Abstract
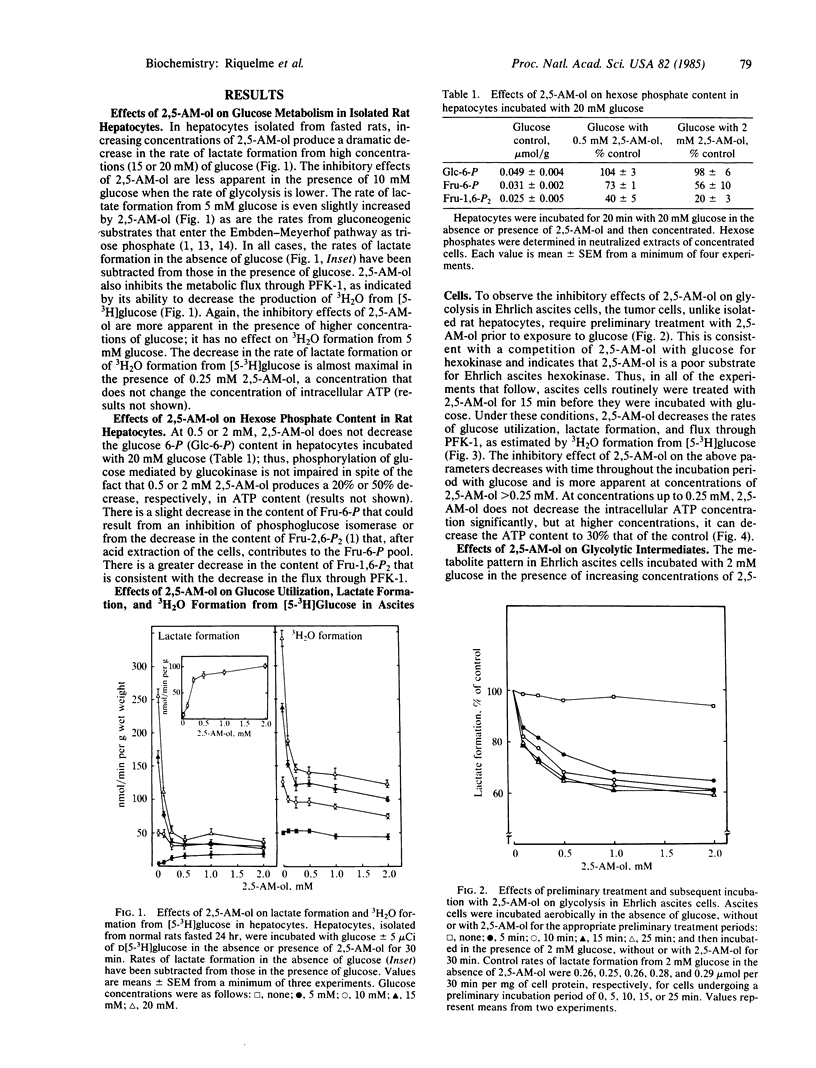
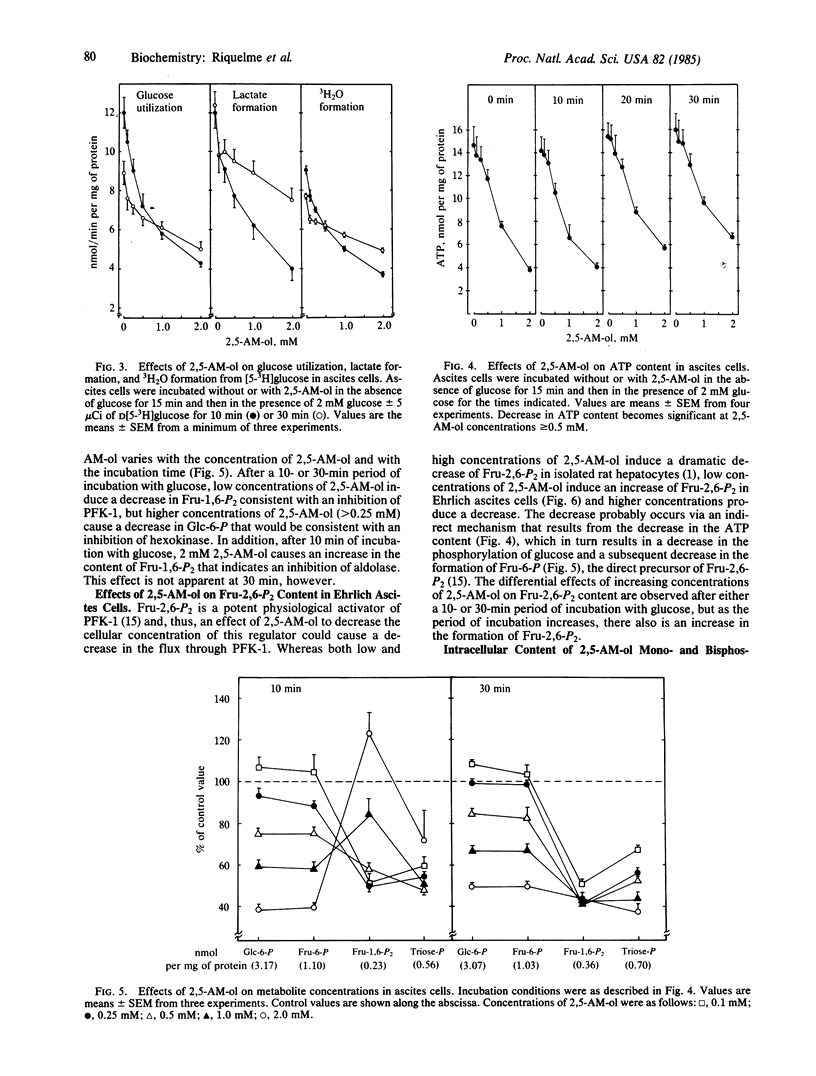
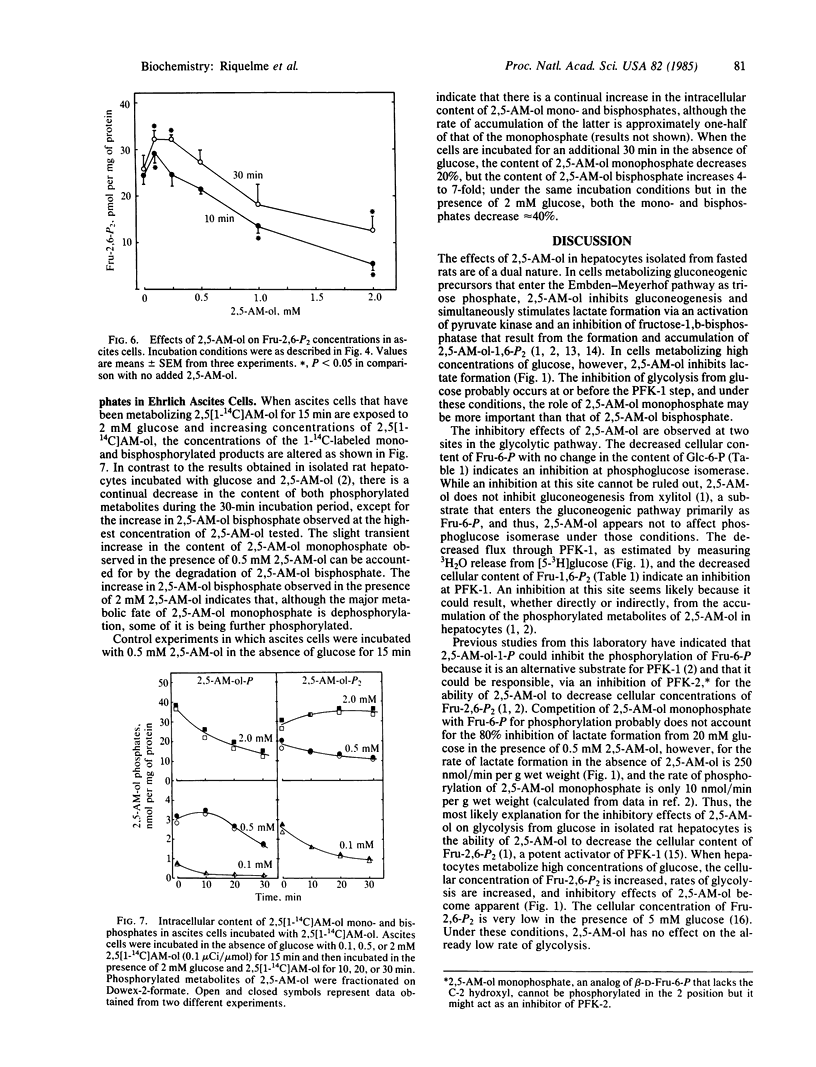
2,5-Anhydromannitol decreases lactate formation and 3H2O formation from [5-3H]glucose in isolated rat hepatocytes metabolizing high concentrations of glucose. The inhibition of glycolysis is accompanied by a slight decrease in the cellular content of fructose-6-P and a more substantial decrease in the cellular content of fructose-1,6-P2, with no change in the content of glucose-6-P. The 3H2O release data and changes in hexosephosphate distribution indicate possible inhibitions at phosphofructokinase-1 and phosphoglucose isomerase. 2,5-Anhydromannitol also inhibits glycolysis in Ehrlich ascites cells, but the tumor cells, unlike hepatocytes, must be treated with 2,5-anhydromannitol prior to exposure to glucose to obtain the inhibition. The decrease in 3H2O formation from [5-3H]glucose and the metabolite pattern that results from the addition of low concentrations (less than or equal to 0.25 mM) of 2,5-anhydromannitol indicate an inhibition at phosphofructokinase-1 that cannot be attributed to a decrease in the cellular content of fructose-2,6-P2. Higher concentrations (greater than or equal to 0.5 mM) of 2,5-anhydromannitol cause a substantial decrease in the cellular content of ATP that is accompanied by decreases in the content of glucose-6-P and fructose-6-P and transient increases in fructose-1,6-P2. In Ehrlich ascites cells, 2,5-anhydromannitol is metabolized to 2,5-anhydromannitol mono- and bisphosphate. The inhibition of glycolysis caused by 2,5-anhydromanitol decreases with time, because the phosphorylated metabolites formed during the preliminary incubation in the absence of glucose are rapidly dephosphorylated during the incubation in the presence of glucose.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartrons R., Van Schaftingen E., Vissers S., Hers H. G. The stimulation of yeast phosphofructokinase by fructose 2,6-bisphosphate. FEBS Lett. 1982 Jun 21;143(1):137–140. doi: 10.1016/0014-5793(82)80290-1. [DOI] [PubMed] [Google Scholar]
- Boscá L., Aragón J. J., Sols A. Specific activation by fructose 2,6-bisphosphate and inhibition by P-enolpyruvate of ascites tumor phosphofructokinase. Biochem Biophys Res Commun. 1982 May 31;106(2):486–491. doi: 10.1016/0006-291x(82)91136-6. [DOI] [PubMed] [Google Scholar]
- Bény M., Dolivo M. Separation of firefly luciferase using an anion exchanger. FEBS Lett. 1976 Nov;70(1):167–170. doi: 10.1016/0014-5793(76)80750-8. [DOI] [PubMed] [Google Scholar]
- Clark M. G., Kneer N. M., Bosch A. L., Lardy H. A. The fructose 1,6-diphosphatase-phosphofructokinase substrate cycle. A site of regulation of hepatic gluconeogenesis by glucagon. J Biol Chem. 1974 Sep 25;249(18):5695–5703. [PubMed] [Google Scholar]
- Cornell N. W. Rapid fractionation of cell suspensions with the use of brominated hydrocarbons. Anal Biochem. 1980 Mar 1;102(2):326–331. doi: 10.1016/0003-2697(80)90162-1. [DOI] [PubMed] [Google Scholar]
- Hanson R. L., Ho R. S., Wiseberg J. J., Simpson R., Younathan E. S., Blair J. B. Inhibition of gluconeogenesis and glycogenolysis by 2,5-anhydro-D-mannitol. J Biol Chem. 1984 Jan 10;259(1):218–223. [PubMed] [Google Scholar]
- Hers H. G., Van Schaftingen E. Fructose 2,6-bisphosphate 2 years after its discovery. Biochem J. 1982 Jul 15;206(1):1–12. doi: 10.1042/bj2060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kneer N. M., Wagner M. J., Lardy H. A. Regulation by calcium of hormonal effects on gluconeogenesis. J Biol Chem. 1979 Dec 10;254(23):12160–12168. [PubMed] [Google Scholar]
- Riquelme P. T., Wernette-Hammond M. E., Kneer N. M., Lardy H. A. Mechanism of action of 2,5-anhydro-D-mannitol in hepatocytes. Effects of phosphorylated metabolites on enzymes of carbohydrate metabolism. J Biol Chem. 1984 Apr 25;259(8):5115–5123. [PubMed] [Google Scholar]
- Riquelme P. T., Wernette-Hammond M. E., Kneer N. M., Lardy H. A. Regulation of carbohydrate metabolism by 2,5-anhydro-D-mannitol. Proc Natl Acad Sci U S A. 1983 Jul;80(14):4301–4305. doi: 10.1073/pnas.80.14.4301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischler M. E., Hecht P., Williamson J. R. Determination of mitochondrial/cytosolic metabolite gradients in isolated rat liver cells by cell disruption. Arch Biochem Biophys. 1977 May;181(1):278–293. doi: 10.1016/0003-9861(77)90506-9. [DOI] [PubMed] [Google Scholar]
- VINUELA E., SALAS M. L., SOLS A. End-product inhibition of yeast phosphofructokinase by ATP. Biochem Biophys Res Commun. 1963 Jul 18;12:140–145. doi: 10.1016/0006-291x(63)90250-x. [DOI] [PubMed] [Google Scholar]
- Van Schaftingen E., Hue L., Hers H. G. Control of the fructose-6-phosphate/fructose 1,6-bisphosphate cycle in isolated hepatocytes by glucose and glucagon. Role of a low-molecular-weight stimulator of phosphofructokinase. Biochem J. 1980 Dec 15;192(3):887–895. doi: 10.1042/bj1920887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Jett M. F., Hue L., Hers H. G. Control of liver 6-phosphofructokinase by fructose 2,6-bisphosphate and other effectors. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3483–3486. doi: 10.1073/pnas.78.6.3483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Schaftingen E., Lederer B., Bartrons R., Hers H. G. A kinetic study of pyrophosphate: fructose-6-phosphate phosphotransferase from potato tubers. Application to a microassay of fructose 2,6-bisphosphate. Eur J Biochem. 1982 Dec;129(1):191–195. doi: 10.1111/j.1432-1033.1982.tb07039.x. [DOI] [PubMed] [Google Scholar]
- Wehrle J. P., Pedersen P. L. Characteristics of phosphate uptake by Ehrlich ascites tumor cells. J Biol Chem. 1982 Aug 25;257(16):9698–9703. [PubMed] [Google Scholar]


