bopassa JC, Stefani E, Toro L. Visualization and quantification of cardiac mitochondrial protein clusters with STED microscopy. Mitochondrion. 2012;12:230–236. doi: 10.1016/j.mito.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh H, Stefani E, Toro L. Intracellular BK(Ca) (iBK(Ca)) channels. J Physiol. 2012;590:5937–5947. doi: 10.1113/jphysiol.2011.215533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sokolowski B, Orchard S, Harvey M, Sridhar S, Sakai Y. Conserved BK channel-protein interactions reveal signals relevant to cell death and survival. PLoS One. 2011;6:e28532. doi: 10.1371/journal.pone.0028532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sokolowski S, Harvey M, Sakai Y, Jordan A, Sokolowski B. The large conductance calcium-activated K(+) channel interacts with the small GTPase Rab11b. Biochem Biophys Res Commun. 2012;426:221–225. doi: 10.1016/j.bbrc.2012.08.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Tatro E, Hefler S, Shumaker-Armstrong S, Soontornniyomkij B, Yang M, Yermanos A, Wren N, Moore D, Achim C. Modulation of BK Channel by MicroRNA-9 in Neurons After Exposure to HIV and Methamphetamine. J Neuroimmune Pharmacol. 2013:1–14. doi: 10.1007/s11481-013-9446-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian L, Chen L, McClafferty H, Sailer CA, Ruth P, Knaus HG, Shipston MJ. A noncanonical SH3 domain binding motif links BK channels to the actin cytoskeleton via the SH3 adapter cortactin. FASEB J. 2006;20:2588–2590. doi: 10.1096/fj.06-6152fje. [DOI] [PubMed] [Google Scholar]
- 75.Tian L, Coghill LS, MacDonald SH, Armstrong DL, Shipston MJ. Leucine zipper domain targets cAMP-dependent protein kinase to mammalian BK channels. J Biol Chem. 2003;278:8669–8677. doi: 10.1074/jbc.M211661200. [DOI] [PubMed] [Google Scholar]
- 76.Tian L, Coghill LS, McClafferty H, MacDonald SH, Antoni FA, Ruth P, Knaus HG, Shipston MJ. Distinct stoichiometry of BKCa channel tetramer phosphorylation specifies channel activation and inhibition by cAMP-dependent protein kinase. Proc Natl Acad Sci U S A. 2004;101:11897–11902. doi: 10.1073/pnas.0402590101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tian L, Duncan RR, Hammond MS, Coghill LS, Wen H, Rusinova R, Clark AG, Levitan IB, Shipston MJ. Alternative splicing switches potassium channel sensitivity to protein phosphorylation. J Biol Chem. 2001;276:7717–7720. doi: 10.1074/jbc.C000741200. [DOI] [PubMed] [Google Scholar]
- 78.Tomas M, Vazquez E, Fernandez-Fernandez JM, Subirana I, Plata C, Heras M, Vila J, Marrugat J, Valverde MA, Senti M. Genetic variation in the KCNMA1 potassium channel alpha subunit as risk factor for severe essential hypertension and myocardial infarction. J Hypertens. 2008;26:2147–2153. doi: 10.1097/HJH.0b013e32831103d8. [DOI] [PubMed] [Google Scholar]
- 79.Toro L, Amador M, Stefani E. ANG II inhibits calcium-activated potassium channels from coronary smooth muscle in lipid bilayers. Am J Physiol. 1990;258:H912–H915. doi: 10.1152/ajpheart.1990.258.3.H912. [DOI] [PubMed] [Google Scholar]
- 80.Toro L, Ramos-Franco J, Stefani E. GTP-dependent regulation of myometrial KCa channels incorporated into lipid bilayers. J Gen Physiol. 1990;96:373–394. doi: 10.1085/jgp.96.2.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Uebele VN, Lagrutta A, Wade T, Figueroa DJ, Liu Y, McKenna E, Austin CP, Bennett PB, Swanson R. Cloning and functional expression of 2 families of {beta}-subunits of the large conductance calcium-activated K+ channel. J Biol Chem. 2000;275:23211–23218. doi: 10.1074/jbc.M910187199. [DOI] [PubMed] [Google Scholar]
- 82.Wade GR, Sims SM. Muscarinic stimulation of tracheal smooth muscle cells activates large-conductance Ca2+-dependent K+ channel. Am J Physiol Cell Physiol. 1993;265:C658–C665. doi: 10.1152/ajpcell.1993.265.3.C658. [DOI] [PubMed] [Google Scholar]
- 83.Wallner M, Meera P, Ottolia M, Kaczorowski GJ, Latorre R, Garcia ML, Stefani E, Toro L. Characterization of and modulation by a beta-subunit of a human maxi KCa channel cloned from myometrium. Receptors Channels. 1995;3:185–199. [PubMed] [Google Scholar]
- 84.Wallner M, Meera P, Toro L. Molecular basis of fast inactivation in voltage and Ca2+-activated K+ channels: A transmembrane β–subunit homolog. Proc Natl Acad Sci USA. 1999;96:4137–4142. doi: 10.1073/pnas.96.7.4137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Weaver AK, Liu X, Sontheimer H. Role for calcium-activated potassium channels (BK) in growth control of human malignant glioma cells. J Neurosci Res. 2004;78:224–234. doi: 10.1002/jnr.20240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Werner ME, Zvara P, Meredith AL, Aldrich RW, Nelson MT. Erectile dysfunction in mice lacking the large conductance calcium-activated potassium (BK) channel. J Physiol. 2005;567:545–556. doi: 10.1113/jphysiol.2005.093823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Wojtovich AP, Nadtochiy SM, Urciuoli WR, Smith CO, Grunnet M, Nehrke K, Brookes PS. A non-cardiomyocyte autonomous mechanism of cardioprotection involving the SLO1 BK channel. PeerJ. 2013;1:e48. doi: 10.7717/peerj.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wood LS, Vogeli G. Mutations and deletions within the S8–S9 interdomain region abolish complementation of N- and C-terminal domains of Ca(2+)-activated K+ (BK) channels. Biochem Biophys Res Commun. 1997;240:623–628. doi: 10.1006/bbrc.1997.7714. [DOI] [PubMed] [Google Scholar]
- 89.Xia XM, Ding JP, Lingle CJ. Inactivation of BK channels by the NH2 terminus of the beta2 auxiliary subunit: an essential role of a terminal peptide segment of three hydrophobic residues. J Gen Physiol. 2003;121:125–148. doi: 10.1085/jgp.20028667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Xu W, Liu Y, Wang S, McDonald T, Van Eyk JE, Sidor A, O’Rourke B. Cytoprotective role of Ca2+- activated K+ channels in the cardiac inner mitochondrial membrane. Science. 2002;298:1029–1033. doi: 10.1126/science.1074360. [DOI] [PubMed] [Google Scholar]
- 91.Yan J, Aldrich RW. LRRC26 auxiliary protein allows BK channel activation at resting voltage without calcium. Nature. 2010;466:513–516. doi: 10.1038/nature09162. [DOI] [PubMed] [Google Scholar]
- 92.Yan J, Aldrich RW. BK potassium channel modulation by leucine-rich repeat-containing proteins. Proc Natl Acad Sci U S A. 2012;109:7917–7922. doi: 10.1073/pnas.1205435109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yan J, Olsen JV, Park KS, Li W, Bildl W, Schulte U, Aldrich RW, Fakler B, Trimmer JS. Profiling the phospho-status of the BKCa channel alpha subunit in rat brain reveals unexpected patterns and complexity. Mol Cell Proteomics. 2008;7:2188–2198. doi: 10.1074/mcp.M800063-MCP200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Yang Y, Li PY, Cheng J, Mao L, Wen J, Tan XQ, Liu ZF, Zeng XR. Function of BKCa channels is reduced in human vascular smooth muscle cells from Han Chinese patients with hypertension. Hypertension. 2013;61:519–525. doi: 10.1161/HYPERTENSIONAHA.111.00211. [DOI] [PubMed] [Google Scholar]
- 95.Yu X, Ma H, Barman SA, Liu AT, Sellers M, Stallone JN, Prossnitz ER, White RE, Han G. Activation of G protein-coupled estrogen receptor induces endothelium-independent relaxation of coronary artery smooth muscle. Am J Physiol Endocrinol Metab. 2011;301:E882–E888. doi: 10.1152/ajpendo.00037.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Yuan P, Leonetti MD, Hsiung Y, MacKinnon R. Open structure of the Ca2+ gating ring in the high-conductance Ca2+-activated K+ channel. Nature. 2012;481:94–97. doi: 10.1038/nature10670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Zarei MM, Eghbali M, Alioua A, Song M, Knaus HG, Stefani E, Toro L. An endoplasmic reticulum trafficking signal prevents surface expression of a voltage- and Ca2+-activated K+ channel splice variant. Proc Natl Acad Sci U S A. 2004;101:10072–10077. doi: 10.1073/pnas.0302919101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Zarei MM, Zhu N, Alioua A, Eghbali M, Stefani E, Toro L. A novel MaxiK splice variant exhibits dominant-negative properties for surface expression. J Biol Chem. 2001;276:16232–16239. doi: 10.1074/jbc.M008852200. [DOI] [PubMed] [Google Scholar]
- 99.Zhou X, Wulfsen I, Korth M, McClafferty H, Lukowski R, Shipston MJ, Ruth P, Dobrev D, Wieland T. Palmitoylation and membrane association of the stress axis regulated insert (STREX) controls BK channel regulation by protein kinase C. J Biol Chem. 2012;287:32161–32171. doi: 10.1074/jbc.M112.386359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhou XB, Wulfsen I, Lutz S, Utku E, Sausbier U, Ruth P, Wieland T, Korth M. M2 muscarinic receptors induce airway smooth muscle activation via a dual, Gbetagamma-mediated inhibition of large conductance Ca2+-activated K+ channel activity. J Biol Chem. 2008;283:21036–21044. doi: 10.1074/jbc.M800447200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou XB, Wulfsen I, Utku E, Sausbier U, Sausbier M, Wieland T, Ruth P, Korth M. Dual role of protein kinase C on BK channel regulation. Proc Natl Acad Sci U S A. 2010;107:8005–8010. doi: 10.1073/pnas.0912029107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zhu N, Eghbali M, Helguera G, Song M, Stefani E, Toro L. Alternative splicing of Slo channel gene programmed by estrogen, progesterone and pregnancy. FEBS Lett. 2005;579:4856–4860. doi: 10.1016/j.febslet.2005.07.069. [DOI] [PubMed] [Google Scholar]
- 103.Zou S, Jha S, Kim EY, Dryer SE. A novel actin-binding domain on Slo1 calcium-activated potassium channels is necessary for their expression in the plasma membrane. Mol Pharmacol. 2008;73:359–368. doi: 10.1124/mol.107.039743. [DOI] [PubMed] [Google Scholar]
- 104.Zou S, Jha S, Kim EY, Dryer SE. The beta 1 subunit of L-type voltage-gated Ca2+ channels independently binds to and inhibits the gating of large-conductance Ca2+-activated K+ channels. Mol Pharmacol. 2008;73:369–378. doi: 10.1124/mol.107.040733. [DOI] [PubMed] [Google Scholar]