Abstract
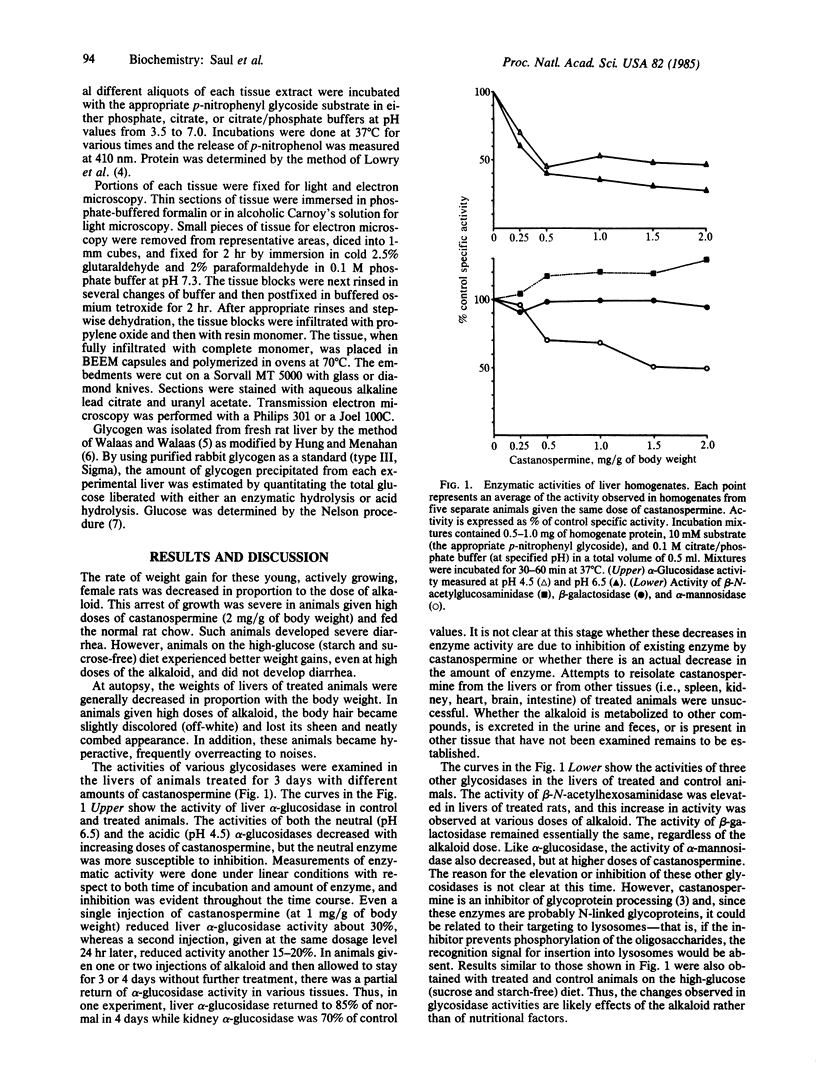
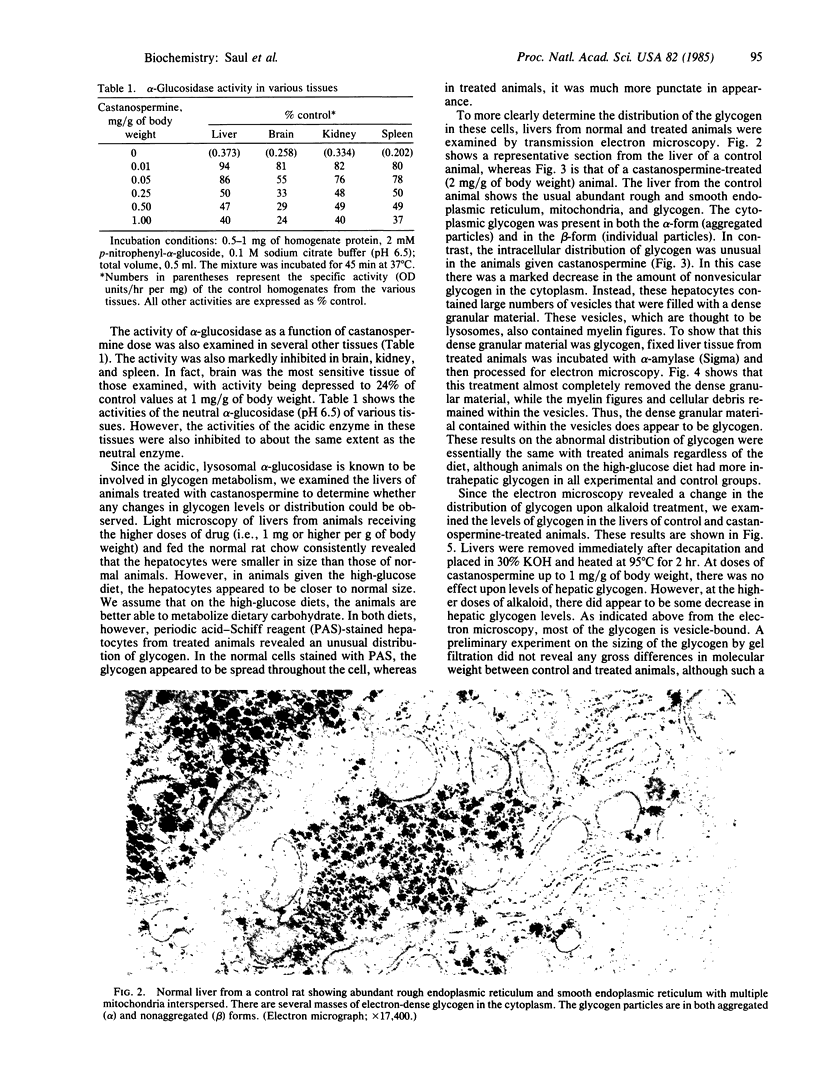
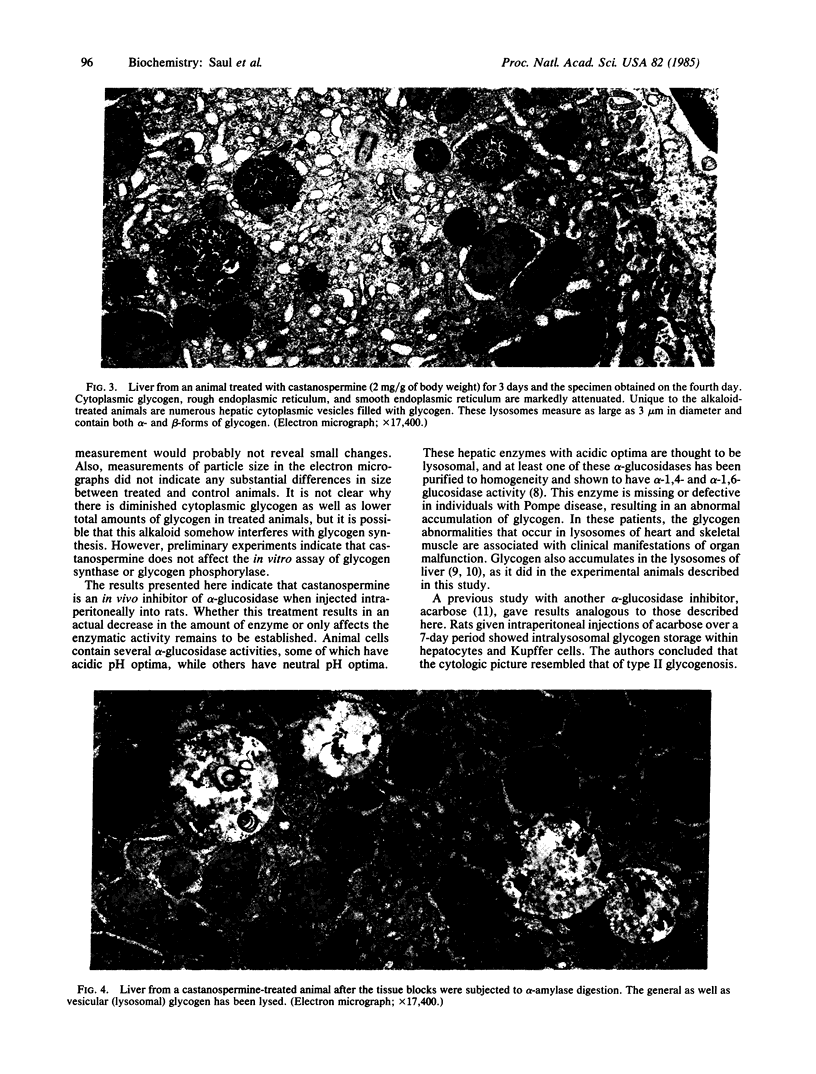
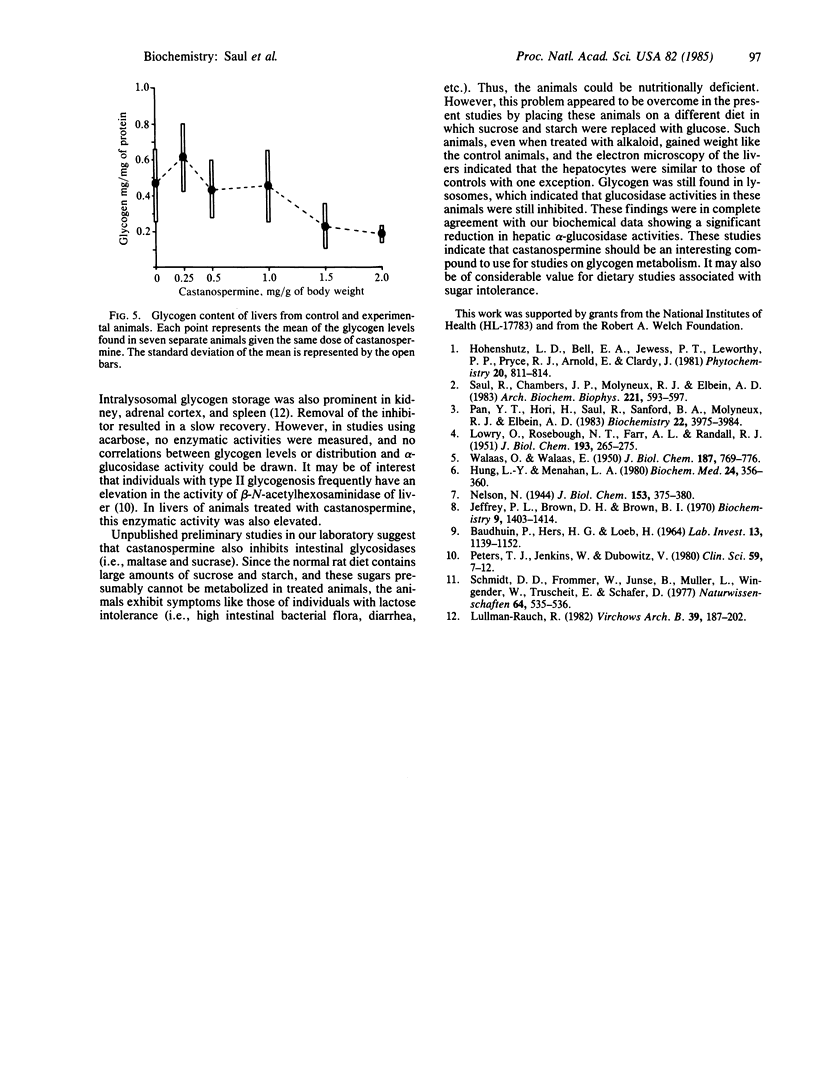
Castanospermine, an inhibitor of alpha-glucosidase activity, was injected into rats to determine its effects in vivo. Daily injections of alkaloid, at levels of 0.5 mg/g of body weight, or higher, for 3 days decreased hepatic alpha-glucosidase to 40% of control values, whereas alpha-glucosidase in brain was reduced to 25% of control values and that in spleen and kidney was reduced to about 40%. In liver, both the neutral (pH 6.5) and the acidic (pH 4.5) alpha-glucosidase activities were inhibited, but the former was more susceptible. On the other hand, beta-N-acetylhexosaminidase activity was elevated in the livers of treated animals, whereas beta-galactosidase activity was unchanged and alpha-mannosidase activity was somewhat inhibited. Livers of treated animals were examined by light and electron microscopy and compared to control animals to determine whether changes in morphology had occurred. In treated animals fed normal rat chow, the hepatocytes were smaller in size and simplified in structure, whereas the high-glucose diet lessened these alterations. Furthermore, in those animals receiving castanospermine at 1.0 mg or higher per g of body weight for 3 days, there was a marked decrease in the amount of glycogen in the cytoplasm, while a large number of lysosomes were observed that were full of dense, granular material. That this dense material was indeed glycogen was shown by the fact that it disappeared when blocks of fixed tissue were pretreated with alpha-amylase. Glycogen levels in liver, as measured either colorimetrically or enzymatically, were somewhat depressed at the higher levels of castanospermine.
Full text
PDF
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BAUDHUIN P., HERS H. G., LOEB H. AN ELECTRON MICROSCOPIC AND BIOCHEMICAL STUDY OF TYPE II GLYCOGENOSIS. Lab Invest. 1964 Sep;13:1139–1152. [PubMed] [Google Scholar]
- Hung L. Y., Menahan L. a. A comparison of direct enzymatic determination of glycogen in liver and heart. Biochem Med. 1980 Dec;24(3):356–360. doi: 10.1016/0006-2944(80)90030-7. [DOI] [PubMed] [Google Scholar]
- Jeffrey P. L., Brown D. H., Brown B. I. Studies of lysosomal alpha-glucosidase. I. Purification and properties of the rat liver enzyme. Biochemistry. 1970 Mar 17;9(6):1403–1415. doi: 10.1021/bi00808a015. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lüllmann-Rauch R. Lysosomal glycogen storage mimicking the cytological picture of Pompe's disease as induced in rats by injection of an alpha-glycosidase inhibitor. II. Alterations in kidney, adrenal gland, spleen and soleus muscle. Virchows Arch B Cell Pathol Incl Mol Pathol. 1982;39(2):187–202. doi: 10.1007/BF02892847. [DOI] [PubMed] [Google Scholar]
- Pan Y. T., Hori H., Saul R., Sanford B. A., Molyneux R. J., Elbein A. D. Castanospermine inhibits the processing of the oligosaccharide portion of the influenza viral hemagglutinin. Biochemistry. 1983 Aug 2;22(16):3975–3984. doi: 10.1021/bi00285a038. [DOI] [PubMed] [Google Scholar]
- Peters T. J., Jenkins W., Dubowitz V. Subcellular fractionation studies on hepatic tissue from a patient with Pompe's disease (type II glycogen-storage disease). Clin Sci (Lond) 1980 Jul;59(1):7–12. doi: 10.1042/cs0590007. [DOI] [PubMed] [Google Scholar]
- Saul R., Chambers J. P., Molyneux R. J., Elbein A. D. Castanospermine, a tetrahydroxylated alkaloid that inhibits beta-glucosidase and beta-glucocerebrosidase. Arch Biochem Biophys. 1983 Mar;221(2):593–597. doi: 10.1016/0003-9861(83)90181-9. [DOI] [PubMed] [Google Scholar]
- Schmidt D. D., Frommer W., Junge B., Müller L., Wingender W., Truscheit E., Schäfer D. alpha-Glucosidase inhibitors. New complex oligosaccharides of microbial origin. Naturwissenschaften. 1977 Oct;64(10):535–536. doi: 10.1007/BF00483561. [DOI] [PubMed] [Google Scholar]
- WALAAS O., WALAAS E. Effect of epinephrine on rat diaphragm. J Biol Chem. 1950 Dec;187(2):769–776. [PubMed] [Google Scholar]





