Abstract
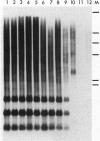
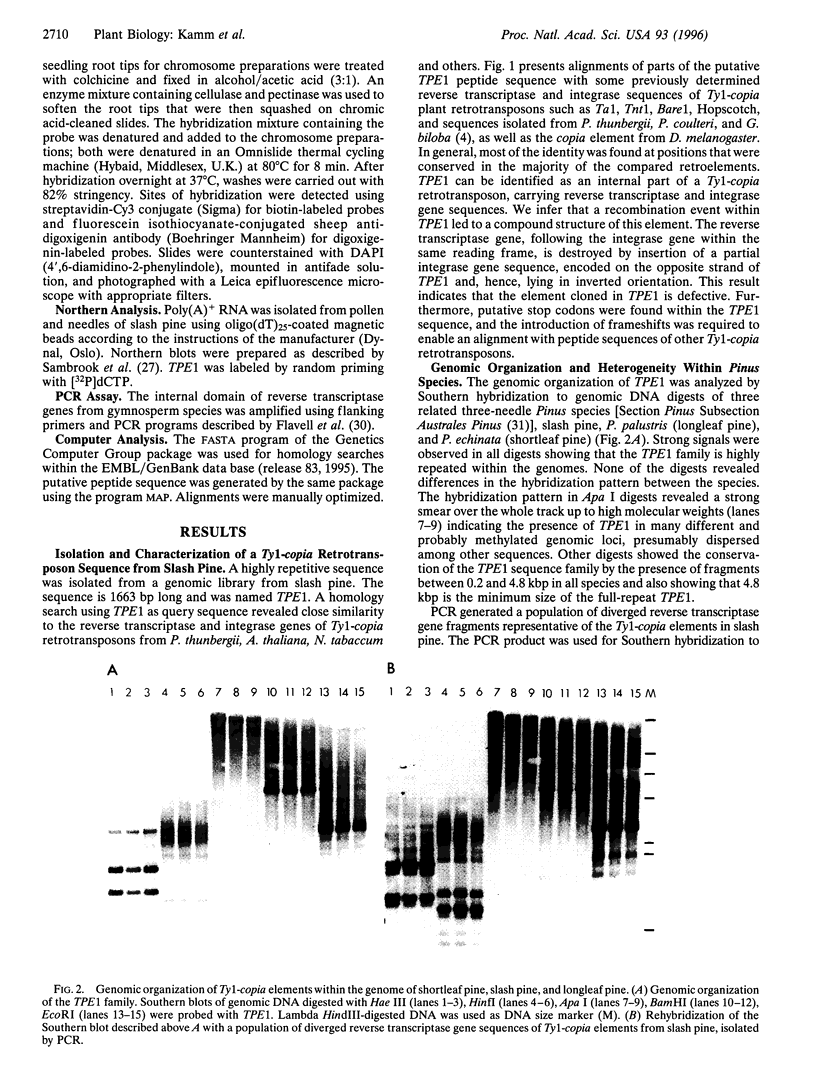
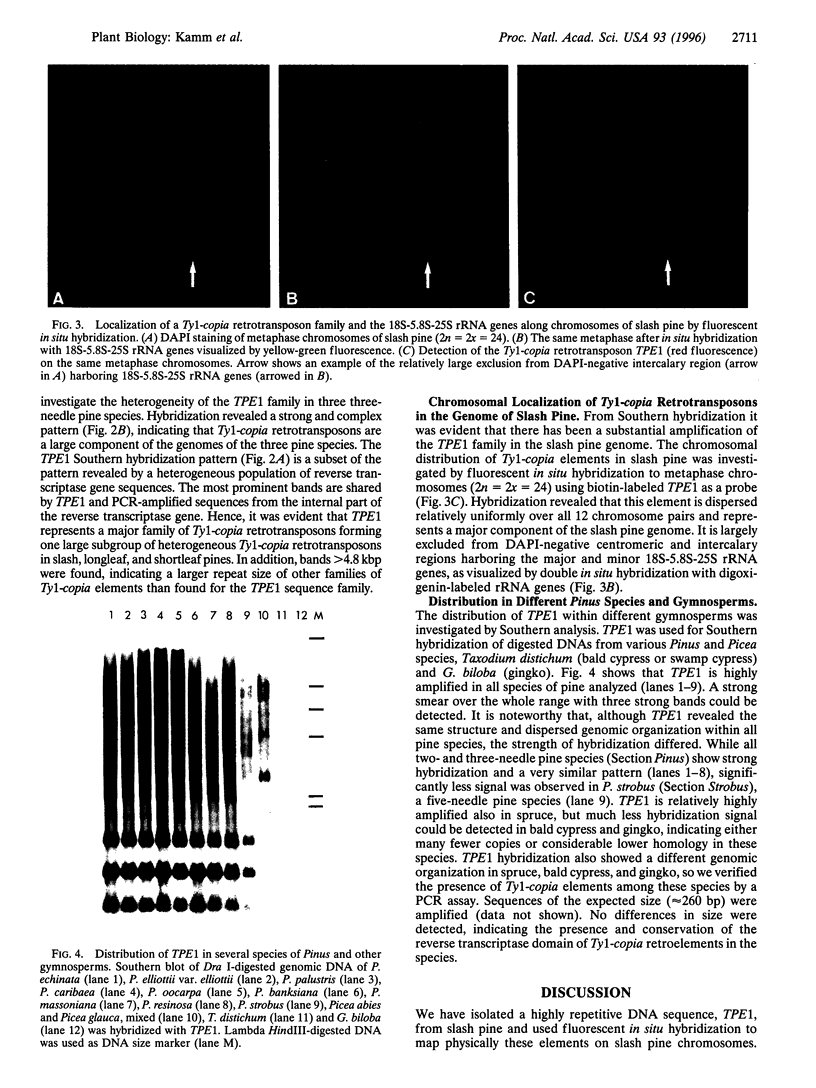
A DNA sequence, TPE1, representing the internal domain of a Ty1-copia retroelement, was isolated from genomic DNA of Pinus elliottii Engelm. var. elliottii (slash pine). Genomic Southern analysis showed that this sequence, carrying partial reverse transcriptase and integrase gene sequences, is highly amplified within the genome of slash pine and part of a dispersed element >4.8 kbp. Fluorescent in situ hybridization to metaphase chromosomes shows that the element is relatively uniformly dispersed over all 12 chromosome pairs and is highly abundant in the genome. It is largely excluded from centromeric regions and intercalary chromosomal sites representing the 18S-5.8S-25S rRNA genes. Southern hybridization with specific DNA probes for the reverse transcriptase gene shows that TPE1 represents a large subgroup of heterogeneous Ty1-copia retrotransposons in Pinus species. Because no TPE1 transcription could be detected, it is most likely an inactive element--at least in needle tissue. Further evidence for inactivity was found in recombinant reverse transcriptase and integrase sequences. The distribution of TPE1 within different gymnosperms that contain Ty1-copia group retrotransposons, as shown by a PCR assay, was investigated by Southern hybridization. The TPE1 family is highly amplified and conserved in all Pinus species analyzed, showing a similar genomic organization in the three- and five-needle pine species investigated. It is also present in spruce, bald cypress (swamp cypress), and in gingko but in fewer copies and a different genomic organization.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Camirand A., Brisson N. The complete nucleotide sequence of the Tst1 retrotransposon of potato. Nucleic Acids Res. 1990 Aug 25;18(16):4929–4929. doi: 10.1093/nar/18.16.4929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doolittle R. F., Feng D. F., Johnson M. S., McClure M. A. Origins and evolutionary relationships of retroviruses. Q Rev Biol. 1989 Mar;64(1):1–30. doi: 10.1086/416128. [DOI] [PubMed] [Google Scholar]
- Flavell A. J., Dunbar E., Anderson R., Pearce S. R., Hartley R., Kumar A. Ty1-copia group retrotransposons are ubiquitous and heterogeneous in higher plants. Nucleic Acids Res. 1992 Jul 25;20(14):3639–3644. doi: 10.1093/nar/20.14.3639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flavell A. J., Smith D. B., Kumar A. Extreme heterogeneity of Ty1-copia group retrotransposons in plants. Mol Gen Genet. 1992 Jan;231(2):233–242. doi: 10.1007/BF00279796. [DOI] [PubMed] [Google Scholar]
- Flavell A. J. Ty1-copia group retrotransposons and the evolution of retroelements in the eukaryotes. Genetica. 1992;86(1-3):203–214. doi: 10.1007/BF00133721. [DOI] [PubMed] [Google Scholar]
- Gerlach W. L., Bedbrook J. R. Cloning and characterization of ribosomal RNA genes from wheat and barley. Nucleic Acids Res. 1979 Dec 11;7(7):1869–1885. doi: 10.1093/nar/7.7.1869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandbastien M. A., Spielmann A., Caboche M. Tnt1, a mobile retroviral-like transposable element of tobacco isolated by plant cell genetics. Nature. 1989 Jan 26;337(6205):376–380. doi: 10.1038/337376a0. [DOI] [PubMed] [Google Scholar]
- Hirochika H., Fukuchi A., Kikuchi F. Retrotransposon families in rice. Mol Gen Genet. 1992 May;233(1-2):209–216. doi: 10.1007/BF00587581. [DOI] [PubMed] [Google Scholar]
- Hirochika H., Hirochika R. Ty1-copia group retrotransposons as ubiquitous components of plant genomes. Jpn J Genet. 1993 Feb;68(1):35–46. doi: 10.1266/jjg.68.35. [DOI] [PubMed] [Google Scholar]
- Knapp D. W., Richardson R. C., Chan T. C., Bottoms G. D., Widmer W. R., DeNicola D. B., Teclaw R., Bonney P. L., Kuczek T. Piroxicam therapy in 34 dogs with transitional cell carcinoma of the urinary bladder. J Vet Intern Med. 1994 Jul-Aug;8(4):273–278. doi: 10.1111/j.1939-1676.1994.tb03232.x. [DOI] [PubMed] [Google Scholar]
- Konieczny A., Voytas D. F., Cummings M. P., Ausubel F. M. A superfamily of Arabidopsis thaliana retrotransposons. Genetics. 1991 Apr;127(4):801–809. doi: 10.1093/genetics/127.4.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manninen I., Schulman A. H. BARE-1, a copia-like retroelement in barley (Hordeum vulgare L.). Plant Mol Biol. 1993 Aug;22(5):829–846. doi: 10.1007/BF00027369. [DOI] [PubMed] [Google Scholar]
- Moore G., Cheung W., Schwarzacher T., Flavell R. BIS 1, a major component of the cereal genome and a tool for studying genomic organization. Genomics. 1991 Jun;10(2):469–476. doi: 10.1016/0888-7543(91)90334-b. [DOI] [PubMed] [Google Scholar]
- Mount S. M., Rubin G. M. Complete nucleotide sequence of the Drosophila transposable element copia: homology between copia and retroviral proteins. Mol Cell Biol. 1985 Jul;5(7):1630–1638. doi: 10.1128/mcb.5.7.1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pouteau S., Huttner E., Grandbastien M. A., Caboche M. Specific expression of the tobacco Tnt1 retrotransposon in protoplasts. EMBO J. 1991 Jul;10(7):1911–1918. doi: 10.1002/j.1460-2075.1991.tb07717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt T., Kubis S., Heslop-Harrison J. S. Analysis and chromosomal localization of retrotransposons in sugar beet (Beta vulgaris L.): LINEs and Ty1-copia-like elements as major components of the genome. Chromosome Res. 1995 Sep;3(6):335–345. doi: 10.1007/BF00710014. [DOI] [PubMed] [Google Scholar]
- Tulsieram L. K., Glaubitz J. C., Kiss G., Carlson J. E. Single tree genetic linkage mapping in conifers using haploid DNA from megagametophytes. Biotechnology (N Y) 1992 Jun;10(6):686–690. doi: 10.1038/nbt0692-686. [DOI] [PubMed] [Google Scholar]
- VanderWiel P. L., Voytas D. F., Wendel J. F. Copia-like retrotransposable element evolution in diploid and polyploid cotton (Gossypium L.). J Mol Evol. 1993 May;36(5):429–447. doi: 10.1007/BF02406720. [DOI] [PubMed] [Google Scholar]
- Vieira J., Messing J. The pUC plasmids, an M13mp7-derived system for insertion mutagenesis and sequencing with synthetic universal primers. Gene. 1982 Oct;19(3):259–268. doi: 10.1016/0378-1119(82)90015-4. [DOI] [PubMed] [Google Scholar]
- Voytas D. F., Ausubel F. M. A copia-like transposable element family in Arabidopsis thaliana. Nature. 1988 Nov 17;336(6196):242–244. doi: 10.1038/336242a0. [DOI] [PubMed] [Google Scholar]
- Voytas D. F., Cummings M. P., Koniczny A., Ausubel F. M., Rodermel S. R. copia-like retrotransposons are ubiquitous among plants. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):7124–7128. doi: 10.1073/pnas.89.15.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner D. B., Furnier G. R., Saghai-Maroof M. A., Williams S. M., Dancik B. P., Allard R. W. Chloroplast DNA polymorphisms in lodgepole and jack pines and their hybrids. Proc Natl Acad Sci U S A. 1987 Apr;84(7):2097–2100. doi: 10.1073/pnas.84.7.2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White S. E., Habera L. F., Wessler S. R. Retrotransposons in the flanking regions of normal plant genes: a role for copia-like elements in the evolution of gene structure and expression. Proc Natl Acad Sci U S A. 1994 Dec 6;91(25):11792–11796. doi: 10.1073/pnas.91.25.11792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y., Eickbush T. H. Origin and evolution of retroelements based upon their reverse transcriptase sequences. EMBO J. 1990 Oct;9(10):3353–3362. doi: 10.1002/j.1460-2075.1990.tb07536.x. [DOI] [PMC free article] [PubMed] [Google Scholar]