Abstract
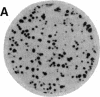
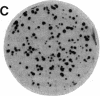
We have isolated genetic variants of the C2 muscle cell line that are defective in expressing the acetylcholine receptor (AcChoR). Because the AcChoR is expressed only after C2 myoblasts have fused to form myotubes, we employed a replica technique to detect the variants. This technique yields two copies of each clone, one of which can be used for screening and the other, as a source of dividing cells. In a screening of about 10,000 clones derived from mutagenized cells, we found 2 that fused normally and expressed normal levels of acetylcholinesterase but had reduced amounts of AcChoR on their surface. One of these also had a reduced level of intracellular AcChoR, but, in the other, the amount of intracellular AcChoR was 5-fold higher than normal. Several variants were found that failed to fuse and had reduced levels of both AcChoR and acetylcholinesterase. Though we relied on 125I-labeled alpha-bungarotoxin to distinguish wild-type from deficient clones, we found that an antiserum to the AcChoR, followed by a biotinylated second antibody and a horseradish peroxidase-avidin complex, could also be used. Therefore, it should be possible to obtain muscle cell variants defective in the expression of a variety of proteins for which specific antibodies are available.
Full text
PDF

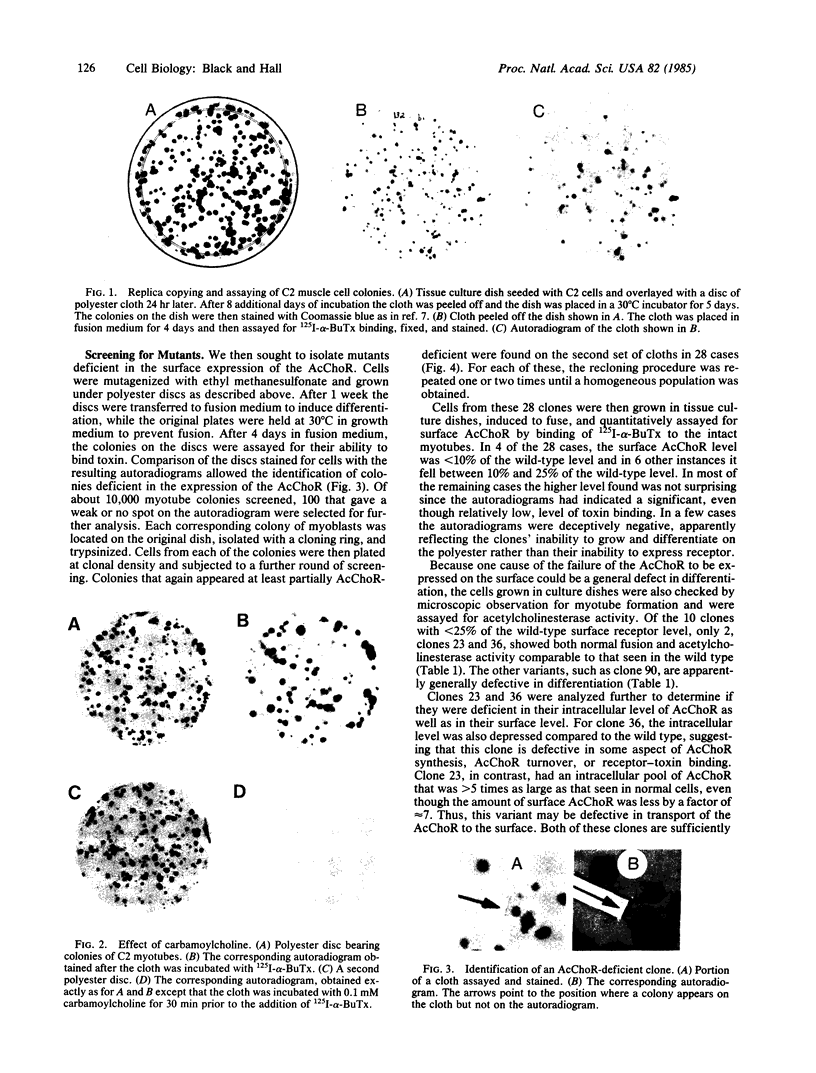


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brockes J. P., Hall Z. W. Acetylcholine receptors in normal and denervated rat diaphragm muscle. II. Comparison of junctional and extrajunctional receptors. Biochemistry. 1975 May 20;14(10):2100–2106. doi: 10.1021/bi00681a009. [DOI] [PubMed] [Google Scholar]
- ELLMAN G. L., COURTNEY K. D., ANDRES V., Jr, FEATHER-STONE R. M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961 Jul;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- Henning U., Schwarz H., Chen R. Radioimmunological screening method for specific membrane proteins. Anal Biochem. 1979 Aug;97(1):153–157. doi: 10.1016/0003-2697(79)90339-7. [DOI] [PubMed] [Google Scholar]
- Inestrosa N. C., Miller J. B., Silberstein L., Ziskind-Conhaim L., Hall Z. W. Developmental regulation of 16S acetylcholinesterase and acetylcholine receptors in a mouse muscle cell line. Exp Cell Res. 1983 Sep;147(2):393–405. doi: 10.1016/0014-4827(83)90221-5. [DOI] [PubMed] [Google Scholar]
- Inestrosa N. C., Silberstein L., Hall Z. W. Association of the synaptic form of acetylcholinesterase with extracellular matrix in cultured mouse muscle cells. Cell. 1982 May;29(1):71–79. doi: 10.1016/0092-8674(82)90091-5. [DOI] [PubMed] [Google Scholar]
- KARNOVSKY M. J., ROOTS L. A "DIRECT-COLORING" THIOCHOLINE METHOD FOR CHOLINESTERASES. J Histochem Cytochem. 1964 Mar;12:219–221. doi: 10.1177/12.3.219. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Merlie J. P. Biogenesis of the acetylcholine receptor, a multisubunit integral membrane protein. Cell. 1984 Mar;36(3):573–575. doi: 10.1016/0092-8674(84)90335-0. [DOI] [PubMed] [Google Scholar]
- Raetz C. R., Wermuth M. M., McIntyre T. M., Esko J. D., Wing D. C. Somatic cell cloning in polyester stacks. Proc Natl Acad Sci U S A. 1982 May;79(10):3223–3227. doi: 10.1073/pnas.79.10.3223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiness C. G., Hall Z. W. The developmental change in immunological properties of the acetylcholine receptor in rat muscle. Dev Biol. 1981 Jan 30;81(2):324–331. doi: 10.1016/0012-1606(81)90295-5. [DOI] [PubMed] [Google Scholar]
- Silberstein L., Inestrosa N. C., Hall Z. W. Aneural muscle cell cultures make synaptic basal lamina components. Nature. 1982 Jan 14;295(5845):143–145. doi: 10.1038/295143a0. [DOI] [PubMed] [Google Scholar]
- Siminovitch L., Thompson L. H. The nature of conditionally lethal temperature-sensitive mutations in somatic cells. J Cell Physiol. 1978 Jun;95(3):361–364. doi: 10.1002/jcp.1040950314. [DOI] [PubMed] [Google Scholar]
- Thompson L. H. Mutant isolation. Methods Enzymol. 1979;58:308–322. doi: 10.1016/s0076-6879(79)58147-6. [DOI] [PubMed] [Google Scholar]
- Yaffe D., Saxel O. Serial passaging and differentiation of myogenic cells isolated from dystrophic mouse muscle. Nature. 1977 Dec 22;270(5639):725–727. doi: 10.1038/270725a0. [DOI] [PubMed] [Google Scholar]