Abstract
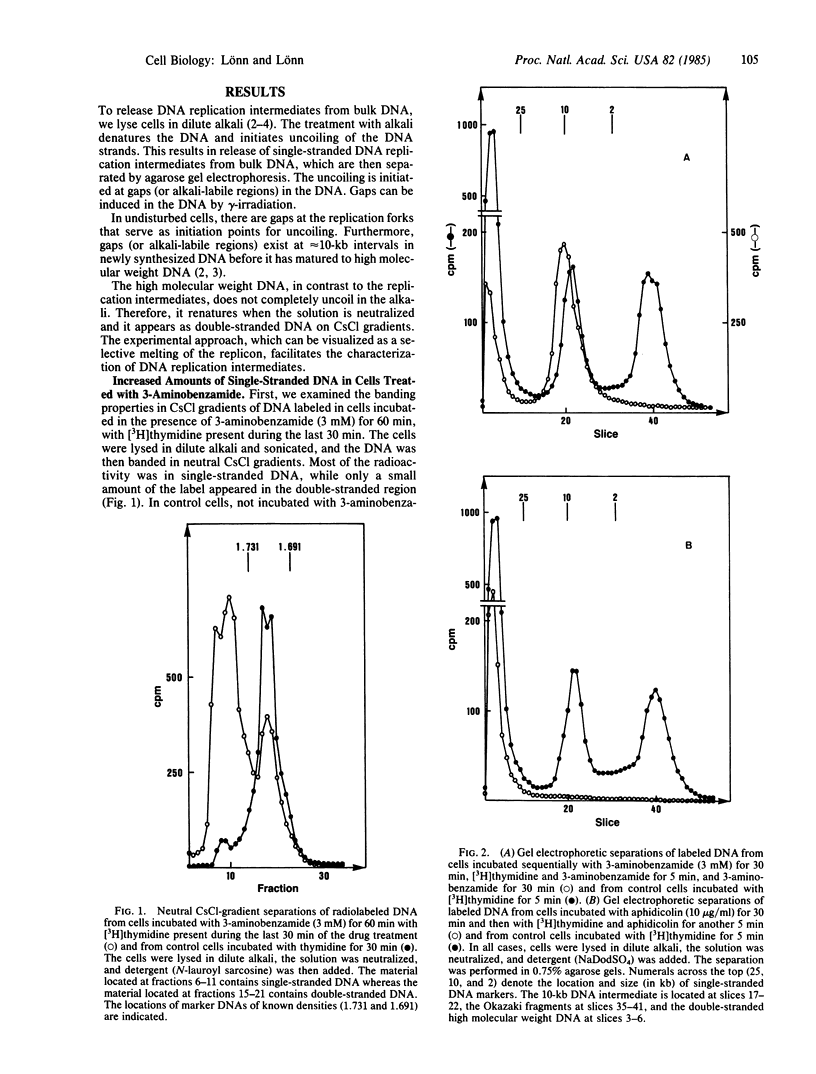
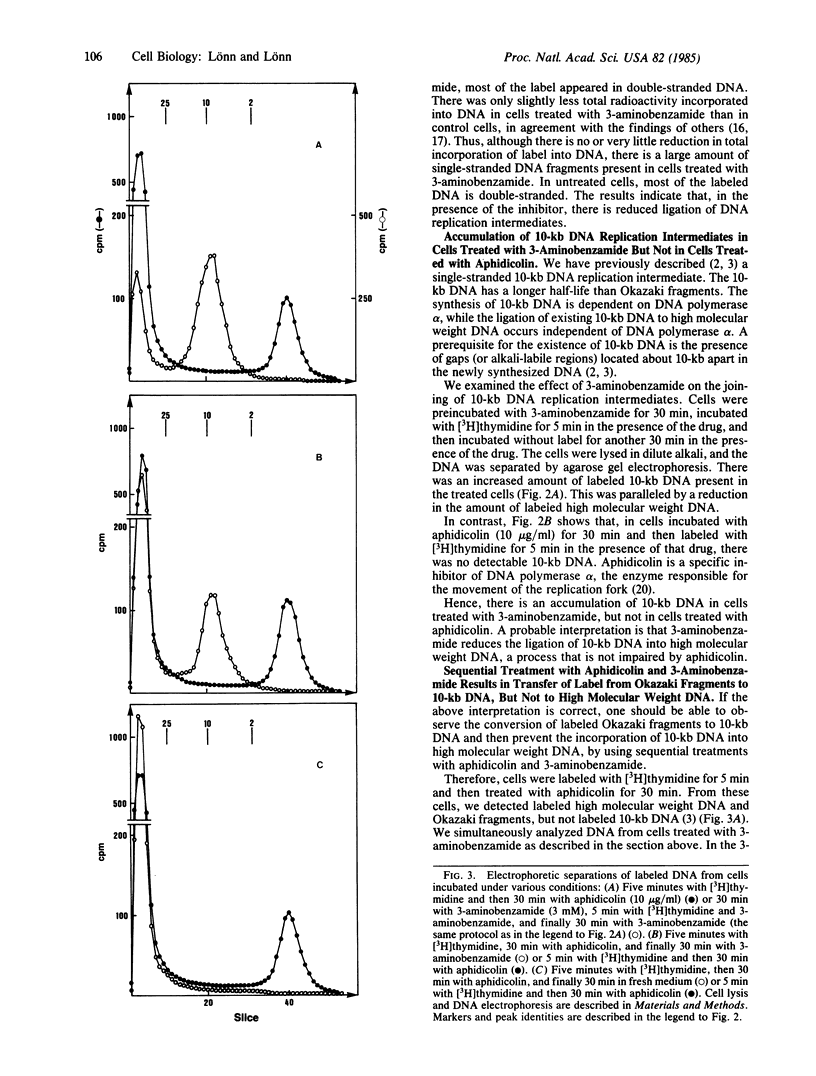
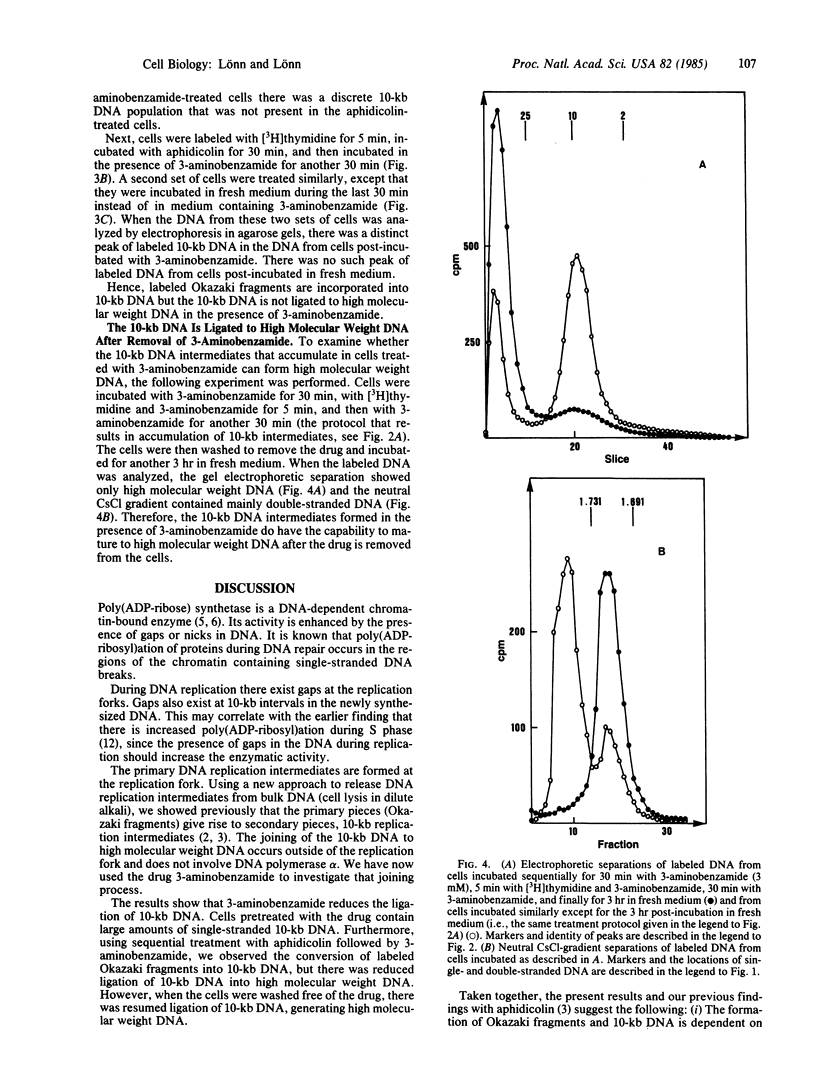
During eukaryotic DNA synthesis there is formation of, in addition to Okazaki fragments, discrete 10-kilobase (kb) DNA replication intermediates. We have investigated the ligation of 10-kb DNA replication intermediates to high molecular weight DNA, using the drug 3-aminobenzamide, an inhibitor of poly(ADP-ribose) synthetase. In human melanoma cells treated with this inhibitor, there is an accumulation of 10-kb DNA. In contrast, in cells treated with aphidicolin, which inhibits DNA polymerase alpha, there is continued ligation of 10-kb DNA to high molecular weight DNA. Furthermore, using sequential treatment with aphidicolin and 3-aminobenzamide, one can observe the conversion of radiolabeled Okazaki fragments into 10-kb intermediates. The 10-kb DNA pieces are, however, not ligated to high molecular weight DNA in the presence of 3-aminobenzamide. Our results imply that functioning poly(ADP-ribose) synthetase is necessary for the ligation process.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aubin R. J., Fréchette A., de Murcia G., Mandel P., Lord A., Grondin G., Poirier G. G. Correlation between endogenous nucleosomal hyper(ADP-ribosyl)ation of histone H1 and the induction of chromatin relaxation. EMBO J. 1983;2(10):1685–1693. doi: 10.1002/j.1460-2075.1983.tb01643.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin R. C., Gill D. M. Poly(ADP-ribose) synthesis in vitro programmed by damaged DNA. A comparison of DNA molecules containing different types of strand breaks. J Biol Chem. 1980 Nov 10;255(21):10502–10508. [PubMed] [Google Scholar]
- Creissen D., Shall S. Regulation of DNA ligase activity by poly(ADP-ribose). Nature. 1982 Mar 18;296(5854):271–272. doi: 10.1038/296271a0. [DOI] [PubMed] [Google Scholar]
- Durkacz B. W., Omidiji O., Gray D. A., Shall S. (ADP-ribose)n participates in DNA excision repair. Nature. 1980 Feb 7;283(5747):593–596. doi: 10.1038/283593a0. [DOI] [PubMed] [Google Scholar]
- Giri C. P., West M. H., Ramirez M. L., Smulson M. Nuclear protein modification and chromatin substructure. 2. Internucleosomal localization of poly(adenosine diphosphate-ribose) polymerase. Biochemistry. 1978 Aug 22;17(17):3501–3504. doi: 10.1021/bi00610a012. [DOI] [PubMed] [Google Scholar]
- Hayaishi O., Ueda K. Poly(ADP-ribose) and ADP-ribosylation of proteins. Annu Rev Biochem. 1977;46:95–116. doi: 10.1146/annurev.bi.46.070177.000523. [DOI] [PubMed] [Google Scholar]
- Huberman J. A. New views of the biochemistry of eucaryotic DNA replication revealed by aphidicolin, an unusual inhibitor of DNA polymerase alpha. Cell. 1981 Mar;23(3):647–648. doi: 10.1016/0092-8674(81)90426-8. [DOI] [PubMed] [Google Scholar]
- James M. R., Lehmann A. R. Role of poly(adenosine diphosphate ribose) in deoxyribonucleic acid repair in human fibroblasts. Biochemistry. 1982 Aug 17;21(17):4007–4013. doi: 10.1021/bi00260a016. [DOI] [PubMed] [Google Scholar]
- Kidwell W. R., Mage M. G. Changes in poly(adenosine diphosphate-ribose) and poly(adenosine diphosphate-ribose) polymerase in synchronous HeLa cells. Biochemistry. 1976 Mar 23;15(6):1213–1217. doi: 10.1021/bi00651a006. [DOI] [PubMed] [Google Scholar]
- Lönn U. Delayed flow-through cytoplasm of newly synthesized Balbiani ring 75S RNA. Cell. 1978 Apr;13(4):727–733. doi: 10.1016/0092-8674(78)90222-2. [DOI] [PubMed] [Google Scholar]
- Lönn U. Detection of a 10 kb DNA replication intermediate in human melanoma cells. Chromosoma. 1982;84(5):663–673. doi: 10.1007/BF00286332. [DOI] [PubMed] [Google Scholar]
- Lönn U., Lönn S. Aphidicolin inhibits the synthesis and joining of short DNA fragments but not the union of 10-kilobase DNA replication intermediates. Proc Natl Acad Sci U S A. 1983 Jul;80(13):3996–3999. doi: 10.1073/pnas.80.13.3996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan W. F., Cleaver J. E. Effect of 3-aminobenzamide on the rate of ligation during repair of alkylated DNA in human fibroblasts. Cancer Res. 1983 Jul;43(7):3104–3107. [PubMed] [Google Scholar]
- Mullins D. W., Jr, Giri C. P., Smulson M. Poly(adenosine diphosphate-ribose) polymerase: the distribution of a chromosome-associated enzyme within the chromatin substructure. Biochemistry. 1977 Feb 8;16(3):506–513. doi: 10.1021/bi00622a026. [DOI] [PubMed] [Google Scholar]
- Ohashi Y., Ueda K., Kawaichi M., Hayaishi O. Activation of DNA ligase by poly(ADP-ribose) in chromatin. Proc Natl Acad Sci U S A. 1983 Jun;80(12):3604–3607. doi: 10.1073/pnas.80.12.3604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poirier G. G., de Murcia G., Jongstra-Bilen J., Niedergang C., Mandel P. Poly(ADP-ribosyl)ation of polynucleosomes causes relaxation of chromatin structure. Proc Natl Acad Sci U S A. 1982 Jun;79(11):3423–3427. doi: 10.1073/pnas.79.11.3423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purnell M. R., Stone P. R., Whish W. J. ADP-ribosylation of nuclear proteins. Biochem Soc Trans. 1980 Apr;8(2):215–227. doi: 10.1042/bst0080215. [DOI] [PubMed] [Google Scholar]
- Purnell M. R., Whish W. J. Novel inhibitors of poly(ADP-ribose) synthetase. Biochem J. 1980 Mar 1;185(3):775–777. doi: 10.1042/bj1850775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueda K., Omachi A., Kawaichi M., Hayaishi O. Natural occurrence of poly(ADP-ribosyl) histones in rat liver. Proc Natl Acad Sci U S A. 1975 Jan;72(1):205–209. doi: 10.1073/pnas.72.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]


