Abstract
Human mesenchymal stem cells (MSC) hold great promise for cellular replacement therapies. Despite their contributing to phenotypically distinct cells in multiple tissues, controversy remains regarding whether the phenotype switch results from a true differentiation process. Here, we studied the events occurring during the first 120 h after human MSC transplantation into a large animal model. We demonstrate that MSC, shortly after engrafting different tissues, undergo proliferation and rapidly initiate the differentiative process, changing their phenotype into tissue-specific cells. Thus, the final level of tissue-specific cell contribution is not determined solely by the initial level of engraftment of the MSC within that organ, but rather by the proliferative capability of the ensuing tissue-specific cells into which the MSC rapidly differentiate. Furthermore, we show that true differentiation, and not cell fusion or transfer of mitochondria or membrane-derived vesicles between transplanted and resident cells, is the primary mechanism contributing to the change of phenotype of MSC upon transplantation.
Introduction
The knowledge that mammalian stem cells can differentiate into cell types other than those of the original organ, and even across different embryonic layers, opened the possibility of using these cells as a tool for the development of novel therapeutic strategies. Not only human embryonic stem cells, with their acknowledged broad differentiative potential (Lerou and Daley, 2005), but also adult cells such as multipotent adult progenitor cells (Jiang et al., 2002) or the very small embryonic-like stem cells (Kucia et al., 2006a) have all now been shown to contribute to and/or improve repair in a broad range of tissues. Another cell that has received a great deal of attention as being very promising for cellular replacement therapies is the bone marrow-derived mesenchymal stem cell (MSC) (also interchangeably referred to as marrow stromal cells and stromal precursor cells), the existence of which was first suggested in the pioneering studies of Friedenstein (1990). The in vitro and in vivo differentiation of MSC into the various mesenchymal cell types found within the bone marrow has now been described by numerous laboratories, and the conditions to bring about each of these differentiative pathways have been delineated in detail (Caplan, 1994; Pittenger et al., 1999). Recently, MSC-like cells with differentiative potential similar to those derived from the bone marrow have been isolated and characterized in numerous tissues, such as cord blood (Zvaifler et al., 2000), peripheral blood (O’Donoghue et al., 2003; Ukai et al., 2007), placenta (Fukuchi et al., 2004), liver (Airey et al., 2004), kidney (Almeida-Porada et al., 2002), lung (In ‘t Anker et al., 2003), fat (Zuk et al., 2002), and even amniotic fluid (In ‘t Anker et al., 2004). All of these cells have in common the cellular and morphologic characteristics of bone-marrow-derived MSC, as well as the ability to expand considerably and differentiate into different tissue-specific cell types and/or contribute to the functional improvement of the engrafted organ(s) (Airey et al., 2004; Chamberlain et al., 2007; Kogler et al., 2004; Liechty et al., 2000; Meyerrose et al., 2007). However, since in many instances MSC appear to play a role in the repair of the injured tissue without evidence of engraftment or differentiation, the ability of MSC to contribute significantly to the organ’s specific cell types has been questioned. It is clear that the characteristics of the tissue itself and the kind of deficiency or injury, if injury is the stimulus for cell differentiation, are important variables in determining whether true MSC differentiation can be observed after engraftment (Camargo et al., 2004; Scott, 2004; Theise and Krause, 2002; Wagers and Weissman, 2004; Wang et al., 2003a). While it is probable that MSC contribute to organ repair through a variety of mechanisms, including suppression of inflammatory and immune responses and/or secretion of factors stimulating the regeneration of endogenous cells, other mechanisms such as cell fusion and transfer of mitochondrial or membrane derived-vesicles between cells (Aliotta et al., 2007; Almeida-Porada et al., 2004; Alvarez-Dolado et al., 2003; Camargo et al., 2004; Kucia et al., 2005, 2006b; Ratajczak et al., 2006; Scott, 2004; Spees et al., 2006; Theise and Krause, 2002; Vassilopoulos et al., 2003; Verfaillie, 2005; Wagers and Weissman, 2004; Wang et al., 2003b; Weimann et al., 2003) have also been put forward as possible explanations for the seemingly broad differentiative capacity of MSC.
In this study, we followed the early events that occur after MSC transplantation, and we demonstrated in the fetal sheep transplantation model that human MSC proliferate and promptly commence the differentiative process into tissue-specific cell types. Furthermore, we determined that cell fusion and/or transfer of mitochondria or membrane-derived vesicles between transplanted and resident cells is not the primary mechanism contributing to the change of phenotype of MSC upon transplantation. By demonstrating that MSC do in fact harbor the intrinsic ability to differentiate into tissue-specific cell types in multiple organs following transplantation, we hope that the stage will now be set for beginning to delineate the means of increasing the efficiency of both delivery and selective differentiation of MSC into desired target cell types for their use in stem-cell-based regenerative therapies.
Results
Several studies have been performed to examine the differentiative potential and the dynamic in vivo distribution of human MSC after transplantation. However, thus far, these studies have either focused on merely establishing homing patterns of MSC to various organs soon after transplant (Schrepfer et al., 2007) or evaluated the differentiative potential of MSC only at several weeks or months after the transplant, making it likely that numerous events, such as proliferation, multistep differentiation, or even cell death could already have taken place prior to analysis (Chamberlain et al., 2007; Liechty et al., 2000; Meyerrose et al., 2007; Phinney and Prockop, 2007). In the present study, we sought to define the therapeutic potential of MSC better by elucidating the mechanistic pathways through which MSC appear capable of giving rise to functional differentiated tissue-specific cells and thus contribute to organ regeneration. To answer this fundamental question, we focused on defining the fate of MSC at very early time points after transplantation, when MSC first begin colonizing their target tissues.
Human MSC isolated based on Stro-1+, CD45−, Gly-A−(Airey et al., 2004) were labeled prior to transplantation with either carboxyfluorescein (diacetate) succinimidyl ester (CFSE), which irreversibly couples to both intracellular and cell-surface proteins (Quah et al., 2007; Slavik et al., 2004), or 1,1′-dioctadecyl-3,3,3′,3′-tetramethylindocarbocyanine perchlorate (DiD), which efficiently labels all cell membranes, membrane-derived vesicles, and intracellular organelles such as mitochondria (Anderson and Trgovcich-Zacok, 1995; Onfelt et al., 2006; Zorov et al., 2004). Efficiency of labeling and viability of cells were assessed prior to cell transplantation. Ninety-nine to one hundred percent of the MSC were efficiently labeled with CFSE or DiD, while cell viability remained 98–100% (Supplementary Figs. 1A–C).
Fetal sheep (n=36) at 55–60 days of gestation were then transplanted by intraperitoneal injection with either 2 × 106 CFSE-positive MSC alone (n= 11) or 1 × 106 CFSE-positive MSC in combination with 1 × 106 DiD-positive MSC (n=25) corresponding to a dose of 2 × 107 cells/kg fetal weight. This transplantation procedure has been used by us and others successfully, resulting in minimal fetal losses posttransplantation (Chamberlain et al., 2007; Liechty et al., 2000). Flow-cytometric analysis of peripheral blood and peritoneal lavage showed that transplanted cells migrate into the systemic circulation as early as 20 h posttransplant and were absent from the peritoneal cavity by 96 h after injection. Thus, evaluation of the liver, lung, and brain for the presence of donor CFSE+ or DiD+ MSC by confocal microscopy, as described under Materials and Methods, commenced at 20 h posttransplant and continued at 25, 30, 40, 60, and 120 h after transplantation in all of the studies.
Small percentages of primary MSC engraftment translate into substantive absolute numbers of tissue-resident MSC
Analysis of liver, lung, and brain at 20 h postinjection demonstrated that the transplanted MSC, despite being detected in the circulation, had not reached any of these organs. The percentage of engraftment and total number of human MSC detected in each organ at different times posttransplantation are shown in Table 1. Transplanted MSC were first detected in the liver parenchyma at 25 h posttransplantation at levels of 0.033 ± 0.007%. To determine the absolute number of cells migrating to each of the tissues, we normalized the number of engrafted cells per tissue mass. Thus, after accounting for the average number of cells comprising a fetal sheep liver at this gestational age, it was calculated that a total of 1.16 × 105 MSC had reached this organ at this first time point. At 30 h postinjection the number of MSC found in the recipient’s liver doubled, with 0.062 ± 0.006%, or 2.15 × 105, of the cells within this organ being of donor origin. This number continued to increase in the next hours, to reach the maximal level of engraftment of 0.13 ± 0.02% at 40 h posttransplant and a total number of donor-derived cells of 4.64 × 105. MSC reached the lung only at 30 h posttransplantation, constituting 0.028 ± 0.007%, or 13.4 × 105, of the total cells in this organ. The number of human MSC continued to increase at 40 and 60 h, reaching 0.097 ± 0.014% or 45 × 105 at 120 h. Settlement of MSC in the brain started at 40 h, a later time point than the liver and lung, with a level of engraftment of 0.034 ± 0.008%, or 1.7 × 105 total cells. This number rose to its maximum of 0.079 ± 0.006% or 3.28 × 105 total cells at 60 h.
Table 1.
Percentage of engraftment and total number of human MSC detected in each organ at various times posttransplantation
| Hours post-Tx | No. of animals | Liver
|
Lung
|
Brain
|
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| % Engraftment | Total No. MSC | Total No. Ki67+ | % Engraftment | Total No. MSC | Total No. Ki67+ | % Engraftment | Total No. MSC | Total No. Ki67+ | ||
| 20 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 25 | 3 | 0.0334 ± 0.0073 | 1.16 ± 0.008 × 105 | 1.1 ± 0.0 × 105 | 0 | 0 | 0 | 0 | 0 | 0 |
| 30 | 3 | 0.0621 ± 0.0061 | 2.15 ± 0.01 × 105 | 2.1 ± 0.0 × 105 | 0.0289 ± 0.0073 | 13.45 ± 0.09 × 105 | 12.9 ± 0.0 × 105 | 0 | 0 | 0 |
| 40 | 10 | 0.1343 ± 0.0244 | 4.64 ± 0.11 × 105 | 4.5 ± 0.1 × 105 | 0.0668 ± 0.0089 | 31.02 ± 0.28 × 105 | 29.5 ± 0.1 × 105 | 0.0340 ± 0.0078 | 1.71 ± 0.01 × 105 | 1.6 ± 0.0 × 105 |
| 60 | 10 | 0.1045 ± 0.0154 | 3.61 ± 0.06 × 105 | 3.5 ± 0.1 × 105 | 0.0681 ± 0.0224 | 31.61 ± 0.69 × 105 | 30 ± 0.0 × 105 | 0.0795 ± 0.0064 | 4.00 ± 0.02 × 105 | 3.8 ± 0.0 × 105 |
| 120 | 7 | 0.1041 ± 0.0155 | 3.96 ± 0.06 × 105 | 3.8 ± 0.0 × 105 | 0.0974 ± 0.0150 | 45.18 ± 0.67 × 105 | 42.5 ± 0.1 × 105 | 0.0652 ± 0.0157 | 3.29 ± 0.05 × 105 | 2.9 ± 0.0 × 105 |
To investigate the relative biodistribution of the injected MSC, we determined, at the time when donor cells were first detected, what percentage of the total transplanted MSC were present in the different organs. While only 5.8% of the transplanted MSC homed to the liver and 8.6% to the brain, 67% of the transplanted MSC were found in the lung.
These data show that, even at a dose of 2 × 107cells/kg, the levels of primary engraftment of human MSC in the liver, lung, and brain are very low. The observed levels would often be considered negligible, or even absent, depending on the sensitivity of the method of detection utilized. However, after taking into consideration the inherent tissue cellularity, these seemingly low percentages of primary engraftment in fact correspond to substantive absolute numbers of MSC lodged in each organ.
Human MSC proliferate and differentiate into tissue-specific cells in various organs
We next investigated what events took place within the transplanted cells upon lodging within their target organs. We began by evaluating whether the transplanted cells underwent proliferation upon engraftment and determining whether this proliferation took place before or after the MSC had initiated differentiation into tissue-specific cell types within the respective tissue. Tissue sections from the engrafted organs, collected at 25, 30, 40, 60, and 120 h after transplantation, were stained with Ki67, a marker of cell division, and analyzed under confocal microscopy as described under Materials and Methods. At all time points, 95% of the CFSE+ or DiD+ cells in each tissue also exhibited Ki67 positivity. Since only 30% of the MSC in a parallel culture exhibited Ki67 positivity at the time of culture harvest prior to transplant (Supplementary Fig. 2), this finding suggests that many of the engrafted cells either began or continued to proliferate upon lodging within the various organs. Figs. 1E–G (liver), Figs. 2H–K (lung), and Figs. 3D–F (brain) show representative tissue sections with DiD+ and CFSE+ cells undergoing division in close association with proliferating endogenous sheep cells (Ki67 positive, CFSE/DiD negative). These studies demonstrate the ability of MSC to proliferate within the host tissues, suggesting that the higher levels of engraftment observed at later time points were due, at least in part, to the proliferation of the MSC that had engrafted earlier on and were not solely a result of the continued lodging of transplanted cells within the tissue.
Figure 1.

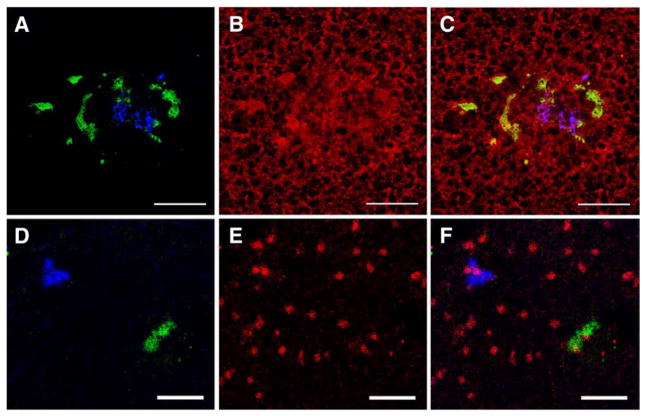
Human MSC initiate the differentiative process into liver cells (A–D) and proliferate (E, F) within the liver parenchyma. (A) A cluster of MSC DiD+ and CFSE+ cells expressing α-fetoprotein can be seen in a representative section of liver tissue, harvested at 30 h posttransplant and stained with antibody against α-fetoprotein (AlexaFluor 594, red). (B) Detail at 40 × original magnification from (A) showing the cluster of DiD+ (blue) and CFSE+ (green) MSC. (C) Expression of α-fetoprotein (red) by the liver cells. (D) Merge of (B) and (C) showing DiD+ and CFSE+ cells expressing α-fetoprotein (magenta and yellow, respectively). (E) Representative liver section with DiD+ and CFSE+ MSC. (F) Same field of view with liver section stained with anti-Ki67 antibody. (G) Merge of (E) and (F) showing DiD+ and CFSE+ cells undergoing division in close association with proliferating endogenous sheep cells (Ki67 positive, CFSE/DiD negative). Scale bars: (A) 100 μm, (B–G) 25 μm.
Figure 2.
Human MSC differentiate into type II pneumocytes (A–D) and proliferate (H–K) shortly after engraftment. (A) Representative lung tissue section at 40 h posttransplant, stained with an antibody against prosurfactant-B (AlexaFluor 594, red); a cluster of MSC CFSE+ cells can be seen (green). (B) Detail at 40 × original magnification from (A) showing the cluster of CFSE+ (green) MSC. (C) Expression of prosurfactant-B (red) by cells in the lung parenchyma. (D) Merge of (B) and (C) showing CFSE+ cells expressing prosurfactant-B (double-positive cells appear yellow). (E) Detail at 40 × original magnification showing MSC DiD+ in the lung parenchyma at 40 h posttransplant. (F) Expression of caveolin-1 (red) by cells in the lung parenchyma. (G) Merge of (E) and (F) showing that DiD-positive MSC fail to express caveolin-1. (H) Representative lung tissue with CFSE+ MSC. (J) Same field of view with lung tissue section stained with anti-Ki67 antibody. (K) Merge of (H) and (J) showing CFSE+ cell dividing in close association with proliferating endogenous sheep cells (Ki67 positive, CFSE negative). Scale bars: (A) 100 μm, (B–K) 25 μm.
Figure 3.
Human MSC up-regulate the expression of proteins related to neural differentiation (A–C) and proliferate (D–F) soon after engraftment. (A) A cluster of DiD+ and CFSE+ MSC can be seen in a representative section of brain tissue, harvested at 40 h posttransplant. (B) Same field of view as (A), stained with an antibody against Tau (AlexaFluor 594, red). (C) Merge of (A) and (B) showing DiD+ and CFSE+ cells expressing Tau (magenta and yellow, respectively). (D) Representative brain tissue with CFSE+ and DiD+ MSC. (E) Same field of view as (D), stained with anti-Ki67 antibody. (F) Merge of (D) and (E) showing DiD+ and CFSE+ cells undergoing division in close association with proliferating endogenous sheep cells (Ki67 positive, CFSE/DiD negative). Scale bars: (A) 100 μm, (B–G) 25 μm.
To determine the timeline of MSC differentiation into organ-specific cell types, we examined liver, brain, and lung tissue sections by immunofluorescence using various cell-specific markers that were not expressed by MSC prior to transplant (Supplementary Figs. 1D–F). We then examined these tissues by confocal microscopy for evidence of cells that were positive for CFSE or DiD and were simultaneously expressing cell-specific markers for each of the engrafted tissues. α-Fetoprotein was chosen as the marker of MSC induction toward a hepatic phenotype, since during normal liver development, the up-regulation of gene transcripts encoding α-fetoprotein and albumin is thought to mark the first evidence of hepatic specification and emergence of the hepatoblast (Gouon-Evans et al., 2006). But, because during this period protein expression of albumin occurs later than that of α-fetoprotein, we used expression of the latter as evidence of initiation of differentiation of MSC into a liver-like phenotype (Nava et al., 2005). At 25 h posttransplant, the first time point at which MSC were detected in the liver, CFSE+ or DiD+ cells (1.16 ± 0.0 × 105) were already expressing α-fetoprotein, demonstrating that MSC rapidly switched to a fetal hepatocyte-like phenotype upon liver engraftment. Fig. 1A shows a representative section of liver tissue, harvested at 30 h posttransplant, stained with antibody against α-fetoprotein. As can be seen, most of the recipient’s hepatic cells are already expressing α-fetoprotein. This figure also shows a cluster of CFSE- and DiD-positive cells expressing α-fetoprotein (AFP), demonstrating their differentiation toward a hepatoblast-like phenotype (total No. of MSC expressing α-fetoprotein: 2.1 ± 0.0 × 105). To evaluate MSC differentiation in the lung, we examined the expression of surfactant protein B, since this protein is expressed in type II pneumocytes early in gestation (Khoor et al., 1994). We also looked at caveolin-1 expression since it is a marker of maturation and differentiation of lung alveolar epithelial type II cells into a type I phenotype (Barar et al., 2007). CFSE- and DiD-positive cells were found to express surfactant protein B (13.4 ± 0.0 × 105) as soon as they first engrafted in the lung at 30 h posttransplantation, and they continued to express this protein throughout the evaluation period. By contrast, up to 120 h posttransplant, the last time point of our analysis, caveolin-1 was still not being expressed by the transplanted MSC. This demonstrates that the transplanted MSC assumed a phenotype that is consistent with differentiation to a type II epithelial cell, but not to a more mature type I. Figs. 2A–D show representative lung tissue sections at 40 h posttransplant, stained with an antibody against prosurfactant-B. This figure shows that a cluster of CFSE-labeled MSC is expressing prosurfactant-B, while Figs. 2E–G show that DiD-positive MSC failed to express caveolin-1 throughout our study (total No. of MSC expressing prosurfactant-B: 31 ± 0.3 × 105).
To investigate the differentiation of MSC into cells with a neural phenotype upon engraftment into the brain tissue, we examined whether CFSE- and DiD-positive cells were also expressing Tau and/or synaptophysin. Tau is widely expressed in the fetal brain during development and correlates with neurite growth and axonal development in neurons and neural cell lines upon in vitro culture (Kempf et al., 1996), while synaptophysin is a presynaptic vesicle glycoprotein that is a reliable marker of nerve terminal differentiation (Sarnat and Born, 1999). All of the transplanted DiD- and CFSE-positive MSC expressed Tau promptly at the first time point of 40 h posttransplantation (1.7 ± 0.0 × 105), the first point at which these cells were found in the brain. Figs. 3A–C show DiD- and CFSE-positive MSC expressing Tau at 40 h posttransplant, demonstrating that MSC quickly up-regulated the expression of proteins related to neural differentiation. By contrast, at the same time point of 40 h posttransplant, synaptophysin was found to be expressed in approximately 56% of the transplanted MSC (9.6 ± 0.0 × 104). Figs. 4B–D show a DiD-positive cell that was negative for synaptophysin, while Figs. 4E–G show a CFSE-positive cell that is expressing synaptophysin. Nevertheless, at 60 h posttransplant, all of the MSC visualized in the brain were expressing synaptophysin (4 ± 0.0 × 105). Overall, these results show that transplanted MSC, after reaching brain, liver, or lung, rapidly undergo proliferation and differentiation, leading to a change in MSC phenotype toward tissue-specific cell types. To confirm further that expression of these markers by the engrafted MSC was indeed due to differentiation into tissue-specific cells, additional experiments were performed in which antibodies presumed to be tissue-specific were used to stain sections from unrelated tissues exhibiting human cell engraftment. Supplementary Figs. 3–5 show the results of these swapped antibody experiments in different tissues and clearly demonstrate that while engrafted MSC in each of these tissues express markers specific to the tissue in question, they do not express markers normally present in other tissues. For example, MSC engrafted within the liver express AFP, but they do not express Tau. Likewise, MSC that have engrafted the lung express prosurfactant-B, but do not express AFP, while MSC that engraft within the brain express Tau, but do not express prosurfactant-B. These studies thus demonstrate the specificity of the antibodies we employed and confirm that the transplanted MSC were not expressing multiple “tissue-specific” antigens as a result of transplantation into a fetal environment, but rather, began to express each of these markers only upon engraftment within the respective tissue.
Figure 4.
Human MSC engrafted in brain start expressing synaptophysin at later time points posttransplant. (A) DiD+ and CFSE+ MSC can be seen in a representative section of brain tissue, harvested at 40 h posttransplant and stained with antibody against synaptophysin (AlexaFluor 594, red). (B) Detail at 40 × original magnification from (A) showing DiD+ MSC (blue). (C) Expression of synaptophysin (red) by the brain cells. (D) Merge of (B) and (C) showing that these DiD+ cells are not yet expressing synaptophysin. (E) Detail at 40 × original magnification from (A) showing CFSE+ MSC (green). (F) Expression of synaptophysin (red) by the brain cells. (G) Merge of (E) and (F) showing CFSE+ MSC expressing synaptophysin. Scale bars: (A) 100 μm, (B–G) 25 μm.
Human mesenchymal stem cells differentiate into tissue-specific cells in the absence of fusion or membrane/mitochondrial transfer
In an effort to delineate the mechanisms responsible for the observed generation of tissue-specific cells by MSC, we next investigated whether the rapid expression of tissue-specific cell markers was due to true differentiation of the transplanted MSC into tissue-specific cells or instead resulted from the transfer of membrane vesicles/mitochondria or fusion of MSC with resident tissue-specific cells within each organ. To this end, we used confocal microscopy to visualize cells that were positive for CFSE, which irreversibly couples to both intracellular and cell-surface proteins (Quah et al., 2007; Slavik et al., 2004), or DiD, which efficiently labels all cell membranes and intracellular organelles, such as mitochondria (Anderson and Trgovcich-Zacok, 1995; Onfelt et al., 2006; Zorov et al., 2004), and performed fluorescence in situ hybridization (FISH) using either a human- or a sheep-specific probe. By examining the species origin of the genomic DNA present within the nuclei of the DiD+ or CFSE+ cells in each engrafted organ, we were able to determine whether the transplanted MSC had undergone fusion or membrane/mitochondrial transfer. Figs. 5, 6, and 7 show tissue sections from liver, lung, and brain, respectively. Columns A–C in each of these figures illustrate the result of FISH using a sheep-specific probe, and columns D–F show tissue sections analyzed by FISH using a human-specific probe. CFSE+ cells in the liver, lung, and brain all hybridized exclusively to the human probe, demonstrating that no fusion had occurred between the transplanted MSC and the endogenous sheep cells within the examined organs. Since the majority of studies thus far have focused on the role of mitochondrial transfer from donor cells to recipient tissues as a means of providing respiratory rescue to damaged or injured host tissues/cells, and have suggested that this may in fact be the mechanism whereby transplanted MSC provide therapeutic benefit without the need for actual engraftment (Spees et al., 2006), we also examined whether membrane vesicles/mitochondria were transferred from the transplanted human cells to the host sheep tissues. As can be seen in columns G–J in Figs. 5, 6, and 7, all of the DiD-positive cells were negative for sheep genomic DNA, while columns K–M prove that every DiD-positive cell hybridized exclusively to the human probe. This combinatorial approach thus demonstrated that in the absence of disease or injury, MSC gave rise to tissue-specific cell types in the absence of cellular fusion or donor-to-recipient transfer of mitochondria or membrane vesicles. Nevertheless, despite the sensitivity of these methods, the possibility exists that minute levels of vesicle/organelle transfer could have occurred and fallen below our limits of detection.
Figure 5.
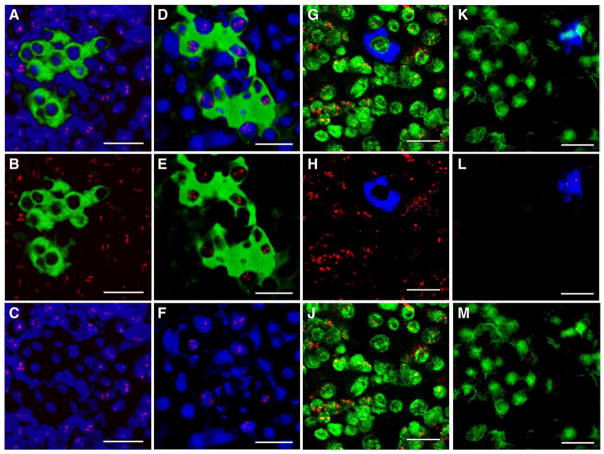
Human MSC differentiate into liver cells in the absence of fusion (A–F) or membrane vesicular or mitochondria transfer (G–M). (A) A cluster of CFSE+ (green) MSC in a representative section of liver tissue obtained at 60 h posttransplant and stained by FISH with a sheep-specific probe (red), nuclei counterstained with DAPI are pseudocolored blue. (B) The same tissue section, showing only the green (CFSE) and red (sheep-specific FISH probe) channels. (C) The same liver tissue section, showing only the red (sheep-specific FISH probe) and blue (DAPI nuclear counterstain) channels. (D) Serial section of the same MSC CFSE+ cluster (green) analyzed by FISH using a human-specific probe (red), with nuclei counterstained with DAPI (pseudocolored blue). (E) The same tissue section, showing only the green (CFSE) and red (human-specific FISH probe) channels. (F) The same liver tissue section, showing only the red (human-specific FISH probe) and blue (DAPI nuclear counterstain) channels. (G) DiD+ (blue) MSC in a representative liver tissue section stained by FISH with a sheep-specific probe (red), nuclei counterstained with DAPI are pseudocolored green. (H) The same tissue section, showing only the blue (DiD) and red (sheep-specific FISH probe) channels. (J) The same liver tissue section, showing only the red (sheep-specific FISH probe) and green (DAPI nuclear counterstain) channels. (K) A cluster of DiD+ (blue) MSC in a representative liver tissue section stained by FISH with a human-specific probe (red), nuclei counterstained with DAPI are pseudocolored green. (L) The same tissue section, showing only the blue (DiD) and red (human-specific FISH probe) channels. (M) The same liver tissue section, showing only the red (human-specific FISH probe) and green (DAPI nuclear counterstain) channels. These pictures collectively show that CFSE+ and DiD+ donor cells in the liver hybridized exclusively to the human probe, demonstrating that no fusion had occurred between the transplanted MSC and the endogenous sheep cells. Furthermore, the exclusive hybridization of DiD+ cells to the human probe demonstrates that no membrane or mitochondrial transfer took place between the donor human MSC and the neighboring recipient sheep liver cells. Scale bars: 25 μm.
Figure 6.
Human MSC differentiate into lung cells in the absence of fusion (A–F) or membrane vesicular or mitochondria transfer (G–M). (A) A cluster of CFSE+ (green) MSC in a representative section of lung tissue obtained at 60 h posttransplant and stained by FISH with a sheep-specific probe (red), nuclei counterstained with DAPI are pseudocolored blue. (B) The same tissue section, showing only the green (CFSE) and red (sheep-specific FISH probe) channels. (C) The same tissue section, showing only the red (sheep-specific FISH probe) and blue (DAPI nuclear counterstain) channels. (D) A cluster of CFSE+ (green) MSC in a representative lung tissue section analyzed by FISH using a human-specific probe (red), with nuclei counterstained with DAPI (pseudocolored blue). (E) The same tissue section, showing only the green (CFSE) and red (human-specific FISH probe) channels. (F) The same lung tissue section, showing only the red (human-specific FISH probe) and blue (DAPI nuclear counterstain) channels. (G) A cluster of DiD+ (blue) MSC in a representative lung tissue section stained by FISH with a sheep-specific probe (red), nuclei counterstained with DAPI are pseudocolored green. (H) The same tissue section, showing only the blue (DiD) and red (sheep-specific FISH probe) channels. (J) The same lung tissue section, showing only the red (sheep-specific FISH probe) and green (DAPI nuclear counterstain) channels. (K) A cluster of DiD+ (blue) MSC in a representative lung tissue section stained by FISH with a human-specific probe (red), nuclei counterstained with DAPI are pseudocolored green. (L) The same tissue section, showing only the blue (DiD) and red (human-specific FISH probe) channels. (M) The same lung tissue section, showing only the red (human-specific FISH probe) and green (DAPI nuclear counterstain) channels. Similar to the results obtained for liver, these pictures collectively show that CFSE+ and DiD+ donor cells in the lung hybridized exclusively to the human probe, demonstrating that no fusion or membrane/mitochondrial transfer had occurred between the transplanted MSC and the endogenous sheep cells. Scale bars: 25 μm.
Figure 7.
Human MSC differentiate into neural cells in the absence of fusion (A–F) or membrane vesicular or mitochondria transfer (G–M). (A) A cluster of CFSE+ (green) MSC in a representative section of brain tissue obtained at 60 h posttransplant and stained by FISH with a sheep-specific probe (red), nuclei counterstained with DAPI are pseudocolored blue. (B) The same tissue section, showing only the green (CFSE) and red (sheep-specific FISH probe) channels. (C) The same tissue section, showing only the red (sheep-specific FISH probe) and blue (DAPI nuclear counterstain) channels. (D) A cluster of CFSE+ (green) MSC in a representative brain tissue section analyzed by FISH using a human-specific probe (red), with nuclei counterstained with DAPI (pseudocolored blue). (E) The same tissue section, showing only the green (CFSE) and red (human-specific FISH probe) channels. (F) The same brain tissue section, showing only the red (human-specific FISH probe) and blue (DAPI nuclear counterstain) channels. (G) A cluster of DiD+ (blue) MSC in a representative brain tissue section stained by FISH with a sheep-specific probe (red), nuclei counterstained with DAPI are pseudocolored green. (H) The same tissue section, showing only the blue (DiD) and red (sheep-specific FISH probe) channels. (J) The same brain tissue section, showing only the red (sheep-specific FISH probe) and green (DAPI nuclear counterstain) channels. (K) A cluster of DiD+ (blue) MSC in a representative brain tissue section stained by FISH with a human-specific probe (red), nuclei counterstained with DAPI are pseudocolored green. (L) The same tissue section, showing only the blue (DiD) and red (human-specific FISH probe) channels. (M) The same brain tissue section, showing only the red (human-specific FISH probe) and green (DAPI nuclear counterstain) channels. Similar to the results obtained for liver and lung, these pictures collectively show that CFSE+ and DiD+ donor cells in the brain hybridized exclusively to the human probe, demonstrating that no fusion or membrane/mitochondrial transfer had occurred between the transplanted MSC and the endogenous sheep cells. Scale bars: 25 μm.
Discussion
In the present studies we show that, upon transplantation into a noninjury fetal model, human MSC engrafted in all of the examined organs, began or continued proliferating, and started differentiation into multiple tissue-specific cell types. Nevertheless, even when we transplanted 20 million cells/kg, the percentages of engrafted donor cells in the different organs were low. It is important to note, however, that the final level of tissue-specific cells generated by the transplanted MSC within a given organ may not be determined solely by the initial level of engraftment and proliferation of the MSC within that organ, but rather on the ensuing proliferative capability of the tissue-specific cells into which the MSC rapidly differentiate upon engraftment. For example, in the case of the liver, the MSC rapidly differentiate to form hepatoblasts, which, like the MSC themselves, possess a high intrinsic proliferative capacity (Mahieu-Caputo et al., 2004). For this reason, even relatively low initial levels of donor MSC engraftment within this organ can result in high long-term levels of donor-derived hepatocytes through subsequent proliferation of MSC-derived hepatoblast progeny (Chamberlain et al., 2007). In contrast, in an organ such as the brain, the rapid conversion of the engrafted MSC into a phenotype consistent with terminally differentiated neurons would probably preclude any further proliferation/expansion of the donor cells, limiting the ultimate levels of engraftment achieved in this tissue. This suggests that to achieve clinically relevant levels of MSC engraftment able to provide for suitable therapy, it will probably be necessary to find ways to substantially increase the efficiency with which the infused cells can be delivered to the specific desired organ. For instance, the work by Sackstein et al., showing that converting the native CD44 glycoform found on MSC into an E-selectin/L-selectin ligand resulted in enhanced tropism of MSC to the bone (Sackstein et al., 2008), elegantly illustrates how it may be possible in the near future to unlock new pathways for increasing the trafficking and homing of these cells to other organs.
These studies also suggest that transplanted MSC go through a gradual program of differentiation, with the subsequent cells sequentially expressing markers indicative of progressive cell maturation. Because the differentiation process of MSC into tissue-specific cells occurs promptly upon engraftment, a deeper understanding of how the differentiative process by which MSC give rise to tissue-specific cells comes about is needed, so that novel strategies can be developed to drive the proliferation of the engrafted cells at specific steps of the differentiation process, to achieve therapeutic levels of a particular cell needed for correction of the disease in question.
Furthermore, we have also demonstrated that the formation of tissue-specific cells from transplanted adult human MSC occurs in several organs in the absence of either fusion or donor-to-recipient mitochondrial/membrane transfer. Despite the sensitivity of the methods we employed in the present study to address this issue, the possibility still exists that minute levels of vesicle/organelle transfer could have occurred and fallen below our limits of detection. Nevertheless, it is likely that in the fetal sheep model system, in the absence of injury or disease, there are sufficient developmental cues present within the physiologic inductive microenvironment to induce up-regulation of tissue-specific genes within the transplanted MSC that then lead to differentiation into cells of the specific desired organ. This would be in agreement with recent studies in which investigators showed that by forcing adult cells to overexpress a small number of genes normally associated with embryonic stem (ES) cells, they were able to induce these adult cells to achieve totipotency, thus providing strong evidence that adult stem cells, under very specific conditions, possess the ability to change the developmental clock and, in this case, to become an ES-like cell (Takahashi et al., 2007; Wernig et al., 2007; Yu et al., 2007). It is envisioned that further studies elucidating precisely which genes are required for adult stem cells to differentiate into each of the tissue-specific cell types will ultimately lead to the discovery of the means of specifically up-regulating only the genes required to produce the cell type needed for benefit in the disease state in question. In our present studies, we have taken advantage of the unique proliferative/inductive nature of the fetal microenvironment to show that transplanted adult MSC have the inherent ability to generate tissue-specific cells rapidly in several tissues. It is important to note, however, that while this model is well suited to defining the mechanisms involved in this process, translation to clinical application will require experiments in adult animals exhibiting a specific defect/disease, to assess the impact the diseased/proapoptotic microenvironment may have on stem cell engraftment and differentiation. Thus, once the requisite gene pathways have been elucidated and the means of circumventing the hurdles that are present in injured/diseased adult tissues have been developed, the stage will be set for beginning to delineate the means of increasing the efficiency of both delivery and selective differentiation of MSC into desired target cell types, to be able finally to fully exploit the potential of human MSC for their use in stem cell-based regenerative therapies.
Materials and methods
MSC isolation and characterization
Human fetal liver (18 to 22 weeks gestational age) was purchased from Advanced Bioscience Resources (Alameda, CA, USA). Fetal tissues were homogenized to yield single-cell suspensions and enriched for Stro-1+ cells (Simmons et al., 1994) (Stro-1 antibody was a kind gift from Dr. Paul Simmons) by magnetic cell sorting (Miltenyi Biotec, Auburn, CA, USA). Stro-1+ cells were plated at low density in MSC growth medium (MSCGM) (Lonza, Allendale, NJ, USA). After enrichment, MSC were expanded in MSCGM and cultured at 1 × 104 to 5 × 105 cells/cm2. Similar to bone-marrow-derived MSC (Chamberlain et al., 2007), liver-derived MSC expressed high levels of CD90, CD13, CD29, and CD105 and were negative for CD14, CD33, CD34, CD45, glycophorin A, and HLA-DR. Liver-derived MSC were also evaluated for their ability to fulfill the criteria of MSC by confirming their ability to undergo differentiation along osteogenic and adipogenic pathway in vitro, as previously described (Chamberlain et al., 2007).
MSC labeling with CFSE and DiD
Cultures of MSC were suspended in HBSS containing 0.1% bovine serum albumin (BSA) at a concentration of 2 × 106 cells/ml in 2 ml of medium. A stock solution of 3 mM CFSE (Sigma–Aldrich, St. Louis, MO, USA) in DMSO was diluted to 6 μM in PBS containing 0.1% BSA. One milliliter of CFSE solution was mixed with 1 ml of MSC cell suspension to give a final concentration of 3 μM and was allowed to incubate for 10 min at 37 °C. MSCGM containing 13% fetal bovine serum was used to quench the CFSE protein binding reaction. The free CFSE was then removed by washing and cells were suspended in injection medium, Hepes-buffered salt solution with 0.1% BSA.
For DiD labeling, adherent MSC were cultured in tissue culture flasks to a concentration of 5 × 105 cells/cm2 in MSCGM. To label cells, 25 μl of a stock solution of 1 mM DiD oil (DiIC18(5) oil; Molecular Probes, Carlsbad, CA, USA) in DMSO was added to 2 ml of serum-free medium to give a final concentration of 12.5 μM. The growth medium was removed from the flasks, then the MSC cultures were incubated in the DiD labeling solution for 20 min at 37 °C. The labeling solution was removed; the cells were rinsed with medium and then allowed to incubate for 10 min in warmed growth medium. The MSC were detached using trypsin/EDTA (Lonza). Cells were then suspended in injection medium as described above. Efficiency of labeling and viability of cells were assessed prior to cell transplantation (Supplementary Fig. 1).
In utero transplantation of sheep fetuses
Thirty-six fetal sheep (bred and housed at the University of Nevada Agricultural Experimental Station) at 55–60 days of gestation (term: 145 days) were injected intraperitoneally with MSC labeled with CFSE alone (n=11) or in combination with DiD (n=25) at a dose of 2 × 107cells/kg (estimated fetal body wt) in 0.5–1.0 ml as previously described (Airey et al., 2004; Chamberlain et al., 2007; Liechty et al., 2000). Animals were euthanized and tissues were harvested starting at 20 h posttransplant and continued at 25, 30, 40, 60, and 120 h (Table 1). All procedures were in accordance with University of Nevada IACUC guidelines.
Tissue preparation
Tissues were dissected and washed with ice-cold PBS, cut into 5-mm cubes, and immersed in ice-cold PBS containing 4% paraformaldehyde for 1 h. After cryoprotection in PBS containing increasing sucrose concentrations, from 5, 10, 15, to 20%, tissues were incubated in 2 parts 20% sucrose, 1 part OCT compound (TissueTec, Torrance, CA, USA) for 1 h and then embedded in fresh solution by rapid freezing in isopentane-cooled in liquid nitrogen. A Leica Minotome was used to section each tissue, and cryosections 7 to 10 μm thick were adhered to variously coated slides (Fisher Superfrost, Surgipath Xtra, or Vectabond treated) according to tissue adherence preferences.
Immunohistochemical analysis
Tissue sections were washed with PBS and blocked in PBS containing 10% normal serum from the species of origin of the secondary antibody or 2% BSA. Sections were then incubated in PBS with 2% normal goat serum and primary antibody overnight at 4 °C. Primary antibodies were against the following proteins: Tau (BioGenex), Ki67 (Neomakers), prosurfactant-B (Chemicon), caveolin-1 (BD Transduction Laboratories), α-fetoprotein (Abcam), and synaptophysin (Biogenex). Slides were washed with PBS with 2% BSA and then incubated with secondary antibody in PBS with 2% BSA for 1 h at 4 °C. Secondary antibodies were conjugated to Alexa 488, 568, 594, or 633.
Data analysis and statistics
At least five different tissue sections were analyzed from each of the organs of the transplanted animals at each time point. An average of at least 50,000 total cells were counted per tissue section and the numbers of human CFSE- and/or DiD-positive cells in each section determined as a percentage of the total number of cells. The mean percentages of engraftment were then calculated by averaging the results obtained from all five sections from the animals at each time point of analysis, and standard deviation was determined for each time point to obtain the presented results. To determine the percentage of the total transplanted MSC that migrated to a specific tissue we used the following formula:
Fluorescence in situ hybridization
FISH analysis of tissue sections was performed as previously described (Chamberlain et al., 2007). Briefly, human- and sheep-specific probes, generated as described in the Supplementary Materials and Methods, were denatured at 95°C for 5 min and then allowed to renature at 37 °C for 3 h. Frozen sections 10 μm thick were washed in 2 × SSC at 37 °C for 30 min and then dehydrated in ethanol. Sections for sheep FISH analysis were treated with 12.5 μg/ml proteinase K for 10 min at 37 °C, while sections for human FISH analysis were treated with 10 μg/ml proteinase K and incubated at room temperature. Sections were washed with water for 5 min and then 2 × SSC for 5 min and dehydrated in ice-cold ethanol. Sections were denatured at 85 °C for 3 min in preheated 70% formamide in 2 × SSC, pH 7.0, and then dehydrated with ice-cold ethanol. Probe was applied to sections at 45 °C, sealed with a coverslip, and incubated overnight at 42 °C. Coverslips were removed by immersing slides in 2 × SSC at 45 °C. Sections hybridized with sheep-specific probe were washed twice with preheated 50% formamide in 2 × SSC, pH 7.0, for 5 min and then washed with 0.1 × SSC twice, 5 min each, at 45 °C. Sections hybridized with human-specific probe were washed once with 2 × SSC, pH 7.0, at 45°C for 5 min and then washed twice with room-temperature PBS with 0.1% Triton X. Finally, sections were washed with PBS, treated with DAPI (Biogenex), and sealed with Cytoseal 60 for use with confocal microscopy.
Supplementary Material
Acknowledgments
This work was supported by HL70566 and HL73737 from the National Institutes of Health. E. Colletti is supported by CAT32 09563-19 from the National Institutes of Health. The authors thank Eileen Meredith for her help with the sheep model.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.scr.2008.08.002.
References
- Airey JA, Almeida-Porada G, Colletti EJ, Porada CD, Chamberlain J, Movsesian M, Sutko JL, Zanjani ED. Human mesenchymal stem cells form Purkinje fibers in fetal sheep heart. Circulation. 2004;109:1401–1407. doi: 10.1161/01.CIR.0000124222.16321.26. [DOI] [PubMed] [Google Scholar]
- Aliotta JM, Sanchez-Guijo FM, Dooner GJ, Johnson KW, Dooner MS, Greer KA, Greer D, Pimentel J, Kolankiewicz LM, Puente N, et al. Alteration of marrow cell gene expression, protein production, and engraftment into lung by lung-derived microvesicles: a novel mechanism for phenotype modulation. Stem Cells. 2007;25:2245–2256. doi: 10.1634/stemcells.2007-0128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida-Porada G, El Shabrawy D, Porada C, Zanjani ED. Differentiative potential of human metanephric mesenchymal cells. Exp Hematol. 2002;30:1454–1462. doi: 10.1016/s0301-472x(02)00967-0. [DOI] [PubMed] [Google Scholar]
- Almeida-Porada G, Porada C, Zanjani ED. Plasticity of human stem cells in the fetal sheep model of human stem cell transplantation. Int J Hematol. 2004;79:1–6. doi: 10.1007/BF02983526. [DOI] [PubMed] [Google Scholar]
- Alvarez-Dolado M, Pardal R, Garcia-Verdugo JM, Fike JR, Lee HO, Pfeffer K, Lois C, Morrison SJ, Alvarez-Buylla A. Fusion of bone-marrow-derived cells with Purkinje neurons, cardiomyocytes and hepatocytes. Nature. 2003;425:968–973. doi: 10.1038/nature02069. [DOI] [PubMed] [Google Scholar]
- Anderson WM, Trgovcich-Zacok D. Carbocyanine dyes with long alkyl side-chains: broad spectrum inhibitors of mitochondrial electron transport chain activity. Biochem Pharmacol. 1995;49:1303–1311. doi: 10.1016/0006-2952(95)00060-d. [DOI] [PubMed] [Google Scholar]
- Barar J, Campbell L, Hollins AJ, Thomas NP, Smith MW, Morris CJ, Gumbleton M. Cell selective glucocorticoid induction of caveolin-1 and caveolae in differentiating pulmonary alveolar epithelial cell cultures. Biochem Biophys Res Commun. 2007;359:360–366. doi: 10.1016/j.bbrc.2007.05.106. [DOI] [PubMed] [Google Scholar]
- Camargo FD, Chambers SM, Goodell MA. Stem cell plasticity: from transdifferentiation to macrophage fusion. Cell Prolif. 2004;37:55–65. doi: 10.1111/j.1365-2184.2004.00300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AI. The mesengenic process. Clin Plast Surg. 1994;21:429–435. [PubMed] [Google Scholar]
- Chamberlain J, Yamagami T, Colletti E, Theise ND, Desai J, Frias A, Pixley J, Zanjani ED, Porada CD, Almeida-Porada G. Efficient generation of human hepatocytes by the intrahepatic delivery of clonal human mesenchymal stem cells in fetal sheep. Hepatology. 2007;46:1935–1945. doi: 10.1002/hep.21899. [DOI] [PubMed] [Google Scholar]
- Friedenstein AJ. Osteogenic stem cells in the bone marrow. Bone Miner Res. 1990;7:243–272. [Google Scholar]
- Fukuchi Y, Nakajima H, Sugiyama D, Hirose I, Kitamura T, Tsuji K. Human placenta-derived cells have mesenchymal stem/progenitor cell potential. Stem Cells. 2004;22:649–658. doi: 10.1634/stemcells.22-5-649. [DOI] [PubMed] [Google Scholar]
- Gouon-Evans V, Boussemart L, Gadue P, Nierhoff D, Koehler CI, Kubo A, Shafritz DA, Keller G. BMP-4 is required for hepatic specification of mouse embryonic stem cell-derived definitive endoderm. Nat Biotechnol. 2006;24:1402–1411. doi: 10.1038/nbt1258. [DOI] [PubMed] [Google Scholar]
- In ‘t Anker PS, Noort WA, Kruisselbrink AB, Scherjon SA, Beekhuizen W, Willemze R, Kanhai HH, Fibbe WE. Nonexpanded primary lung and bone marrow-derived mesenchymal cells promote the engraftment of umbilical cord blood-derived CD34(+) cells in NOD/SCID mice. Exp Hematol. 2003;31:881–889. doi: 10.1016/s0301-472x(03)00202-9. [DOI] [PubMed] [Google Scholar]
- In ‘t Anker PS, Scherjon SA, Kleijburg-van der Keur C, de Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells. 2004;22:1338–1345. doi: 10.1634/stemcells.2004-0058. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Jahagirdar BN, Reinhardt RL, Schwartz RE, Keene CD, Ortiz-Gonzalez XR, Reyes M, Lenvik T, Lund T, Blackstad M, et al. Pluripotency of mesenchymal stem cells derived from adult marrow. Nature. 2002;418:41–49. doi: 10.1038/nature00870. [DOI] [PubMed] [Google Scholar]
- Kempf M, Clement A, Faissner A, Lee G, Brandt R. Tau binds to the distal axon early in development of polarity in a microtubule- and microfilament-dependent manner. J Neurosci. 1996;16:5583–5592. doi: 10.1523/JNEUROSCI.16-18-05583.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoor A, Stahlman MT, Gray ME, Whitsett JA. Temporal–spatial distribution of SP-B and SP-C proteins and mRNAs in developing respiratory epithelium of human lung. J Histochem Cytochem. 1994;42:1187–1199. doi: 10.1177/42.9.8064126. [DOI] [PubMed] [Google Scholar]
- Kogler G, Sensken S, Airey JA, Trapp T, Muschen M, Feldhahn N, Liedtke S, Sorg RV, Fischer J, Rosenbaum C, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kucia M, Ratajczak J, Ratajczak MZ. Are bone marrow stem cells plastic or heterogenous—that is the question. Exp Hematol. 2005;33:613–623. doi: 10.1016/j.exphem.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Kucia M, Halasa M, Wysoczynski M, Baskiewicz-Masiuk M, Moldenhawer S, Zuba-Surma E, Czajka R, Wojakowski W, Machalinski B, Ratajczak MZ. Morphological and molecular characterization of novel population of CXCR4+ SSEA-4+ Oct-4+ very small embryonic-like cells purified from human cord blood—preliminary report. Leukemia. 2006a;21:297–303. doi: 10.1038/sj.leu.2404470. [DOI] [PubMed] [Google Scholar]
- Kucia M, Reca R, Campbell FR, Zuba-Surma E, Majka M, Ratajczak J, Ratajczak MZ. A population of very small embryonic-like (VSEL) CXCR4(+)SSEA-1(+)Oct-4+ stem cells identified in adult bone marrow. Leukemia. 2006b;20:857–869. doi: 10.1038/sj.leu.2404171. [DOI] [PubMed] [Google Scholar]
- Lerou PH, Daley GQ. Therapeutic potential of embryonic stem cells. Blood Rev. 2005;19:321–331. doi: 10.1016/j.blre.2005.01.005. [DOI] [PubMed] [Google Scholar]
- Liechty KW, MacKenzie TC, Shaaban AF, Radu A, Moseley AM, Deans R, Marshak DR, Flake AW. Human mesenchymal stem cells engraft and demonstrate site-specific differentiation after in utero transplantation in sheep. Nat Med. 2000;6:1282–1286. doi: 10.1038/81395. [DOI] [PubMed] [Google Scholar]
- Mahieu-Caputo D, Allain JE, Branger J, Coulomb A, Delgado JP, Andreoletti M, Mainot S, Frydman R, Leboulch P, Di Santo JP, et al. Repopulation of athymic mouse liver by cryopreserved early human fetal hepatoblasts. Hum Gene Ther. 2004;15:1219–1228. doi: 10.1089/hum.2004.15.1219. [DOI] [PubMed] [Google Scholar]
- Meyerrose TE, De Ugarte DA, Hofling AA, Herrbrich PE, Cordonnier TD, Shultz LD, Eagon JC, Wirthlin L, Sands MS, Hedrick MA, et al. In vivo distribution of human adipose-derived mesenchymal stem cells in novel xenotransplan-tation models. Stem Cells. 2007;25:220–227. doi: 10.1634/stemcells.2006-0243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nava S, Westgren M, Jaksch M, Tibell A, Broome U, Ericzon BG, Sumitran-Holgersson S. Characterization of cells in the developing human liver. Differentiation. 2005;73:249–260. doi: 10.1111/j.1432-0436.2005.00019.x. [DOI] [PubMed] [Google Scholar]
- O’Donoghue K, Choolani M, Chan J, de la Fuente J, Kumar S, Campagnoli C, Bennett PR, Roberts IA, Fisk NM. Identification of fetal mesenchymal stem cells in maternal blood: implications for non-invasive prenatal diagnosis. Mol Hum Reprod. 2003;9:497–502. doi: 10.1093/molehr/gag063. [DOI] [PubMed] [Google Scholar]
- Onfelt B, Nedvetzki S, Benninger RK, Purbhoo MA, Sowinski S, Hume AN, Seabra MC, Neil MA, French PM, Davis DM. Structurally distinct membrane nanotubes between human macrophages support long-distance vesicular traffic or surfing of bacteria. J Immunol. 2006;177:8476–8483. doi: 10.4049/jimmunol.177.12.8476. [DOI] [PubMed] [Google Scholar]
- Phinney DG, Prockop DJ. Mesenchymal stem/multipotent stromal cells: the state of transdifferentiation and modes of tissue repair—current views. Stem Cells. 2007;25:2896–2902. doi: 10.1634/stemcells.2007-0637. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Quah BJ, Warren HS, Parish CR. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye carboxyfluorescein diacetate succinimidyl ester. Nat Protoc. 2007;2:2049–2056. doi: 10.1038/nprot.2007.296. [DOI] [PubMed] [Google Scholar]
- Ratajczak J, Miekus K, Kucia M, Zhang J, Reca R, Dvorak P, Ratajczak MZ. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- Sackstein R, Merzaban JS, Cain DW, Dagia NM, Spencer JA, Lin CP, Wohlgemuth R. Ex vivo glycan engineering of CD44 programs human multipotent mesenchymal stromal cell trafficking to bone. Nat Med. 2008;14:181–187. doi: 10.1038/nm1703. [DOI] [PubMed] [Google Scholar]
- Sarnat HB, Born DE. Synaptophysin immunocytochemistry with thermal intensification: a marker of terminal axonal maturation in the human fetal nervous system. Brain Dev. 1999;21:41–50. doi: 10.1016/s0387-7604(98)00068-0. [DOI] [PubMed] [Google Scholar]
- Schrepfer S, Deuse T, Reichenspurner H, Fischbein MP, Robbins RC, Pelletier MP. Stem cell transplantation: the lung barrier. Transplant Proc. 2007;39:573–576. doi: 10.1016/j.transproceed.2006.12.019. [DOI] [PubMed] [Google Scholar]
- Scott EW. Stem cell plasticity or fusion: two approaches to targeted cell therapy. Blood Cells Mol Dis. 2004;32:65–67. doi: 10.1016/j.bcmd.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Simmons PJ, Gronthos S, Zannettino A, Ohta S, Graves S. Isolation, characterization and functional activity of human marrow stromal progenitors in hemopoiesis. Prog Clin Biol Res. 1994;389:271–280. [PubMed] [Google Scholar]
- Slavik JM, Lim DG, Burakoff SJ, Hafler DA. Rapamycin-resistant proliferation of CD8+ T cells correlates with p27kip1 down-regulation and bcl-xL induction, and is prevented by an inhibitor of phosphoinositide 3-kinase activity. J Biol Chem. 2004;279:910–919. doi: 10.1074/jbc.M209733200. [DOI] [PubMed] [Google Scholar]
- Spees JL, Olson SD, Whitney MJ, Prockop DJ. Mitochondrial transfer between cells can rescue aerobic respiration. Proc Natl Acad Sci USA. 2006;103:1283–1288. doi: 10.1073/pnas.0510511103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131:861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- Theise ND, Krause DS. Toward a new paradigm of cell plasticity. Leukemia. 2002;16:542–548. doi: 10.1038/sj.leu.2402445. [DOI] [PubMed] [Google Scholar]
- Ukai R, Honmou O, Harada K, Houkin K, Hamada H, Kocsis JD. Mesenchymal stem cells derived from peripheral blood protects against ischemia. J Neurotrauma. 2007;24:508–520. doi: 10.1089/neu.2006.0161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vassilopoulos G, Wang PR, Russell DW. Transplanted bone marrow regenerates liver by cell fusion. Nature. 2003;422:901–904. doi: 10.1038/nature01539. [DOI] [PubMed] [Google Scholar]
- Verfaillie C. Stem cell plasticity. Hematology. 2005;10 (Suppl 1):293–296. doi: 10.1080/10245330512331390113. [DOI] [PubMed] [Google Scholar]
- Wagers AJ, Weissman IL. Plasticity of adult stem cells. Cell. 2004;116:639–648. doi: 10.1016/s0092-8674(04)00208-9. [DOI] [PubMed] [Google Scholar]
- Wang X, Ge S, McNamara G, Hao QL, Crooks GM, Nolta JA. Albumin-expressing hepatocyte-like cells develop in the livers of immune-deficient mice that received transplants of highly purified human hematopoietic stem cells. Blood. 2003a;101:4201–4208. doi: 10.1182/blood-2002-05-1338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Willenbring H, Akkari Y, Torimaru Y, Foster M, Al-Dhalimy M, Lagasse E, Finegold M, Olson S, Grompe M. Cell fusion is the principal source of bone-marrow-derived hepatocytes. Nature. 2003b;422:897–901. doi: 10.1038/nature01531. [DOI] [PubMed] [Google Scholar]
- Weimann JM, Johansson CB, Trejo A, Blau HM. Stable reprogrammed heterokaryons form spontaneously in Purkinje neurons after bone marrow transplant. Nat Cell Biol. 2003;5:959–966. doi: 10.1038/ncb1053. [DOI] [PubMed] [Google Scholar]
- Wernig M, Meissner A, Foreman R, Brambrink T, Ku M, Hochedlinger K, Bernstein BE, Jaenisch R. In vitro reprogramming of fibroblasts into a pluripotent ES-cell-like state. Nature. 2007;448:318–324. doi: 10.1038/nature05944. [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, et al. Induced pluripotent stem cell lines derived from human somatic cells. Science. 2007;318:1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- Zorov DB, Kobrinsky E, Juhaszova M, Sollott SJ. Examining intracellular organelle function using fluorescent probes: from animalcules to quantum dots. Circ Res. 2004;95:239–252. doi: 10.1161/01.RES.0000137875.42385.8e. [DOI] [PubMed] [Google Scholar]
- Zuk PA, Zhu M, Ashjian P, De Ugarte DA, Huang JI, Mizuno H, Alfonso ZC, Fraser JK, Benhaim P, Hedrick MH. Human adipose tissue is a source of multipotent stem cells. Mol Biol Cell. 2002;13:4279–4295. doi: 10.1091/mbc.E02-02-0105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvaifler NJ, Marinova-Mutafchieva L, Adams G, Edwards CJ, Moss J, Burger JA, Maini RN. Mesenchymal precursor cells in the blood of normal individuals. Arthritis Res. 2000;2:477–488. doi: 10.1186/ar130. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.