Abstract
Amyloid formation in the pancreas by islet amyloid polypeptide (IAPP) leads to β-cell death and dysfunction, contributing to islet transplant failure and to type-2 diabetes. IAPP is stored in the β-cell insulin secretory granules and cosecreted with insulin in response to β-cell secretagogues. IAPP is believed to play a role in the control of food intake, in controlling gastric emptying and in glucose homeostasis. The polypeptide is natively unfolded in its monomeric state, but is one of the most amyloidogenic sequences known. The mechanisms of IAPP amyloid formation in vivo and in vitro are not understood; the mechanisms of IAPP induced cell death are unclear; and the nature of the toxic species is not completely defined. Recent work is shedding light on these important issues.
Introduction
The presence of amyloid in the pancreatic islets of Langerhans is a pathophysiological feature of type-2 diabetes. Pancreatic islet amyloid deposits were first reported more than 110 years ago [1], but it was not until 1987 that a 37-residue polypeptide hormone, denoted as amylin or islet amyloid polypeptide (IAPP), was shown to be the protein component of islet amyloid [2,3]. IAPP is found in all mammals and is believed to play a role in controlling gastric emptying, glucose homeostasis and in the suppression of glucagon release [4]. IAPP is synthesized as a pre-proform [5], is processed in the Golgi and in the insulin secretory granule (Figure 1), and is released in response to stimuli which trigger insulin release. The concentration of IAPP in the granule is about 1–2% that of insulin. This is much higher than required to lead to rapid amyloid formation in vitro, so there must be mechanisms which inhibit irreversible aggregation in the granule [4].
Figure 1.

Post translational modification of human PreProIAPP to form the mature IAPP sequence: (a) The primary sequence of the 89-residue human PreProIAPP. The 22 residue signaling sequence is shown in black, the N-terminal and C-terminal proIAPP flanking regions are shown in blue, and the mature IAPP sequence is shown in red. (b) The primary sequence of the 67-residue human proIAPP. Before secretion, proIAPP is cleaved by the prohormone convertases PC2 and PC(1/3) at two dibasic sites, indicated by arrows. Further processing by the CPE/PAM complex results in an amidated Tyr at the C-terminus of mature IAPP. (c) The mature 37-residue human IAPP. The biologically active peptide has an intramolecular disulfide bridge between Cys-2 and Cys-7 and an amidated C-terminus. Positively charged residues are underlined in the ProIAPP and mature IAPP sequences.
The polypeptide is normally soluble and is natively unfolded in its monomeric state, but forms amyloid in type-2 diabetes (T2D) [2–4]. The process of islet amyloid formation leads to pancreatic β-cell dysfunction, cell death and the loss of islet β-cell mass [6–8]. Islet amyloid is not the cause of T2D; however it contributes to β-cell failure in T2D and the failure of islet cell transplantation [4,9,10•].
There is a large and growing body of work on the biophysics of IAPP amyloid formation and on the biological consequences of islet amyloid deposition. Unfortunately, space limitations prevent a detailed discussion of all aspects of the IAPP field and in this review we focus on the factors which control IAPP amyloid formation, on structural models of IAPP amyloid fibrils, on the nature of early intermediates, and on mechanisms of IAPP induced cytotoxicity. We provide citations to review articles which cover other topics.
Not all species form islet amyloid and its presence or absence correlates with differences in the primary sequence of IAPP
Mature IAPP is a 37 residue polypeptide that contains an intramolecular disulfide bridge between residues two and seven, and an amidated C-terminus (Figure 1). The only known polymorphism of mature human IAPP (hIAPP) that impacts amyloid formation in vivo is a Ser to Gly mutation at position 20, found at low levels in certain Asian populations [4]. This mutation accelerates amyloid formation in vitro [4,11]. Other factors leading to accelerated amyloid formation by hIAPP in vitro include spontaneous Asn deamidation. Asn deamidation can also lead to changes in the morphology of amyloid fibrils [12].
There is a correlation between the sequence of IAPP and its propensity to form amyloid (Figure 2) [13]. hIAPP, for example, forms amyloid readily while rat/mouse IAPP does not. The differences between the human and rat/ mouse sequences occur at only six out of 37 positions, five of which are located between residues 20–29. Rat/mouse IAPP contains three Pro residues at positions 25, 28 and 29, while the human sequence does not contain any. The inability of rat/mouse IAPP to form amyloid is attributed to the Pro substitutions, consistent with the secondary structure disrupting effect of Pro. A non-aggregating variant of hIAPP, Pramlintide, which contains proline residues at the same positions as found in the rat/mouse sequence, has been approved by the FDA for treatment of diabetes [14].
Figure 2.
Primary sequences of IAPP from different species: residues that differ from the human sequence are underlined and highlighted in red. Only partial sequences are available for rabbit and hare. The biologically active mature sequence has a disulfide bridge between Cys-2 and Cys-7 and an amidated C-terminus. Primates and cats have been reported to form islet amyloid while dogs, rodents and cows do not. Porcine and ferret IAPP are significantly less amyloidogenic than human IAPP. The degue forms islet amyloid, but the deposits are derived from aggregation of insulin, not IAPP.
Multiple Pro substitutions outside of the 20–29 region can abolish amyloid formation by hIAPP, as can replacement of Asn-14 or Asn-21 [15,16]. Conversely, replacement of residues Arg-18, Leu-23, and Val-26 in rat/mouse IAPP by their human counterparts leads to a weakly amyloidogenic polypeptide [17]. These studies show that the 20–29 sequence is not the only region of the polypeptide governing in vitro amyloid formation.
Monomeric IAPP does not adopt a compact globular structure, but it is not a random coil. The region encompassing residues 5–20 transiently populates helical φ, ψ angles in aqueous solution even though the level of persistent helical structure is low [18,19]. More persistent helical structure can be induced by negatively charged membranes [20] and NMR studies have delineated the conformation of IAPP in membrane mimetic environments [21].
Models of the hIAPP protofibril propose a parallel in-register β-structure with U-shaped monomers
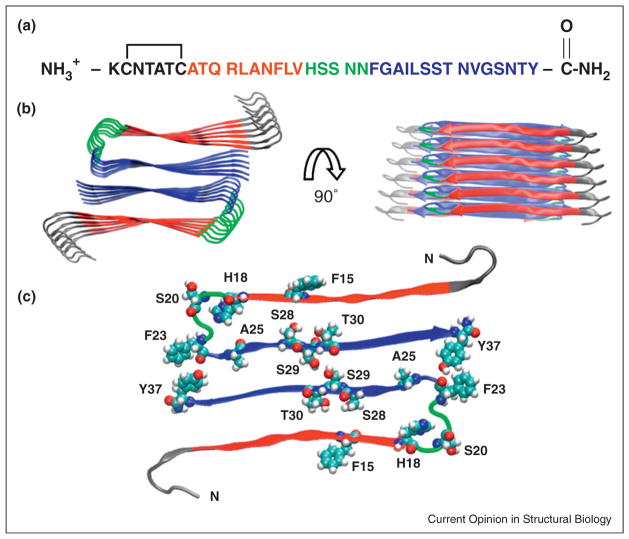
hIAPP amyloid fibrils consist of a cross-β arrangement of β-strands that run perpendicular to the fibril axis with the hydrogen bonds oriented parallel to the long axis of the fibril. Residues one to seven probably do not participate in β-structure because of conformational constraints imposed by the disulfide bridge. Several atomic models have been proposed for the hIAPP fibril and they share some common features. Two columns of symmetry related hIAPP monomers pack against each other in the basic assembly (Figure 3). Each polypeptide adopts a U-shaped structure and contains two β-strands connected by a bend-loop. There are no intrachain backbone hydrogen bonds. The β-strands form hydrogen bonds with adjacent polypeptide chains within the same column generating an intermolecular, parallel, in-register β-sheet (Figure 3). In the solid state NMR derived model from the NIH group the β-strands are comprised of residues 8–17 and 28–37 while the bend involves residues 18–27 [22].
Figure 3.
The structure of human IAPP: (a) The primary sequence of human IAPP. Residues 8–17 form intermolecular β-sheets in models of the IAPP amyloid fiber and are color coded in red. Residues 18–22, colored in green, form a bend in the UCLA model while residues 23–37, colored in blue, take part in an intermolecular β-sheet. The color coding corresponds to the IAPP amyloid structural model developed by the UCLA group [23]. The first 7 residues do not take part in β-sheet structure in existing models of IAPP amyloid. (b) A structural model of the IAPP fiber developed by the UCLA group [23]. Two views are shown: a top down view and an image rotated by 90 degrees. The color coding corresponds to that used in panel A. (c) A view of one layer of the stacked structure shown in panel (b). His-18, Ser-20, Ser-28, Ser-29, Thr-30 are shown in space-filling representation. The protonation state of His-18 affects the rate of amyloid formation [77]. A Ser-20 to Gly mutant is the only reported mutation in mature IAPP found in humans and has been shown to accelerate amyloid formation [4,11]. Ser-28, Ser-29 and Thr-30 form key inter-peptide contacts in the UCLA model. IAPP contains three aromatic residues (Phe-15, Phe-23, and Tyr-37) which are also highlighted.
Two other models have been proposed recently. The UCLA group has developed a model based on crystallographic studies of fragments of hIAPP [23] that shares many features with the solid state NMR model, but differs in how the two columns of hIAPP monomers pack against each other and in the length of the C-terminal β-strand (Figure 3). EPR studies of variants of hIAPP that lack the disulfide bond have led to a variation on these models. These experiments required the use of non conservative nitroxide spin labeled Cys mutants. The fibrils are still built up of U-shaped stacks of monomers, but the planes of the two β-strand regions within one IAPP molecule are staggered with respect to each other; the spacing between the β-strands is also larger than in other models [24].
An interesting feature of all three models is that a significant fraction of the 20–29 region is not part of a β-sheet, but forms a partially ordered bend that connects the two β-strands. This leads to obvious questions about the sensitivity of hIAPP amyloid formation to substitutions in the 20–29 region. In both the UCLA and NIH models, Ser-28 and Ser-29 make key contacts, suggesting that the Pro substitutions in rat/mouse IAPP could disrupt the interface (Figure 3). The bend may also impact the kinetics of amyloid formation, as structure has been postulated to form early in this region [25].
Amyloid formation in vitro may proceed via helical intermediates, but other mechanisms have been proposed
In vitro studies provide evidence for the population of helical intermediates during amyloid formation in homogenous solution [18,19,26,27]. Helix formation and self-association are linked in many systems; examples include peptides with a tendency to form amphiphilic helices and coiled coils. hIAPP has the potential to form an amphiphilic helix between residues 5–20 and the initial formation of oligomers may be driven by the linkage between helix formation and association. This would lead to a high local concentration of the amyloidogenic C-terminal region of hIAPP, which could promote intermolecular β-sheet formation. The ability of rat/mouse IAPP and some proline mutants of hIAPP to inhibit amyloid formation by wild type hIAPP are consistent with this model. These peptides have a tendency to form amphiphilic helices, but the prolines in their C-terminal region inhibit formation of β-sheet structure. This suggests that they could bind to helical oligomers and inhibit their conversion to β-structure, but it is important to note that their mechanism is not defined [28,29]. There are also small molecule inhibitors that are designed to target helical structure [30].
Ion mobility mass spectroscopy (IM-MS) combined with MD simulations have led to a different model of early intermediates [31]. The model proposes formation of side by side β-hairpin dimers. This structure requires a significant rearrangement of the backbone hydrogen bonding to form the stacked column structures found in the amyloid fibril models. IM-MS has the important advantage that it can separate different conformers in a heterogeneous mixture, but has the disadvantage that one must assume that conformations detected in the gas phase are representative of those populated by the dynamic peptide in solution.
A third model postulates a head to tail dimerization in which His-18 makes critical contacts with the C-terminal tyrosine [32]. A variant of hIAPP with a free C-terminal carboxylate rather than the physiological C-terminal amidated form was used in the studies to facilitate labeling for NMR, suggesting that there may be non-natural electrostatic interactions between the negatively charged C-terminus and His-18. It will be interesting to see if this model holds for the naturally occurring amidated peptide.
Anionic lipids and components of the extracellular matrix accelerate hIAPP amyloid formation in vitro
hIAPP has a net positive charge at all biologically relevant pH’s and interacts with negatively charged biopolymers, membranes and surfaces. Anionic membranes promote amyloid formation by hIAPP in vitro and more highly charged systems have a larger effect for high peptide to lipid ratios [33]. Many of the studies of hIAPP–membrane interactions have used simple model membranes with a mole fraction of anionic lipids that is significantly higher than that found in β-cell membranes, making it difficult to translate the results to the situation in vivo. More complicated model membranes containing phospholipids found in β-cell membranes, but which lack cholesterol, have also been shown to accelerate hIAPP amyloid formation, as have heterogeneous anionic model membranes that are capable of forming lipid rafts [34•,35,36]. The mechanism of membrane catalyzed hIAPP aggregation is not completely understood, but helical intermediates appear to be important [20,33,37•].
hIAPP amyloid formation in vivo has been proposed to be initiated by the binding of proIAPP processing intermediates to the glycosaminoglycan (GAG) chains of the heparan sulfate proteoglycan (HSPG) perlecan [4]. HSPGs are associated with in vivo hIAPP amyloid deposits, and secretion of an incompletely processed proIAPP intermediate, (NproIAPP), containing the N-terminal prosequence is increased in T2D [38,39]. Interactions with model GAGs accelerate amyloid formation by hIAPP and NproIAPP in vitro and NproIAPP amyloid can seed amyloid formation by mature hIAPP [40,41•]. Interestingly, NproIAPP interacts more weakly with anionic membranes than the mature sequence [42]. Inhibition of glycosaminoglycan synthesis reduces amyloid deposition in cultured islets, as does overexpression of heparanse in a double transgenic mouse model that overexpresses hIAPP, suggesting that interactions with HSPGs may be important in vivo [43,44]. Interactions with membranes and GAGs can impact the efficacy of amyloid inhibitors [45,46].
The mechanism of hIAPP induced β-cell toxicity and the initial site of amyloid deposition are open questions
Amyloid deposits observed in T2D appear to be extracellular and early histological studies with transgenic mouse models are consistent with an extracellular origin. Work with models in which IAPP is over expressed suggests that initial aggregation may occur intracellularly in the secretory pathway [4,47,48]. By contrast, a recent study shows that secretion of IAPP is an important determinate of β-cell toxicity and islet amyloid formation, arguing that islet amyloid has an extracellular origin. In that work, agents that increased secretion increased amyloid formation and toxicity, while inhibition of secretion of IAPP had the opposite effect [49•]. The difference between the studies may be related to the level at which IAPP is produced and the methods used to detect aggregates [4,49•,50]. The debate on an intracellular vs extracellular origin of islet amyloid is important since it impacts treatment strategies.
The process of amyloid formation by hIAPP is toxic to cultured β-cells and induces apoptosis and β-cell dysfunction in isolated human islets [6–8,44,51,52,53••]. The literature strongly suggests that there are multiple mechanisms of hIAPP induced β-cell dysfunction and cell death. Many of these overlap and share the same downstream signaling pathways.
IAPP has been proposed to exert its toxic effects by permeabilizing membranes [54–56]. The ability of IAPP to induce membrane leakage depends on lipid composition and on the lipid to peptide ratio as well as pH and ionic strength. The fraction of anionic lipids in the β-cell membrane is much lower than that employed in many biophysical studies of membrane leakage and the type of anionic lipids used are often very different [34•]. Most model systems also lack cholesterol and do not contain gangliosides. This may be important since recent work has shown that cholesterol and gangliosides play a role in mediating hIAPP membrane interactions and in the uptake and clearance of hIAPP [35,57–59]. We do not want to leave the impression that loss of membrane integrity is not important; this may indeed be one mechanism of toxicity, especially at high peptide concentrations. A range of studies have shown that hIAPP amyloid fibrils cluster on or near membranes and there is very good evidence that exogenously added IAPP perturbs cell membranes [54–57,60]. We believe that caution should be employed when extrapolating from mechanistic biophysical studies involving simple model membranes to the situation in vivo. Particularly since variants of hIAPP which do not induce β-cell death in vivo can disrupt some model membranes in vitro. More complicated and physiologically relevant model membrane systems are starting to be used and should provide new mechanistic insight [34–36]. The in vitro mechanism of hIAPP induced membrane disruption is an interesting open question, but space limitations preclude a detailed discussion [33,56,61].
ER stress and defects in ERAD have been proposed to be important factors in hIAPP induced β-cell death [47,48,62]. ProIAPP and not mature hIAPP may be the culprit, as the processing of proIAPP to mature hIAPP is completed in the Golgi and secretory granule [4]. Exogenously added hIAPP has also been reported to induce ER stress [63]. The exact role of ER stress in hIAPP mediated toxicity in vivo is currently unclear. Some of the studies that support a role for ER stress made use of transgenic rodent models that significantly over express hIAPP. By contrast, no ER stress was detected in cultured islets that produce IAPP at lower levels, and the overproduction of hIAPP has been suggested to be responsible for the differences [64].
Other proposed mechanisms of hIAPP toxicity include defects in autophagy, the enhanced production of pro-inflammatory cytokines, mitochondrial membrane damage and receptor-mediated mechanisms linked to oxidative stress and activation of signaling pathways leading to cell death [65–69,70••]. These pathological cellular processes can be triggered by either intracellular or extracellular aggregates. The pathways that mediate β-cell apoptosis in response to hIAPP amyloid formation are not completely characterized, but recent work has shed light on this important point. The cJUN N-terminal kinase (JNK) pathway is a critical pro-apoptotic pathway in β-cells and is activated by a range of stress stimuli. These include ER stress, oxidative stress, exposure to pro-inflammatory cytokines and high glucose. JNK mediates β-cell apoptosis in cultured cells and in islets exposed to high concentrations of hIAPP; it has recently been shown to do the same in response to amyloid generated from endogenous hIAPP [70••]. Downstream mediators have been identified in both intrinsic (Bim) and extrinsic (Fas, Fadd) pathways. Aggregation of both endogenous and exogenous hIAPP upregulates Fas and activates caspase pathways [65,71•], and deletion of Fas protects β-cells from hIAPP toxicity [71•]. hIAPP aggregation has also been shown to upregulate the terminal effector Casp3, and in vivo studies have shown that preventing Casp3 activation protects β-cells from hIAPP amyloid induced apoptosis [72].
Macroautophagy and chaperone-mediated autophagy lead to clearance of ubiquitinated proteins and autodigestion of abnormal or aged organelles by degradation in the lysosome. Defects in autophagy play a role in the toxicity of other amyloidogenic proteins. Recent studies have shown that over expression of hIAPP in β-cells leads to impaired autophagy; this effect has been demonstrated to occur before the development of hyperglycemia [66••,73]. Inhibition of autophagylysosomal degradation promotes hIAPP induced β-cell apoptosis while stimulation of autophagy protects β-cells from IAPP toxicity [66••].
hIAPP aggregates may promote β-cell dysfunction by triggering a localized inflammatory response, as well as by acting directly on β-cells [67••,69]. Recent reports highlight the role of inflammasomes in metabolic syndrome and provide evidence that hIAPP can stimulate their activity [67••]. Inflammasomes are protein complexes that recognize a range of pro-inflammatory stimuli and control the production of key pro-inflammatory cytokines, such as Interleukin-1β (IL-1β). Studies support a role for IL-1β in hIAPP-induced β-cell dysfunction and cell death.
New biophysical approaches hold the promise of a high resolution view of in vitro amyloid formation
Advances in 2DIR and isotopic labeling offer the prospect of obtaining site specific information about aggregation, but currently require high sample concentrations [25,74••]. Single particle methods provide insight into hIAPP membrane interactions [37•,75], while the development of non-invasive fluorescence probes allows the study of side chain solvation and backbone compaction in real time [76]. Advances in mass spectroscopy provide information about heterogeneous populations [31]. Continued refinement of these methods should lead to further advances. A key challenge will be to connect in vitro biophysical studies of model systems to the situation in vivo.
Conclusions
Progress has been made in understanding hIAPP amyloid formation, but important challenges remain. These include elucidating the mechanisms of islet amyloid formation in vivo and in vitro; identifying the initiation sites of amyloid formation in vivo; defining the nature of the toxic species and the mechanisms of cell death; elucidating the mechanisms of hIAPP clearance in vivo and the role such processes play in islet amyloid deposition. The development of inhibitors of hIAPP toxicity is also an area that warrants further effort, especially since many studies have relied on ex vivo assays using cultured cells and islets.
Acknowledgments
We thank Dr. S. Zraika, Dr. P. Marek, Ms H. Wang and Mr. V. Patsalo for helpful discussions and Mr. Patsalo for help in preparing figures. This work was supported by grants from the United States National Institutes of Health GM078114 to D.P.R.; and F32 DK089734-02 to A.A.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Opie EL. The relation of diabetes mellitus to lesions of the pancreas. Hyaline degeneration of the islands of Langerhans. J Exp Med. 1901;5:527–540. doi: 10.1084/jem.5.5.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Westermark P, Wernstedt C, Wilander E, Hayden DW, Obrien TD, Johnson KH. Amyloid fibrils in human insulinoma and islets of langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci USA. 1987;84:3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cooper GJS, Willis AC, Clark A, Turner RC, Sim RB, Reid KBM. Purification and characterization of a peptide from amyloid-rich pancreases of type-2 diabetic-patients. Proc Natl Acad Sci USA. 1987;84:8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Westermark P, Andersson A, Westermark GT. Islet amyloid polypeptide, islet amyloid, and diabetes mellitus. Physiol Rev. 2011;91:795–826. doi: 10.1152/physrev.00042.2009. [DOI] [PubMed] [Google Scholar]
- 5.Sanke T, Bell GI, Sample C, Rubenstein AH, Steiner DF. An islet amyloid peptide is derived from an 89-amino acid precursor by proteolytic processing. J Biol Chem. 1988;263:17243–17246. [PubMed] [Google Scholar]
- 6.Clark A, Wells CA, Buley ID, Cruickshank JK, Vanhegan RI, Matthews DR, Cooper GJS, Holman RR, Turner RC. Islet amyloid, increased alpha-cells, reduced beta-cells and exocrine fibrosis – quantitative changes in the pancreas in type-2 diabetes. Diabetes Res. 1988;9:151–159. [PubMed] [Google Scholar]
- 7.Lorenzo A, Razzaboni B, Weir GC, Yankner BA. Pancreatic-islet cell toxicity of amylin associated with type-2 diabetes-mellitus. Nature. 1994;368:756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- 8.Konarkowska B, Aitken JF, Kistler J, Zhang S, Cooper GJ. The aggregation potential of human amylin determines its cytotoxicity towards islet beta-cells. FEBS J. 2006;273:3614–3624. doi: 10.1111/j.1742-4658.2006.05367.x. [DOI] [PubMed] [Google Scholar]
- 9.Westermark GT, Westermark P, Berne C, Korsgren O, Transpla NNCI. Widespread amyloid deposition in transplanted human pancreatic islets. N Engl J Med. 2008;359:977–979. doi: 10.1056/NEJMc0802893. [DOI] [PubMed] [Google Scholar]
- 10•.Potter KJ, Abedini A, Marek P, Klimek AM, Butterworth S, Driscoll M, Baker R, Nilsson MR, Warnock GL, Oberholzer J, et al. Islet amyloid deposition limits the viability of human islet grafts but not porcine islet grafts. Proc Natl Acad Sci USA. 2010;107:4305–4310. doi: 10.1073/pnas.0909024107. The authors show that preventing amyloid formation enhances islet graft survival. Transplanted porcine islets survive longer than transplanted human islets and the effects correlate with the decreased amyloidogenicity and toxicity of porcine IAPP. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao P, Tu LH, Abedini A, Levsh O, Akter R, Patsalo V, Schmidt AM, Raleigh DP. Sensitivity of amyloid formation by human islet amyloid polypeptide to mutations at residue 20. J Mol Biol. 2012;421:282–295. doi: 10.1016/j.jmb.2011.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dunkelberger EB, Buchanan LE, Marek P, Cao P, Raleigh DP, Zanni MT. Deamidation accelerates amyloid formation and alters amylin fiber structure. J Am Chem Soc. 2012;134:12658–12667. doi: 10.1021/ja3039486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Westermark P, Engstrom U, Johnson KH, Westermark GT, Betsholtz C. Islet amyloid polypeptide – pinpointing aminoacid-residues linked to amyloid fibril formation. Proc Natl Acad Sci USA. 1990;87:5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ratner RE, Dickey R, Fineman M, Maggs DG, Shen L, Strobel SA, Weyer C, Kolterman OG. Amylin replacement with pramlintide as an adjunct to insulin therapy improves long-term glycaemic and weight control in Type 1 diabetes mellitus: a 1-year, randomized controlled trial. Diabet Med. 2004;21:1204–1212. doi: 10.1111/j.1464-5491.2004.01319.x. [DOI] [PubMed] [Google Scholar]
- 15.Abedini A, Raleigh DP. Destabilization of human IAPP amyloid fibrils by proline mutations outside of the putative amyloidogenic domain: is there a critical amyloidogenic domain in human IAPP? J Mol Biol. 2006;355:274–281. doi: 10.1016/j.jmb.2005.10.052. [DOI] [PubMed] [Google Scholar]
- 16.Koo BW, Hebda JA, Miranker AD. Amide inequivalence in the fibrillar assembly of islet amyloid polypeptide. Protein Eng Des Sel. 2008;21:147–154. doi: 10.1093/protein/gzm076. [DOI] [PubMed] [Google Scholar]
- 17.Green J, Goldsbury C, Min T, Sunderji S, Frey P, Kistler J, Cooper G, Aebi U. Full-length rat amylin forms fibrils following substitution of single residues from human amylin. J Mol Biol. 2003;326:1147–1156. doi: 10.1016/s0022-2836(02)01377-3. [DOI] [PubMed] [Google Scholar]
- 18.Williamson JA, Miranker AD. Direct detection of transient alpha-helical states in islet amyloid polypeptide. Protein Sci. 2007;16:110–117. doi: 10.1110/ps.062486907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Williamson JA, Loria JP, Miranker AD. Helix stabilization precedes aqueous and bilayer-catalyzed fiber formation in islet amyloid polypeptide. J Mol Biol. 2009;393:383–396. doi: 10.1016/j.jmb.2009.07.077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Knight JD, Hebda JA, Miranker AD. Conserved and cooperative assembly of membrane-bound alpha-helical states of islet amyloid polypeptide. Biochemistry. 2006;45:9496–9508. doi: 10.1021/bi060579z. [DOI] [PubMed] [Google Scholar]
- 21.Nanga RP, Brender JR, Xu J, Hartman K, Subramanian V, Ramamoorthy A. Three-dimensional structure and orientation of rat islet amyloid polypeptide protein in a membrane environment by solution NMR spectroscopy. J Am Chem Soc. 2009;131:8252–8261. doi: 10.1021/ja9010095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Luca S, Yau WM, Leapman R, Tycko R. Peptide conformation and supramolecular organization in amylin fibrils: constraints from solid-state NMR. Biochemistry. 2007;46:13505–13522. doi: 10.1021/bi701427q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wiltzius JJW, Sievers SA, Sawaya MR, Cascio D, Popov D, Riekel C, Eisenberg D. Atomic structure of the cross-beta spine of islet amyloid polypeptide (amylin) Protein Sci. 2008;17:1467–1474. doi: 10.1110/ps.036509.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bedrood S, Li YY, Isas JM, Hegde BG, Baxa U, Haworth IS, Langen R. Fibril structure of human islet amyloid polypeptide. J Biol Chem. 2012;287:5235–5241. doi: 10.1074/jbc.M111.327817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shim SH, Gupta R, Ling YL, Strasfeld DB, Raleigh DP, Zanni MT. Two-dimensional IR spectroscopy and isotope labeling defines the pathway of amyloid formation with residue-specific resolution. Proc Natl Acad Sci USA. 2009;106:6614–6619. doi: 10.1073/pnas.0805957106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abedini A, Raleigh DP. A role for helical intermediates in amyloid formation by natively unfolded polypeptides? Phys Biol. 2009;6:015005. doi: 10.1088/1478-3975/6/1/015005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wiltzius JJW, Sievers SA, Sawaya MR, Eisenberg D. Atomic structures of IAPP (amylin) fusions suggest a mechanism for fibrillation and the role of insulin in the process. Protein Sci. 2009;18:1521–1530. doi: 10.1002/pro.145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cao P, Meng F, Abedini A, Raleigh DP. The ability of rodent islet amyloid polypeptide to inhibit amyloid formation by human islet amyloid polypeptide has important implications for the mechanism of amyloid formation and the design of inhibitors. Biochemistry. 2010;49:872–881. doi: 10.1021/bi901751b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Meng FL, Raleigh DP, Abedini A. Combination of kinetically selected inhibitors in trans leads to highly effective inhibition of amyloid formation. J Am Chem Soc. 2010;132:14340–14342. doi: 10.1021/ja1046186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saraogi I, Hebda JA, Becerril J, Estroff LA, Miranker AD, Hamilton AD. Synthetic alpha-helix mimetics as agonists and antagonists of islet amyloid polypeptide aggregation. Angew Chem Int Ed. 2010;49:736–739. doi: 10.1002/anie.200901694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dupuis NF, Wu C, Shea JE, Bowers MT. The amyloid formation mechanism in human IAPP: dimers have beta-strand monomer-monomer interfaces. J Am Chem Soc. 2011;133:7240–7243. doi: 10.1021/ja1081537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wei L, Jiang P, Xu WX, Li H, Zhang H, Yan LY, Chan-Park MB, Liu XW, Tang K, Mu YG, et al. The molecular basis of distinct aggregation pathways of islet amyloid polypeptide. J Biol Chem. 2011;286:6291–6300. doi: 10.1074/jbc.M110.166678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hebda JA, Miranker AD. The interplay of catalysis and toxicity by amyloid intermediates on lipid bilayers: insights from type II diabetes. Annu Rev Biophys. 2009;38:125–152. doi: 10.1146/annurev.biophys.050708.133622. [DOI] [PubMed] [Google Scholar]
- 34•.Seeliger J, Weise K, Opitz N, Winter R. The effect of abeta on IAPP aggregation in the presence of an isolated beta-cell membrane. J Mol Biol. 2012;421:348–363. doi: 10.1016/j.jmb.2012.01.048. Membrane phosolipids from the rat insulinoma-derived INS-1E β-cell line were isolated and characterized. The ability of model membranes derived from these lipids to catalyze amyloid formation by IAPP was examined, and the ability of IAPP to permeabilize these membranes was tested. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wakabayashi M, Matsuzaki K. Ganglioside-induced amyloid formation by human islet amyloid polypeptide in lipid rafts. FEBS Lett. 2009;583:2854–2858. doi: 10.1016/j.febslet.2009.07.044. [DOI] [PubMed] [Google Scholar]
- 36.Weise K, Radovan D, Gohlke A, Opitz N, Winter R. Interaction of hIAPP with model raft membranes and pancreatic beta-cells: cytotoxicity of hIAPP oligomers. Chembiochem. 2010;11:1280–1290. doi: 10.1002/cbic.201000039. [DOI] [PubMed] [Google Scholar]
- 37•.Nath A, Miranker AD, Rhoades E. A membrane-bound antiparallel dimer of rat islet amyloid polypeptide. Angew Chem Int Ed. 2011;50:10859–10862. doi: 10.1002/anie.201102887. The authors characterized membrane-bound dimeric states of rat IAPP using a combination of intermolecular FRET and constrained Monte Carlo simulations. The model is the most detailed yet proposed for a membrane-bound rat IAPP oligomer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Paulsson JF, Westermark GT. Aberrant processing of human proislet amyloid polypeptide results in increased amyloid formation. Diabetes. 2005;54:2117–2125. doi: 10.2337/diabetes.54.7.2117. [DOI] [PubMed] [Google Scholar]
- 39.Marzban L, Rhodes CJ, Steiner DF, Haataja L, Halban PA, Verchere CB. Impaired NH2-terminal processing of human proislet amyloid polypeptide by the prohormone convertase PC2 leads to amyloid formation and cell death. Diabetes. 2006;55:2192–2201. doi: 10.2337/db05-1566. [DOI] [PubMed] [Google Scholar]
- 40.Meng F, Abedini A, Song B, Raleigh DP. Amyloid formation by pro-islet amyloid polypeptide processing intermediates: examination of the role of protein heparan sulfate interactions and implications for islet amyloid formation in type 2 diabetes. Biochemistry. 2007;46:12091–12099. doi: 10.1021/bi7004834. [DOI] [PubMed] [Google Scholar]
- 41•.Jha S, Patil SM, Gibson J, Nelson CE, Alder NN, Alexandrescu AT. Mechanism of amylin fibrillization enhancement by heparin. J Biol Chem. 2011;286:22894–22904. doi: 10.1074/jbc.M110.215814. The interactions of IAPP with GAG heparin fragments were characterized and the effects of oligosaccharide length and charge content on binding, fibrillization kinetics and cytotoxicity were investigated. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Khemtemourian L, Lahoz Casarramona G, Suylen DP, Hackeng TM, Meeldijk JD, de Kruijff B, Hoppener JW, Killian JA. Impaired processing of human pro-islet amyloid polypeptide is not a causative factor for fibril formation or membrane damage in vitro. Biochemistry. 2009;48:10918–10925. doi: 10.1021/bi901076d. [DOI] [PubMed] [Google Scholar]
- 43.Hull RL, Zraika S, Udayasankar J, Kisilevsky R, Szarek WA, Wight TN, Kahn SE. Inhibition of glycosaminoglycan synthesis and protein glycosylation with WAS-406 and azaserine result in reduced islet amyloid formation in vitro. Am J Physiol Cell Physiol. 2007;293:C1586–C1593. doi: 10.1152/ajpcell.00208.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Westermark GT, Westermark P. Localized amyloids important in diseases outside the brain – lessons from the islets of Langerhans and the thoracic aorta. FEBS J. 2011;278:3918–3929. doi: 10.1111/j.1742-4658.2011.08298.x. [DOI] [PubMed] [Google Scholar]
- 45.Engel MF, Vandenakker CC, Schleeger M, Velikov KP, Koenderink GH, Bonn M. The polyphenol EGCG inhibits amyloid formation less efficiently at phospholipid interfaces than in bulk solution. J Am Chem Soc. 2012;134:14781–14788. doi: 10.1021/ja3031664. [DOI] [PubMed] [Google Scholar]
- 46.Meng F, Raleigh DP. Inhibition of glycosaminoglycan-mediated amyloid formation by islet amyloid polypeptide and proIAPP processing intermediates. J Mol Biol. 2011;406:491–502. doi: 10.1016/j.jmb.2010.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Huang CJ, Lin CY, Haataja L, Gurlo T, Butler AE, Rizza RA, Butler PC. High expression rates of human islet amyloid polypeptide induce endoplasmic reticulum stress mediated beta-cell apoptosis, a characteristic of humans with type 2 but not type 1 diabetes. Diabetes. 2007;56:2016–2027. doi: 10.2337/db07-0197. [DOI] [PubMed] [Google Scholar]
- 48.Gurlo T, Ryazantsev S, Huang CJ, Yeh MW, Reber HA, Hines OJ, O’Brien TD, Glabe CG, Butler PC. Evidence for proteotoxicity in beta cells in type 2 diabetes: toxic islet amyloid polypeptide oligomers form intracellularly in the secretory pathway. Am J Pathol. 2010;176:861–869. doi: 10.2353/ajpath.2010.090532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49•.Aston-Mourney K, Hull RL, Zraika S, Udayasankar J, Subramanian SL, Kahn SE. Exendin-4 increases islet amyloid deposition but offsets the resultant beta cell toxicity in human islet amyloid polypeptide transgenic mouse islets. Diabetologia. 2011;54:1756–1765. doi: 10.1007/s00125-011-2143-3. The authors demonstrated that treatments which increase the secretion of IAPP from transgenic islets increased amyloid deposition, while treatments that decreased IAPP release lead to reduced amyloid formation. The results suggest that secretion of IAPP is necessary for amyloid formation and imply an extracellular origin of islet amyloid. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zraika S, Hull RL, Verchere CB, Clark A, Potter KJ, Fraser PE, Raleigh DP, Kahn SE. Toxic oligomers and islet beta cell death: guilty by association or convicted by circumstantial evidence? Diabetologia. 2010;53:1046–1056. doi: 10.1007/s00125-010-1671-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saafi EL, Konarkowska B, Zhang SP, Kistler J, Cooper GJS. Ultrastructural evidence that apoptosis is the mechanism by which human amylin evokes death in RINm5F pancreatic islet beta-cells. Cell Biol Int. 2001;25:339–350. doi: 10.1006/cbir.2000.0643. [DOI] [PubMed] [Google Scholar]
- 52.Butler AE, Janson J, Soeller WC, Butler PC. Increased beta-cell apoptosis prevents adaptive increase in beta-cell mass in mouse model of type 2 diabetes – evidence for role of islet amyloid formation rather than direct action of amyloid. Diabetes. 2003;52:2304–2314. doi: 10.2337/diabetes.52.9.2304. [DOI] [PubMed] [Google Scholar]
- 53••.Cooper GJS, Aitken JF, Zhang S. Is type 2 diabetes an amyloidosis and does it really matter (to patients)? Diabetologia. 2010;53:1011–1016. doi: 10.1007/s00125-010-1715-y. In this commentary, the authors discussed if type 2 diabetes should be classified as a form of amyloidosis, and emphasized that human IAPP aggregation is linked to the pathogenesis of diabetes. [DOI] [PubMed] [Google Scholar]
- 54.Janson J, Ashley RH, Harrison D, McIntyre S, Butler PC. The mechanism of islet amyloid polypeptide toxicity is membrane disruption by intermediate-sized toxic amyloid particles. Diabetes. 1999;48:491–498. doi: 10.2337/diabetes.48.3.491. [DOI] [PubMed] [Google Scholar]
- 55.Mirzabekov TA, Lin MC, Kagan BL. Pore formation by the cytotoxic islet amyloid peptide amylin. J Biol Chem. 1996;271:1988–1992. doi: 10.1074/jbc.271.4.1988. [DOI] [PubMed] [Google Scholar]
- 56.Khemtemourian L, Killian JA, Hoppener JWM, Engel MFM. Recent insights in islet amyloid polypeptide-induced membrane disruption and its role in beta-cell death in type 2 diabetes mellitus. Exp Diabetes Res. 2008;2008:421287. doi: 10.1155/2008/421287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Xu W, Wei G, Su H, Nordenskiold L, Mu Y. Effects of cholesterol on pore formation in lipid bilayers induced by human islet amyloid polypeptide fragments: a coarse-grained molecular dynamics study. Phys Rev E. 2011;84:051922. doi: 10.1103/PhysRevE.84.051922. [DOI] [PubMed] [Google Scholar]
- 58.Trikha S, Jeremic AM. Clustering and internalization of toxic amylin oligomers in pancreatic cells require plasma membrane cholesterol. J Biol Chem. 2011;286:36086–36097. doi: 10.1074/jbc.M111.240762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cho WJ, Jena BP, Jeremic AM. Nano-scale imaging and dynamics of amylin-membrane interactions and its implication in type II diabetes mellitus. Methods Cell Biol. 2008;90:267–286. doi: 10.1016/S0091-679X(08)00813-3. [DOI] [PubMed] [Google Scholar]
- 60.Demuro A, Mina E, Kayed R, Milton SC, Parker I, Glabe CG. Calcium dysregulation and membrane disruption as a ubiquitous neurotoxic mechanism of soluble amyloid oligomers. J Biol Chem. 2005;280:17294–17300. doi: 10.1074/jbc.M500997200. [DOI] [PubMed] [Google Scholar]
- 61.Brender JR, Salamekh S, Ramamoorthy A. Membrane disruption and early events in the aggregation of the diabetes related peptide IAPP from a molecular perspective. Acc Chem Res. 2012;45:454–462. doi: 10.1021/ar200189b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Costes S, Huang CJ, Gurlo T, Daval M, Matveyenko AV, Rizza RA, Butler AE, Butler PC. beta-cell dysfunctional ERAD/ubiquitin/ proteasome system in type 2 diabetes mediated by islet amyloid polypeptide-induced UCH-L1 deficiency. Diabetes. 2011;60:227–238. doi: 10.2337/db10-0522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Casas S, Gomis R, Gribble FM, Altirriba J, Knuutila S, Novials A. Impairment of the ubiquitin-proteasome pathway is a downstream endoplasmic reticulum stress response induced by extracellular human islet amyloid polypeptide and contributes to pancreatic beta-cell apoptosis. Diabetes. 2007;56:2284–2294. doi: 10.2337/db07-0178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Hull RL, Zraika S, Udayasankar J, Aston-Mourney K, Subramanian SL, Kahn SE. Amyloid formation in human IAPP transgenic mouse islets and pancreas, and human pancreas, is not associated with endoplasmic reticulum stress. Diabetologia. 2009;52:1102–1111. doi: 10.1007/s00125-009-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhang S, Liu J, Dragunow M, Cooper GJ. Fibrillogenic amylin evokes islet beta-cell apoptosis through linked activation of a caspase cascade and JNK1. J Biol Chem. 2003;278:52810–52819. doi: 10.1074/jbc.M308244200. [DOI] [PubMed] [Google Scholar]
- 66••.Rivera JF, Gurlo T, Daval M, Huang CJ, Matveyenko AV, Butler PC, Costes S. Human-IAPP disrupts the autophagy/lysosomal pathway in pancreatic beta-cells: protective role of p62 positive cytoplasmic inclusions. Cell Death Differ. 2011;18:415–426. doi: 10.1038/cdd.2010.111. Increased expression of human IAPP is shown to lead to impaired autophagy via disruption of lysosome mediated degradation. Inhibition of lysosomal degradation increased the susceptibility of β-cells to IAPP toxicity while stimulating autophagy had a protective effect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67••.Masters SL, Dunne A, Subramanian SL, Hull RL, Tannahill GM, Sharp FA, Becker C, Franchi L, Yoshihara E, Chen Z, et al. Activation of the NLRP3 inflammasome by islet amyloid polypeptide provides a mechanism for enhanced IL-1beta in type 2 diabetes. Nat Immunol. 2010;11:897–904. doi: 10.1038/ni.1935. The authors showed that human IAPP oligomers activate the NLRP3 inflammasome and thereby lead to the production of mature interleukin-1β and β-cell death. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zraika S, Hull RL, Udayasankar J, Aston-Mourney K, Subramanian SL, Kisilevsky R, Szarek WA, Kahn SE. Oxidative stress is induced by islet amyloid formation and time-dependently mediates amyloid-induced beta cell apoptosis. Diabetologia. 2009;52:626–635. doi: 10.1007/s00125-008-1255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Westwell-Roper C, Dai DL, Soukhatcheva G, Potter KJ, van Rooijen N, Ehses JA, Verchere CB. IL-1 blockade attenuates islet amyloid polypeptide-induced proinflammatory cytokine release and pancreatic islet graft dysfunction. J Immunol. 2011;187:2755–2765. doi: 10.4049/jimmunol.1002854. [DOI] [PubMed] [Google Scholar]
- 70••.Subramanian SL, Hull RL, Zraika S, Aston-Mourney K, Udayasankar J, Kahn SE. cJUN N-terminal kinase (JNK) activation mediates islet amyloid-induced beta cell apoptosis in cultured human islet amyloid polypeptide transgenic mouse islets. Diabetologia. 2012;55:166–174. doi: 10.1007/s00125-011-2338-7. The authors show that activation of JNK by endogenous hIAPP mediates apoptosis in cultured islets. Downstream mediators were identified in both the extrinsic and intrinsic pathways. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71•.Park YJ, Lee S, Kieffer TJ, Warnock GL, Safikhan N, Speck M, Hao Z, Woo M, Marzban L. Deletion of Fas protects islet beta cells from cytotoxic effects of human islet amyloid polypeptide. Diabetologia. 2012;55:1035–1047. doi: 10.1007/s00125-012-2451-2. Treatment of β-cells, human IAPP expressing transgenic mouse islets and human islets with human IAPP lead to upregulation of Fas, caspase-3 activation and apoptosis. Deletion of Fas protected β-cells from the cytotoxicity effects of endogenously secreted and exogenously applied hIAPP. [DOI] [PubMed] [Google Scholar]
- 72.Law E, Lu S, Kieffer TJ, Warnock GL, Ao Z, Woo M, Marzban L. Differences between amyloid toxicity in alpha and beta cells in human and mouse islets and the role of caspase-3. Diabetologia. 2010;53:1415–1427. doi: 10.1007/s00125-010-1717-9. [DOI] [PubMed] [Google Scholar]
- 73.Morita S, Sakagashira S, Shimajiri Y, Eberhardt NL, Kondo T, Kondo T, Sanke T. Autophagy protects against human islet amyloid polypeptide-associated apoptosis. J Diabetes Invest. 2011;2:48–55. doi: 10.1111/j.2040-1124.2010.00065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74••.Middleton CT, Marek P, Cao P, Chiu CC, Singh S, Woys AM, de Pablo JJ, Raleigh DP, Zanni MT. Two-dimensional infrared spectroscopy reveals the complex behaviour of an amyloid fibril inhibitor. Nat Chem. 2012;4:355–360. doi: 10.1038/nchem.1293. The authors used two-dimensional infrared spectroscopy to study the inhibition of hIAPP amyloid formation by rat IAPP. Rat IAPP blocks the N-terminal β-sheet and ultimately forms its own β-sheets on the exterior of the human IAPP fibrils. This study provides the first example of a site specific investigation of the disaggregation of amyloid fibers. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Last NB, Rhoades E, Miranker AD. Islet amyloid polypeptide demonstrates a persistent capacity to disrupt membrane integrity. Proc Natl Acad Sci USA. 2011;108:9460–9465. doi: 10.1073/pnas.1102356108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Marek P, Mukherjee S, Zanni MT, Raleigh DP. Residue-specific, real-time characterization of lag-phase species and fibril growth during amyloid formation: a combined fluorescence and IR study of p-cyanophenylalanine analogs of islet amyloid polypeptide. J Mol Biol. 2010;400:878–888. doi: 10.1016/j.jmb.2010.05.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Abedini A, Raleigh DP. The role of His-18 in amyloid formation by human islet amyloid polypeptide. Biochemistry. 2005;44:16284–16291. doi: 10.1021/bi051432v. [DOI] [PubMed] [Google Scholar]