Abstract
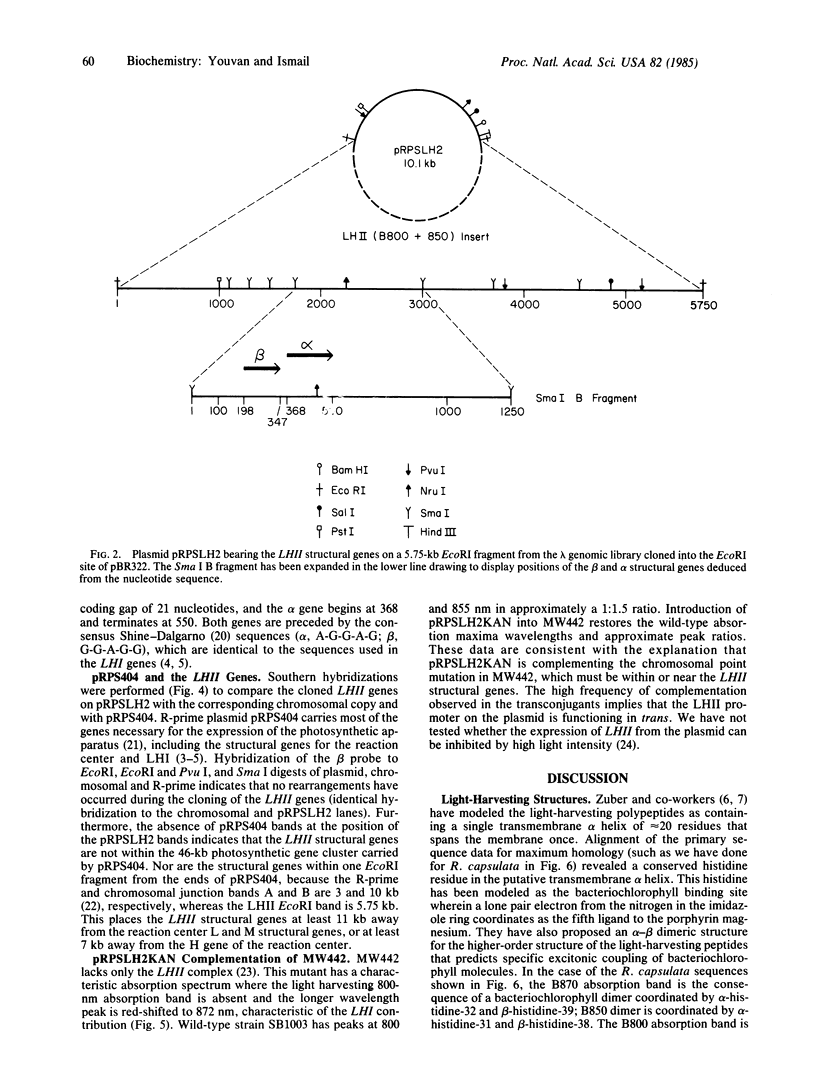
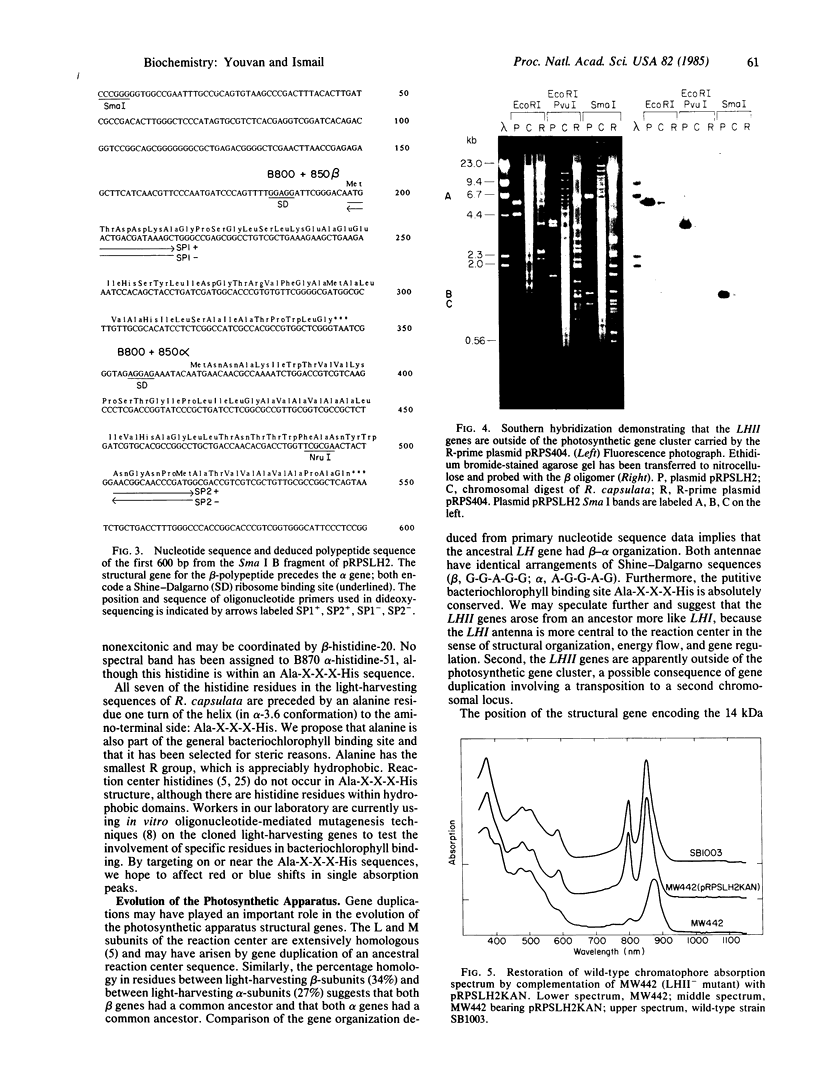
The light-harvesting II (LHII) structural genes coding for the (B800-B850 complex) β- and α-polypeptides have been cloned and the nucleotide and deduced polypeptide sequences have been determined. This completes the sequencing of all seven structural genes coding for the structural polypeptides of the photosynthetic apparatus that bind the pigments and cofactors participating in the primary light reactions of photosynthesis. Unlike the structural genes coding for the reaction center L, M, and H subunits and the light-harvesting I (LHI) (B870 complex) structural polypeptides, the LHII structural genes are not within the 46-kilobase photosynthetic gene cluster carried by the R-prime plasmid pRPS404. Identical organization of the β and α structural genes for both LHI and LHII and sequence homologies between the two β-polypeptides and between the two α-polypeptides suggests that both complexes arose by gene duplication from a single ancestral light-harvesting complex and that the putative bacteriochlorophyll binding sequence Ala-X-X-X-His has been absolutely conserved.
Keywords: photosynthesis, bacteriochlorophyll, antennae, gene duplication, lateral evolution
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ambler R. P., Daniel M., Hermoso J., Meyer T. E., Bartsch R. G., Kamen M. D. Cytochrome c2 sequence variation among the recognised species of purple nonsulphur photosynthetic bacteria. Nature. 1979 Apr 12;278(5705):659–660. doi: 10.1038/278659a0. [DOI] [PubMed] [Google Scholar]
- Beck E., Ludwig G., Auerswald E. A., Reiss B., Schaller H. Nucleotide sequence and exact localization of the neomycin phosphotransferase gene from transposon Tn5. Gene. 1982 Oct;19(3):327–336. doi: 10.1016/0378-1119(82)90023-3. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E. Evolution and gene transfer in purple photosynthetic bacteria. Nature. 1980 Jan 10;283(5743):210–212. doi: 10.1038/283210a0. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Timkovich R., Almassy R. J. The cytochrome fold and the evolution of bacterial energy metabolism. J Mol Biol. 1976 Feb 5;100(4):473–491. doi: 10.1016/s0022-2836(76)80041-1. [DOI] [PubMed] [Google Scholar]
- Engler J. A., van Bree M. P. The nucleotide sequence and protein-coding capability of the transposable element IS5. Gene. 1981 Aug;14(3):155–163. doi: 10.1016/0378-1119(81)90111-6. [DOI] [PubMed] [Google Scholar]
- Fox G. E., Stackebrandt E., Hespell R. B., Gibson J., Maniloff J., Dyer T. A., Wolfe R. S., Balch W. E., Tanner R. S., Magrum L. J. The phylogeny of prokaryotes. Science. 1980 Jul 25;209(4455):457–463. doi: 10.1126/science.6771870. [DOI] [PubMed] [Google Scholar]
- Jorgensen R. A., Rothstein S. J., Reznikoff W. S. A restriction enzyme cleavage map of Tn5 and location of a region encoding neomycin resistance. Mol Gen Genet. 1979;177(1):65–72. doi: 10.1007/BF00267254. [DOI] [PubMed] [Google Scholar]
- Keller C., Corcoran M., Roberts R. J. Computer programs for handling nucleic acid sequences. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):379–386. doi: 10.1093/nar/12.1part1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marrs B. Mobilization of the genes for photosynthesis from Rhodopseudomonas capsulata by a promiscuous plasmid. J Bacteriol. 1981 Jun;146(3):1003–1012. doi: 10.1128/jb.146.3.1003-1012.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell P. Chemiosmotic coupling in oxidative and photosynthetic phosphorylation. Biol Rev Camb Philos Soc. 1966 Aug;41(3):445–502. doi: 10.1111/j.1469-185x.1966.tb01501.x. [DOI] [PubMed] [Google Scholar]
- Olson J. M. Evolution of photosynthetic and respiratory prokaryotes and organelles. Ann N Y Acad Sci. 1981;361:8–19. doi: 10.1111/j.1749-6632.1981.tb46508.x. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher A., Drews G. Effects of light intensity on membrane differentiation in Rhodopseudomonas capsulata. Biochim Biophys Acta. 1979 Sep 11;547(3):417–428. doi: 10.1016/0005-2728(79)90022-7. [DOI] [PubMed] [Google Scholar]
- Scolnik P. A., Haselkorn R. Activation of extra copies of genes coding for nitrogenase in Rhodopseudomonas capsulata. Nature. 1984 Jan 19;307(5948):289–292. doi: 10.1038/307289a0. [DOI] [PubMed] [Google Scholar]
- Scolnik P. A., Zannoni D., Marrs B. L. Spectral and functional comparisons between the carotenoids of the two antenna complexes of Rhodopseudomonas capsulata. Biochim Biophys Acta. 1980 Dec 3;593(2):230–240. doi: 10.1016/0005-2728(80)90061-4. [DOI] [PubMed] [Google Scholar]
- Shine J., Dalgarno L. The 3'-terminal sequence of Escherichia coli 16S ribosomal RNA: complementarity to nonsense triplets and ribosome binding sites. Proc Natl Acad Sci U S A. 1974 Apr;71(4):1342–1346. doi: 10.1073/pnas.71.4.1342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiozawa J. A., Cuendet P. A., Drews G., Zuber H. Isolation and characterization of the polypeptide components from light-harvesting pigment-protein complex B800--850 of Rhodopseudomonas capsulata. Eur J Biochem. 1980 Oct;111(2):455–460. doi: 10.1111/j.1432-1033.1980.tb04960.x. [DOI] [PubMed] [Google Scholar]
- Tadros M. H., Suter F., Drews G., Zuber H. The complete amino-acid sequence of the large bacteriochlorophyll-binding polypeptide from light-harvesting complex II (B800-850) of Rhodopseudomonas capsulata. Eur J Biochem. 1983 Jan 1;129(3):533–536. doi: 10.1111/j.1432-1033.1983.tb07081.x. [DOI] [PubMed] [Google Scholar]
- Taylor D. P., Cohen S. N., Clark W. G., Marrs B. L. Alignment of genetic and restriction maps of the photosynthesis region of the Rhodopseudomonas capsulata chromosome by a conjugation-mediated marker rescue technique. J Bacteriol. 1983 May;154(2):580–590. doi: 10.1128/jb.154.2.580-590.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theiler R., Suter F., Wiemken V., Zuber H. The light-harvesting polypeptides of Rhodopseudomonas sphaeroides R-26.1. I. Isolation, purification and sequence analyses. Hoppe Seylers Z Physiol Chem. 1984 Jul;365(7):703–719. doi: 10.1515/bchm2.1984.365.2.703. [DOI] [PubMed] [Google Scholar]
- Theiler R., Zuber H. The light-harvesting polypeptides of Rhodopseudomonas sphaeroides R-26.1. II. Conformational analyses by attenuated total reflection infrared spectroscopy and the possible molecular structure of the hydrophobic domain of the B 850 complex. Hoppe Seylers Z Physiol Chem. 1984 Jul;365(7):721–729. [PubMed] [Google Scholar]
- Wallace R. B., Johnson M. J., Hirose T., Miyake T., Kawashima E. H., Itakura K. The use of synthetic oligonucleotides as hybridization probes. II. Hybridization of oligonucleotides of mixed sequence to rabbit beta-globin DNA. Nucleic Acids Res. 1981 Feb 25;9(4):879–894. doi: 10.1093/nar/9.4.879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J. C., Steiner L. A., Ogden R. C., Simon M. I., Feher G. Primary structure of the M subunit of the reaction center from Rhodopseudomonas sphaeroides. Proc Natl Acad Sci U S A. 1983 Nov;80(21):6505–6509. doi: 10.1073/pnas.80.21.6505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woese C. R., Gibson J., Fox G. E. Do genealogical patterns in purple photosynthetic bacteria reflect interspecific gene transfer? Nature. 1980 Jan 10;283(5743):212–214. doi: 10.1038/283212a0. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Alberti M., Begusch H., Bylina E. J., Hearst J. E. Reaction center and light-harvesting I genes from Rhodopseudomonas capsulata. Proc Natl Acad Sci U S A. 1984 Jan;81(1):189–192. doi: 10.1073/pnas.81.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Youvan D. C., Bylina E. J., Alberti M., Begusch H., Hearst J. E. Nucleotide and deduced polypeptide sequences of the photosynthetic reaction-center, B870 antenna, and flanking polypeptides from R. capsulata. Cell. 1984 Jul;37(3):949–957. doi: 10.1016/0092-8674(84)90429-x. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Elder J. T., Sandlin D. E., Zsebo K., Alder D. P., Panopoulos N. J., Marrs B. L., Hearst J. E. R-prime site-directed transposon Tn7 mutagenesis of the photosynthetic apparatus in Rhodopseudomonas capsulata. J Mol Biol. 1982 Nov 25;162(1):17–41. doi: 10.1016/0022-2836(82)90160-7. [DOI] [PubMed] [Google Scholar]
- Youvan D. C., Hearst J. E., Marrs B. L. Isolation and characterization of enhanced fluorescence mutants of Rhodopseudomonas capsulata. J Bacteriol. 1983 May;154(2):748–755. doi: 10.1128/jb.154.2.748-755.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zoller M. J., Smith M. Oligonucleotide-directed mutagenesis of DNA fragments cloned into M13 vectors. Methods Enzymol. 1983;100:468–500. doi: 10.1016/0076-6879(83)00074-9. [DOI] [PubMed] [Google Scholar]