Abstract
Background & Aims
Fatty liver is a common problem in children, and increases their risk for cirrhosis, diabetes, and cardiovascular disease. Liver biopsy is the clinical standard for diagnosing and grading fatty liver. However, non-invasive imaging modalities are needed to assess liver fat in children. We performed a systematic review of studies that evaluated imaging of liver fat in children.
Methods
We searched PubMed for original research articles in peer-reviewed journals from January 1, 1982 through December 31, 2012 using the key words “imaging liver fat.” Studies included those in English, and those performed in children from birth to 18 y of age. To be eligible for inclusion, studies were required to measure hepatic steatosis via an imaging modality and a quantitative comparator as the reference standard.
Results
We analyzed 9 studies comprising 610 children; 4 studies assessed ultrasonography and 5 assessed magnetic resonance imaging (MRI). Ultrasonography was used in the diagnosis of fatty liver with positive predictive values of 47–62%. There was not a consistent relationship between ultrasound steatosis score and the reference measurement of hepatic steatosis. Liver fat as measurements by MRI or by spectroscopy varied with the methodologies used. Liver fat measurements by MRI correlated with results from histologic analyses, but sample size did not allow for assessment of diagnostic accuracy.
Conclusions
Available evidence does not support the use of ultrasonography for the diagnosis or grading of fatty liver in children. Although MRI is a promising approach, the data are insufficient to make evidence-based recommendations regarding its use in children for assessment of hepatic steatosis.
Keywords: NAFLD, evidence based medicine, liver imaging
INTRODUCTION
Fatty liver is common in children with an estimated prevalence of 9.6%[1], and is characterized histologically by hepatic steatosis, the accumulation of triglycerides within the cytoplasm of hepatocytes. Because of the high prevalence of obesity, there are many children at risk for fatty liver in the form of Nonalcoholic Fatty Liver disease (NAFLD). NAFLD, which encompasses a broad spectrum of liver disease severity – including steatosis, steatohepatitis with or without fibrosis, and cirrhosis– is only one of many conditions in which hepatic steatosis is an important histologic component. Hepatic steatosis can also occur in drug toxicity, metabolic disease, viral hepatitis, cystic fibrosis, protein malnutrition, and Wilson’s disease. Furthermore, fatty liver is regarded as a cardiometabolic risk factor that can contribute to conditions such as diabetes mellitus and heart disease. The multitude of clinical conditions associated with hepatic steatosis as well as their prevalence and severity create a need to assess liver fat in children.
Histology is considered the most sensitive means of obtaining pertinent, comprehensive information from the liver and surpasses imaging and other laboratory data for several reasons. Liver histology is capable of identifying steatohepatitis and staging liver fibrosis, which have important prognostic implications. Moreover, histology provides complex and integrated information at a cellular level, and can therefore refine a diagnosis when other studies are nonspecific or suggest multiple possible diagnoses. In addition, well-preserved histological samples serve as an accessible ‘tissue bank’ for future investigations. However, the liver biopsy procedure is invasive and requires sedation with potential risks including discomfort, bleeding, and infection.
Given the risks of liver biopsy in children, an alternate means for obtaining diagnostic information and for longitudinal surveillance of liver fat is desirable, and creates a niche for imaging liver fat in children. Diagnostic information about liver abnormalities - including hepatic steatosis - can be obtained from imaging modalities in a non-invasive manner and without the need for sedation in many children. This is particularly relevant for children with fatty liver, in whom disease monitoring is necessary for months, years, or even decades. Although there are many potential advantages, the diagnostic utility of imaging for assessment of liver fat in children has not been systematically evaluated. Although studies in adults have suggested that imaging may be an accurate means of assessing hepatic steatosis, [2–8] children differ from adults in several key aspects including body habitus, breath holding capacity, and ability to tolerate imaging examinations. These factors affect the feasibility, quality, and technical optimization of imaging examinations and may affect their diagnostic performance. Hence, validation in adults does not suffice to establish validity in children. Independent validation of imaging modalities to assess liver fat in children is necessary.
In order to validate an imaging modality as a measure of hepatic steatosis, studies are required in which children are assessed by both the test modality and a comparison modality that correctly classifies each subject. When assessing the ability of a test modality to make a quantitative or semi-quantitative measurement of hepatic steatosis, the study population must use a comparator modality that is sufficiently quantitative and must include a sufficient representation of children across the spectrum of hepatic steatosis, including those without hepatic steatosis.
Our aim was to perform a systematic review of the literature detailing studies that evaluated imaging liver fat in children using ultrasound (US), computed tomography (CT), magnetic resonance imaging (MRI), or magnetic resonance spectroscopy (MRS) compared to a sufficiently quantitative comparator. The preferred reference was liver histology. Due to the limited numbers of studies with histology, we also included studies that used the quantitative methods of MRI or MRS as the reference. Studies using histology as the reference were considered to be of higher quality for the purposes of establishing recommendations, than studies using MRI or MRS as the reference. Our goals were to: 1) critically review and synthesize the collective literature, 2) make recommendations regarding the clinical use of imaging to assess liver fat in children, and 3) identify potential gaps that may be addressed by future research studies.
METHODS
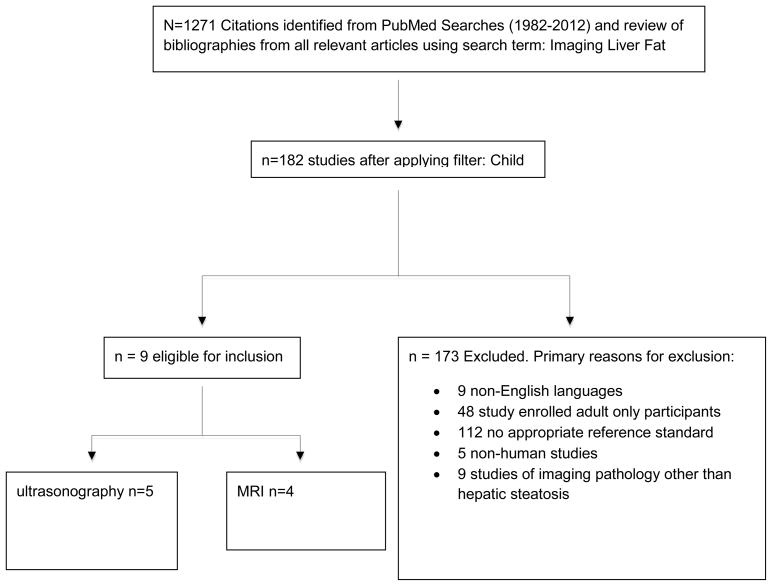
We followed published guidelines for the conduct and reporting of systematic reviews [9]. We performed a structured keyword search in PubMed to identify original research reports published in print or online in English in peer-reviewed journals between January 1, 1982 and December 31, 2012 that evaluated the performance of imaging modalities to assess hepatic steatosis compared against a quantitative or semi-quantitative reference standard. The search term “imaging liver fat” and a review of bibliographies of all relevant articles resulted in 1271 studies. The search was then filtered by age for studies in children (birth to 18 years) resulting in 182 remaining studies. Eligible studies had to assess an imaging modality using a comparator that was sufficiently quantitative. 173 studies were excluded based on various criteria. [Figure 1] Nine studies remained for systematic review. Three independent physicians extracted data from each study to minimize errors and reduce potential biases. Consensus of analyzed data was achieved through a structured review of each article in multiple group meetings. All authors then reviewed and revised the material presented.
Figure 1.
Figure shows the application of the inclusion and exclusion criteria used for the structured review.
Based upon the evidence, recommendations were made in the assessment of fatty liver as a dichotomous outcome (screening, diagnosis, exclusion) and as a continuous measure (grading, monitoring change). We used the classification of the Grading of Recommendation Assessment, Development, and Evaluation (GRADE) workgroup. [10] The GRADE system, developed by a widely representative group of international guideline developers, allows for clear separation between the quality of evidence and the strength of recommendations. The strength of recommendations in the GRADE system is classified as strong (1) or weak (2). The quality of evidence supporting strong or weak recommendations is designated by one of three levels: high- (A), moderate- (B) or low-quality (C). The designation of quality is based upon the likelihood that further research will change the confidence of the estimate with A (unlikely to change the confidence in the estimate of the effect), B (likely to have important impact), and C (very likely to impact to change the estimate).
TECHNICAL DISCUSSION
In order to interpret the results, a brief technical discussion is provided.
Ultrasound
Ultrasound is widely used for evaluation of hepatic steatosis [11–13] and is based upon high-frequency sound wave propagation. The ultrasonic appearance of normal liver is similar to other solid organs. Ordinarily, intrahepatic structures such as hepatic veins can be delineated by ultrasonic waves. In hepatic steatosis, the presence of lipid droplets within hepatocytes disturbs the propagation of the sound wave, causing scatter and attenuation. As waves scatter, more echoes return to the ultrasound transducer. This increase in signal (echogenicity) from liver is processed to yield a liver that is brighter in qualitative comparison to the kidney. Attenuation of the ultrasound waves causes depth-dependent loss of signal, causing obscuration of vessels, bile ducts, and blurring of the diaphragm. Collectively the changes due to scattering and attenuation are used to infer hepatic steatosis, but available ultrasound-based techniques do not permit a direct quantifiable measurement of liver fat. [14]
Magnetic Resonance Imaging
Chemical shift MRI exploits differences in resonance frequencies of water and lipid to differentiate tissues containing only water from those containing both water and lipid. Chemical shift occurs because nuclei in different chemical environments experience slightly different magnetic field strengths. Variation in magnetic field strength results from the intrinsic shielding of surrounding electrons, which partially counteract the force of the main magnetic field. Hydrogen nuclei in water compared to hydrogen nuclei in lipid have fewer surrounding electrons. Hydrogen nuclei in water, therefore, experience a stronger magnetic field, and rotate at a higher resonance frequency.[15] The characteristic resonance frequencies of fat and water and the detection of the signal of fat-specific frequency allows for quantitative measurement of hepatic steatosis.
Several techniques are available for MRI assessment of steatosis. In the 2-point Dixon (or dual-echo) method, signal intensities from tissues such as liver are compared in in-phase (IP) and out-of-phase (OP) images. This requires acquisition of two sets of gradient-echo images, and consideration of the echo-time dependent signal interference between fat and water. In OP echo-time, water and fat signals cancel and therefore the total signal intensity is lower. In IP echo-time, water and fat signals augment and therefore the total signal intensity is higher. Normal liver has similar signal intensity, between the OP and IP images since there is no fat-water interference. In steatosis, the liver signal intensity is lower and the liver appears darker on the OP images because of the fat-water signal interference. In addition to this qualitative measure, MRI can also estimate quantitative fat content. MRI fat and water signal intensities increase proportionately to the respective number of molecules. For quantitative assessment, IP and OP signal intensity comparison can be used to calculate a signal fat fraction (FF) [16–18] which is defined as the portion of the fat signal relative to the total (fat + water) liver signal. The ability to decompose the overall hepatic signal to its water and fat components may give MRI an advantage over other approaches[19]. To achieve necessary accuracy and to permit a standardized measure of liver fat content, MRI assessment must include appropriate sequence, proper parameters, and complete correction for confounding factors such as T1 bias, T2* decay, multi-peak spectral interference effects from protons in fat, noise bias, and eddy currents [6, 7, 18, 20–23].
MR Spectroscopy
Magnetic resonance spectroscopy (MRS) is regarded as the most accurate method for quantification of hepatic fat content in user-specified locations in the liver and has been used in numerous studies as the reference comparator by which the accuracy of other methods, including MRI, is gauged.[2, 5–7, 15, 18, 24]. A volume of at least 4 cm3 is targeted within the liver, and nuclear magnetic resonance spectrum is obtained. The spectral peaks represent chemical moieties of water and triglyceride molecules. Hydrogen protons in water have a dominant frequency in the MR spectrum at 4.7 ppm. Hydrogen protons in the dominant methylene (-CH2-) moiety of triglyceride molecules have a frequency peak at 1.2 ppm[15] while hydrogen protons in other triglyceride moieties have additional peaks at other frequencies. Consequently, triglyceride produces a spectrum of multiple frequency peaks, versus the single large peak created by water [25, 26]. The MR signal of hydrogen protons in water and each triglyceride moiety can be identified by their unique frequencies, and their cumulative signal amplitude can be quantified [25]. From the cumulative signal amplitude of hydrogen in water versus those in fat, the fat fraction can be determined.
RESULTS
Studies of Ultrasonography Assessment of Hepatic Steatosis
US Compared to Liver Histology
There were two studies identified that have compared the evaluation of hepatic steatosis by liver ultrasonography to liver histology in children. The first study evaluated 70 children from a pediatric obesity clinic in Cairo, Egypt.[27] Of these children, 34 were clinically believed to have fatty liver based upon the findings of hepatomegaly, elevated serum ALT activity, and/or an ultrasound that was considered positive for hepatic steatosis. These 34 children underwent liver biopsy. The liver ultrasound images were graded using semi-quantitative criteria for steatosis as none (0), mild (1), moderate (2), or severe (3). Children were classified histologically as having normal liver or fatty liver; however, more specific grading of the degree of hepatic steatosis was not reported. Liver histology revealed that 47% (15/32) of children with a positive liver ultrasound for steatosis had fatty liver. The number of children who had fatty liver but did not have a liver biopsy was unknown. Therefore it was not possible to estimate the negative predictive value of a normal liver ultrasound. Due to the small numbers of children with fatty liver, the accuracy of ultrasound grading of steatosis could not be accurately assessed.
The second study included only children with biopsy-proven NAFLD in Rome, Italy.[28] The study had a large sample size (n = 208), which did support an assessment of the relationship between semi-quantitative measurements of steatosis by liver ultrasound versus liver histology. Steatosis by liver ultrasound was graded as none (0), mild (1), moderate (2), or severe (3). Steatosis by liver histology was graded as negative (<5% of hepatocytes containing fat vacuoles), mild (5–33% of hepatocytes), moderate (>33 – 66% of hepatocytes), or severe (>66% of hepatocytes). The Spearman’s correlation between ultrasound steatosis score and liver histology was good (0.80). However, the individual ultrasound score category did not accurately predict the histological category. There were 12 children who had a negative ultrasound; of these, 10 (83%) had a liver biopsy showing steatosis. Nearly half (29/61) of children with mild steatosis by ultrasound had moderate steatosis by liver histology. For children who had an ultrasound steatosis score of moderate, there was a wide distribution of histological severity spanning mild, moderate, and severe steatosis. The authors also combined the ultrasound grades of moderate and severe together for analyses. Of those children with severe steatosis by liver histology all had an ultrasound steatosis score of moderate to severe steatosis.
US Compared to Liver MRI
Two studies in children used MRI-determined hepatic fat fraction as the quantitative comparator against which ultrasound findings were evaluated. In the first of these studies, Pacifico et al evaluated 50 obese children in Rome, Italy who were pre-selected for suspected fatty liver based on findings of hepatomegaly or liver chemistry values greater than 1.5 times the upper limit of normal for age.[29] Steatosis by liver ultrasound was graded as none (0), mild (1), moderate (2), or severe (3). MRI was performed using a modified Dixon method, and the hepatic signal fat fraction was calculated from mean pixel signal intensities. For the purposes of this study, a child was considered to have fatty liver if the MRI hepatic signal fat fraction was ≥9%. Of the children clinically suspected to have fatty liver, only 40% were determined to have fatty liver by MRI. Of those children with an ultrasound interpreted as positive for fatty liver, slightly more than half (56%) were determined to have fatty liver by MRI. The overall Pearson’s correlation between ultrasound steatosis score and liver MRI-determined hepatic fat fraction was moderate (r = 0.68). There was a low rate of false negatives (1/16). However, there was limited ability to accurately grade the severity of steatosis. The majority of children with mild steatosis by ultrasound (12/18) had a negative MRI for fatty liver. For children who had an ultrasound steatosis score of moderate, there was a wide range of MRI-determined hepatic signal fat fractions with values ranging from 1 to 39%.
In the second study, 60 children were referred to a tertiary care obesity clinic in Milan, Italy.[30] All children underwent liver ultrasound and MRI. Steatosis by liver ultrasound was graded as none (0), mild (1), moderate (2), or severe (3). The MRI protocol, specifically the echo time and flip angle, used in this study differed from that in the Pacifico study. Despite the differences in imaging parameters, the same threshold value (9%) was used to determine fatty liver. Applying the same MRI-based classification threshold may not be valid for techniques that differ in echo time and flip angle, as changes in these parameters alter the MRI-estimated fat fraction [20]. Of those children with an ultrasound interpreted as positive for fatty liver, slightly less than half (48%) were determined to have fatty liver by MRI. The false positive rate was highest amongst those with an ultrasound steatosis score of 1 (mild), with 72% (13/18 participants) having a negative MRI for fatty liver. If one chose to consider only a hepatic steatosis score of 2 or 3 as positive, the false positive rate would be diminished. However, the cost of doing so in this data set would have been missing 43% (6/14) of children with fatty liver.
US Compared to Liver MR Spectroscopy
One study compared liver ultrasonography to MR spectroscopy in 104 obese adolescents from an obesity treatment program in Amsterdam, Netherlands.[31] Liver ultrasound was performed and steatosis was scored as none (0), mild (1), moderate (2), severe (3). MR Spectroscopy was performed using the Point RESolved Spectroscopy (PRESS) technique with T2 correction. In this study, adolescents were classified as having fatty liver if liver fat fraction by spectroscopy was > 1.8%. Despite this low classification threshold value, there were a substantial number of false positive US interpretations. Of those adolescents with an ultrasound interpreted as positive for fatty liver, 22 out of 66 did not have fatty liver by MRS. The authors also assessed the semi-quantitative determination of steatosis by ultrasound. The false positive rate was high; of those with no steatosis on MRS (n=56), 25 out of 56 had ultrasound findings of mild or moderate steatosis. By comparison, the false negative rate was low; of those with a normal ultrasound (n=38), only 7 out of 38 had a positive MRS. However, depending upon the definition used, liver ultrasound correctly identified the severity of steatosis in only 40% to 60% of cases. The authors concluded that liver ultrasound could not be used to predict the presence or severity of hepatic steatosis.
Studies of Computed Tomography Assessment of Hepatic Steatosis
No studies in children compared CT assessment of hepatic steatosis to a quantitative measure. Because of the exposure to ionizing radiation and the existence of other imaging modalities free of ionizing radiation, CT should not be used for the primary purpose of assessing hepatic steatosis.
Studies of Liver MRI Assessment of Hepatic Steatosis
MRI Compared to Liver Histology
One study evaluated 25 obese children in Rome, Italy with biopsy-proven NAFLD who underwent MRI prior to liver biopsy to explore the accuracy of the MRI-determined hepatic signal fat fraction.[32] The MRI method used in this study was a modification of the 2-point Dixon method.[33] Steatosis by liver histology was graded as grade 0 (<5%), grade 1 (mild; 5–33%), grade 2 (moderate; 34–66%), and grade 3 (severe ≥66%). MRI-determined hepatic signal fat fraction was strongly correlated (r = 0.88) with the histological grade of steatosis amongst children with NAFLD. The small sample size precluded more specific determinations of accuracy.
MRI Compared to Liver MR Spectroscopy
One study compared MRI to MRS performed exclusively in pediatric participants. Springer and colleagues studied 29 obese adolescents in Tubingen, Germany.[21] All exams were performed at 1.5 Tesla. Spectroscopy was performed using the stimulated echo acquisition mode (STEAM) technique without T2 correction. MRI was performed using fat-selective imaging. Authors compared MRI to MRS for the ability to detect MRS-determined hepatic fat fraction of > 5%. They also evaluated the correlation of MRI-determined hepatic signal fat fraction with MRS-measured fat fraction. Depending on where the region of interest was selected for MRI, the Spearman correlations coefficient ranged from 0.78 to 0.86. Thus the values derived were highly correlated but not equivalent. In this study sample, MRI had a sensitivity of 95% and specificity of 92% for the detection of an MRS-measured hepatic fat fraction of > 5%.
Two studies compared MRI to MRS and were noted to include some children. Yokoo et al [6] examined 110 participants in California, 30 of whom were children with the goal of assessing the accuracy of four fat quantification methods using low-flip-angle multi-echo gradient-recalled-echo MRI at 1.5 Tesla. In this study, a diagnosis of fatty liver was based upon a spectroscopic fat fraction threshold value of 6.25%. MRI fat fraction was calculated using four analysis methods: 1) dual-echo, 2) triple-echo, 3) multi-echo, and 4) multi-interference. The dual-echo analysis method did not correct for T2* whereas all other methods corrected for T2*. The multi-interference method also corrected for multiple spectral interference effects of fat. Depending on the threshold set to define fatty liver and the MR imaging analytical method used, the diagnostic sensitivity of MRI ranged from 0.76 to 1.0 and the diagnostic specificity ranged from 0.85 to 1.0. Grading accuracy was also assessed. The dual-echo method had a systemic error resulting in underestimation of fat fraction by 2.9%. The triple-echo method had a small but significant error in both correlation and estimation of fat fraction. The multi-echo method had error in correlation but not in estimated fat fraction. The multi-interference method with spectral modeling had the highest quantification accuracy.
In a second study by Yokoo and colleagues a similar methodology was applied at higher field strength, 3.0 Tesla.[7] The authors evaluated MRI methods using 2, 3, or, 6 echoes and each of these were assessed using either single-frequency or multi-frequency fat signal modeling. There were 34 children out of a total sample size of 163 participants from Southern California. In this study, classification accuracy of the six imaging methods for fatty liver was assessed using a range of potential threshold spectroscopic fat fraction values from 4 – 10%. The classification accuracy of all imaging methods included in this study ranged from 83–96%.
MR Spectroscopy
No studies compared hepatic steatosis measured by MRS to steatosis assessed by liver histology in children.
DISCUSSION
We performed a systematic review of the published literature in children assessing hepatic steatosis measured by non-invasive imaging that made use of a quantitative comparator. Nine studies were identified that tested the validity of ultrasound or MRI assessment of liver fat. We analyzed studies for the ability to evaluate the presence or absence of fatty liver as a dichotomous trait as well as the ability to grade steatosis as an ordinal trait. This systematic review provides a framework from which to evaluate the evidence-based utility of these modalities for diagnosis and grading of hepatic steatosis in children.
Ultrasound: Evidence-based Utility
In routine practice, ultrasound is commonly used to determine the presence of fatty liver. However, systematic review revealed a positive predictive value of liver ultrasound for fatty liver in children between 47 and 62%. Thus, the available evidence demonstrated that ultrasound did not meet the standard clinical threshold required to be used as a diagnostic test. This limitation of ultrasound to accurately classify whether or not a child has fatty liver stems in part from an inherent property of ultrasound; it does not measure fat directly, instead, the relation between ultrasound-derived images and liver fat is intrinsically subjective and non-quantitative.
In addition to demonstrating the presence of fatty liver, ultrasound is currently used by many physicians to exclude fatty liver. The design of the included studies limited the ability to evaluate this role for ultrasound. Only one study included children who had a negative ultrasound but still had a liver biopsy[28]. In that study, most of the negative ultrasounds turned out to be falsely negative. However, this was an artifact of the study design, which only included children with known NAFLD. Thus, the data are lacking regarding the ability of ultrasound to exclude fatty liver in children.
Liver ultrasonography has been widely used to grade the severity of steatosis, primarily in research studies; however, the evidence reviewed does not support ultrasound as a semi-quantitative measure of hepatic steatosis. In the studies reviewed comparing ultrasound to histology, children with mild steatosis by ultrasound were found to have moderate steatosis by histology in approximately 50% of cases. Moreover, when ultrasound was compared to MRI, the majority of children with mild steatosis by ultrasound were determined not to have fatty liver by MRI. The grading category of moderate steatosis did not fare better. Children with an ultrasound steatosis grade of moderate had steatosis ranging from mild to severe on liver biopsy, and a liver fat fraction on MRI which ranged from normal to near maximal. Thus, current evidence indicates that liver ultrasound lacks the accuracy to grade steatosis.
MRI Evidence-Based Utility
In pediatric clinical research studies, MRI has overtaken ultrasound as the modality of choice for the non-invasive measurement of hepatic steatosis. In some institutions, MRI is now used in standard clinical practice to measure liver fat fraction. The increasing use of MRI is due to its availability and promise as a quantitative measure of hepatic steatosis. However, the evidence base is extremely limited. Available data do highlight the importance of performing MRI correctly to compensate for confounders that introduce error into both the accuracy and precision of conventional MRI. [5–7, 20, 34, 35] Structured review demonstrated that MRI has the potential for a diagnostic accuracy for steatosis that is on par with spectroscopy [5–7]. Moreover, initial pediatric data comparing MRI measured liver fat fraction to hepatic steatosis assessed by liver histology demonstrated a good correlation between these measures.[32] However, the available evidence is too small to estimate diagnostic performance.
Both clinicians and radiologists need to be aware that – due to the presence of confounding factors such as T1 relaxation, T2(*) decay, multi-frequency interference effects of protons in fat [6, 7, 18, 20, 21], noise bias[22], and eddy currents[23] – conventional MRS and MRI methods may be inaccurate, non-robust, and non-reproducible. Advanced MRS and MRI methods address these confounders to estimate the proton density fat fraction, a standardized biomarker of liver fat content that is accurate[5, 7, 36] reproducible across different scanners[37, 38] and field strengths[37, 38], and robust to routine acquisition parameter changes that are common in clinical practice and may occur in clinical trials. Thus, future studies in children should focus on implementation and validation of these advanced MRI and MRS techniques.
Clinical and Research Recommendations
Recommendations for the use of ultrasound and MRI in clinical care are presented in Table 2. Ultrasound’s primary role is in the evaluation of structural problems within the liver or gallbladder. In addition, ultrasound is widely used for screening, diagnosis, and grading of hepatic steatosis. However, data do not support the use of ultrasound to make a diagnosis of fatty liver. In order to determine the ability of ultrasound to accurately exclude fatty liver, a study must include children who can be determined to not have NAFLD. Because it would not be feasible for a study to perform liver biopsies as a means to prove the absence of liver disease, studies of ultrasound to exclude fatty liver will require a non-invasive test to prove the absence of hepatic steatosis. The best choice would be MRI. However, such studies are limited by the lack of a uniform standard for the MRI liver fat fraction that accurately classifies children as having or not having fatty liver. Therefore, whether or not ultrasound can be used to exclude hepatic steatosis is an unanswered research question. The available data also do not support the use of ultrasound for grading hepatic steatosis in clinical care or research. The high misclassification rate for ultrasound grading precludes its use as a tool for disease monitoring in children at this time and would introduce substantial error into any studies in which it was used. In contrast, MRI showed promise as a means of assessing hepatic steatosis. Unfortunately, the evidence-based data is insufficient to make recommendations for the use of MRI to assess liver fat in a clinical context at this time. In addition, it will be important to consider and evaluate the relative cost of diagnostic imaging studies
Table 2.
Recommendations for Imaging Liver Fat in Clinical Care
| Goal | Modality | Evidence | GRADE | ||
|---|---|---|---|---|---|
| Supports | Does Not Support | Insufficient for Recommendation | |||
| Screen For Fatty Liver | Ultrasonography | • | 2B | ||
| MRI | • | 2C | |||
| Diagnose Fatty Liver | Ultrasonography | • | 1B | ||
| MRI | • | 1C | |||
| Exclude Fatty Liver | Ultrasonography | • | 1C | ||
| MRI | • | 1C | |||
| Grade Hepatic Steatosis | Ultrasonography | • | 1B | ||
| MRI | • | 2C | |||
| Monitor Hepatic Steatosis | Ultrasonography | • | 1B | ||
| MRI | • | 1C | |||
Research focused on imaging liver fat in children is limited. Additional research is urgently needed to assess ultrasound and MRI in large populations of children utilizing appropriate normal controls. Future studies using ultrasound should consider the evaluation of emerging quantitative ultrasound techniques using liver biopsy as the reference standard for diagnosis and grading hepatic steatosis in children. There are also several important research areas to consider for MRI. Firstly, a major priority for MRI research is to determine a uniform standard for MRI-determined liver fat fraction that accurately classifies children as having or not having fatty liver. Proposed value for a cut-point ranges from 1.8% to 9%; however, determining whether such a cut-point exists, and if so, where it lies is a crucial research question. Secondly, MRI is both spatial and quantitative and therefore has the potential to grade the severity of steatosis and assess steatosis across the entire liver; however, strategies on how to best analyze and use such data in a standardized and validated way have yet to be developed. Thirdly, fatty liver monitoring may be the best utilization of MRI. After a liver biopsy is done for purposes of diagnosis and staging, MRI-determined liver fat fraction could be individually calibrated to the index biopsy. MRI could thereafter be used as a component of comprehensive disease monitoring over time. This strategy has not yet been evaluated in children, and thus provides an area for further research.
Conclusion
Fatty liver is a common clinical problem for children and adolescents. Liver biopsy remains the clinical standard for diagnosing and grading fatty liver disease in children. Non-invasive imaging modalities to assess liver fat in children are needed. The available evidence does not support the use of ultrasound for diagnosis or grading of fatty liver in children. Data suggest that MRI may have better utility but sufficient supportive data are lacking. Improvement in the imaging technology for assessment of liver fat in children and the validation of such technology should be considered an urgent need for patient care and research.
Table 1.
Characteristics of Pediatric Studies Comparing Steatosis by Imaging with a Quantitative Reference
| Reference | First Author (year) | Country | N | Age range (y) | Population | Comparator | Definition for fatty liver | Evaluation of severity of steatosis |
|---|---|---|---|---|---|---|---|---|
| Ultrasonograpy | ||||||||
| Pacifico (2007) | Italy | 50 | 5–16 | Children with BMI >95% referred for evaluation for fatty liver upon detection of hepatomegaly and/or elevated liver chemistry | MRI | MRI HFF ≥9% | Continuous hepatic fat fraction | |
| Pozzato (2008) | Italy | 60 | 6–14 | Obese children with White parents admitted for obesity. | MRI | MRI HFF ≥9% | MRI HFF ≥9% | |
| El-Koofy (2011) | Egypt | 70 | 2–13 | Obese children referred from Endocrinology for assessment of hepatic abnormalities; clinical hepatomegaly, raised ALT and/or echogenic liver parenchyma by ultrasound | Histology | >5% of hepatocytes with steatosis | Ordinal histologic steatosis score | |
| Shannon (2011) | Italy | 208 | 3–14 | Children with biopsy proven NAFLD | Histology | >5% of hepatocytes with steatosis | Ordinal histologic steatosis score | |
| Bohte (2012) | Netherlands | 104 | 8–18 | Obese children admitted to a tertiary center lifestyle intervention program based on BMI >30kg/m2 along with obesity-related comorbidity | Spectroscopy | Greater than 1.8% absolute concentration of spectroscopic liver fat | Center defined MRS cut points for mild, moderate, severe steatosis | |
| MRI | ||||||||
| Yokoo (2009) | USA | 30 | 8–18 | Recruited from the institutional hepatology and obesity clinics | Spectroscopy | Threshold value of 6.25% for spectroscopic fat fraction | Continuous spectroscopic fat fraction | |
| Yokoo (2011) | USA | 34 | 10–18 | Referred by hepatologists and surgeons | Spectroscopy | Various MRS thresholds: 0.04, 0.06, 0.08, 0.10 | Continuous spectroscopic fat fraction | |
| Pacifico (2011) | Italy | 25 | 7–16 | Obese Caucasian children with suspected NAFLD based on elevated serum ALT and diffusely hyperechogenic liver on US, and hyperinsulinism | Histology | >5% of hepatocytes with steatosis | Ordinal histologic steatosis score | |
| Springer (2011) | Germany | 29 | 11–16 | Severely obese adolescents referred for treatment | Spectroscopy | Intrahepatic lipid content >5% | Continuous spectroscopic fat fraction | |
Abbreviations: ALT (alanine aminotransferase), HFF (hepatic fat fraction), NAFLD (nonalcoholic fatty liver disease), NASH (nonalcoholic steatohepatitis), MRI (magnetic resonance imaging), US (ultrasound)
Acknowledgments
Grant Support: This work was supported in part by NIH grants DK088925-02S1 and R56-DK090350-01A1. The contents of this work are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- ALT
alanine aminotransferase
- ALT
alanine aminotransferase
- BMI
body mass index
- CT
computed tomography
- FF
fat fraction
- GRADE
Grading of Recommendation Assessment, Development, and Evaluation
- HFF
hepatic fat fraction
- HU
Hounsfield Units
- IP
in phase
- MRI
magnetic resonance imaging
- MRS
magnetic resonance spectroscopy
- OP
out-of-phase
- NAFLD
non-alcoholic fatty liver disease
- NASH
non-alcoholic steatohepatitis
- PRESS
point RESolved spectroscopy
- STEAM
stimulated echo acquisition mode
- US
ultrasound
Footnotes
Disclosures All authors must disclose any potential conflicts (financial, professional, or personal) that are relevant to the manuscript. If the author(s) has nothing to disclose, this must be stated.
Dr. Awai - nothing to disclose
Dr. Newton – nothing to disclose
Dr. Sirlin – research grant from GE
Dr. Behling – nothing to disclose
Dr. Schwimmer - nothing to disclose
Writing Assistance:
No individuals provided writing assistance
Author Contributions:
Dr. Awai – study concept and design, acquisition of data, analysis and interpretation of data; drafting of the manuscript
Dr. Newton – study concept and design, analysis and interpretation of data; critical revision of the manuscript for important intellectual content
Dr. Sirlin – study concept and design, critical revision of the manuscript for important intellectual content
Dr. Behling – study concept and design, drafting of the manuscript; critical revision of the manuscript for important intellectual content
Dr. Schwimmer - study concept and design, analysis and interpretation of data; drafting of the manuscript; critical revision of the manuscript for important intellectual content
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schwimmer JB, et al. Prevalence of fatty liver in children and adolescents. Pediatrics. 2006;118(4):1388–93. doi: 10.1542/peds.2006-1212. [DOI] [PubMed] [Google Scholar]
- 2.Bohte AE, et al. The diagnostic accuracy of US, CT, MRI and 1H-MRS for the evaluation of hepatic steatosis compared with liver biopsy: a meta-analysis. Eur Radiol. 2011;21(1):87–97. doi: 10.1007/s00330-010-1905-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lee SS, et al. Non-invasive assessment of hepatic steatosis: prospective comparison of the accuracy of imaging examinations. J Hepatol. 2010;52(4):579–85. doi: 10.1016/j.jhep.2010.01.008. [DOI] [PubMed] [Google Scholar]
- 4.Mennesson N, et al. Liver steatosis quantification using magnetic resonance imaging: a prospective comparative study with liver biopsy. J Comput Assist Tomogr. 2009;33(5):672–7. doi: 10.1097/RCT.0b013e318199d883. [DOI] [PubMed] [Google Scholar]
- 5.Meisamy S, et al. Quantification of hepatic steatosis with T1-independent, T2-corrected MR imaging with spectral modeling of fat: blinded comparison with MR spectroscopy. Radiology. 2011;258(3):767–75. doi: 10.1148/radiol.10100708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokoo T, et al. Nonalcoholic fatty liver disease: diagnostic and fat-grading accuracy of low-flip-angle multiecho gradient-recalled-echo MR imaging at 1.5 T. Radiology. 2009;251(1):67–76. doi: 10.1148/radiol.2511080666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yokoo T, et al. Estimation of hepatic proton-density fat fraction by using MR imaging at 3.0 T. Radiology. 2011;258(3):749–59. doi: 10.1148/radiol.10100659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Idilman IS, et al. Hepatic steatosis: quantification by proton density fat fraction with MR imaging versus liver biopsy. Radiology. 2013;267(3):767–75. doi: 10.1148/radiol.13121360. [DOI] [PubMed] [Google Scholar]
- 9.Moher D, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–9. W64. doi: 10.7326/0003-4819-151-4-200908180-00135. [DOI] [PubMed] [Google Scholar]
- 10.Guyatt GH, et al. GRADE: an emerging consensus on rating quality of evidence and strength of recommendations. BMJ. 2008;336(7650):924–6. doi: 10.1136/bmj.39489.470347.AD. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chabanova E, et al. (1)H MRS assessment of hepatic steatosis in overweight children and adolescents: comparison between 3T and open 1T MR-systems. Abdom Imaging. 2012 doi: 10.1007/s00261-012-9930-2. [DOI] [PubMed] [Google Scholar]
- 12.Mishra P, Younossi ZM. Abdominal ultrasound for diagnosis of nonalcoholic fatty liver disease (NAFLD) Am J Gastroenterol. 2007;102(12):2716–7. doi: 10.1111/j.1572-0241.2007.01520.x. [DOI] [PubMed] [Google Scholar]
- 13.Saadeh S, et al. The utility of radiological imaging in nonalcoholic fatty liver disease. Gastroenterology. 2002;123(3):745–50. doi: 10.1053/gast.2002.35354. [DOI] [PubMed] [Google Scholar]
- 14.Saverymuttu SH, Joseph AE, Maxwell JD. Ultrasound scanning in the detection of hepatic fibrosis and steatosis. Br Med J (Clin Res Ed) 1986;292(6512):13–5. doi: 10.1136/bmj.292.6512.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mehta SR, et al. Non-invasive means of measuring hepatic fat content. World J Gastroenterol. 2008;14(22):3476–83. doi: 10.3748/wjg.14.3476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mazhar SM, Shiehmorteza M, Sirlin CB. Noninvasive assessment of hepatic steatosis. Clin Gastroenterol Hepatol. 2009;7(2):135–40. doi: 10.1016/j.cgh.2008.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cassidy FH, et al. Fatty liver disease: MR imaging techniques for the detection and quantification of liver steatosis. Radiographics. 2009;29(1):231–60. doi: 10.1148/rg.291075123. [DOI] [PubMed] [Google Scholar]
- 18.Reeder SB, et al. Quantitative Assessment of Liver Fat with Magnetic Resonance Imaging and Spectroscopy. J Magn Reson Imaging. 2011;34(4) doi: 10.1002/jmri.22580. p. spcone. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fishbein M, et al. Hepatic MRI for fat quantitation: its relationship to fat morphology, diagnosis, and ultrasound. J Clin Gastroenterol. 2005;39(7):619–25. doi: 10.1097/00004836-200508000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Bydder M, et al. Relaxation effects in the quantification of fat using gradient echo imaging. Magn Reson Imaging. 2008;26(3):347–59. doi: 10.1016/j.mri.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Springer F, et al. Assessment of relevant hepatic steatosis in obese adolescents by rapid fat-selective GRE imaging with spatial-spectral excitation: a quantitative comparison with spectroscopic findings. Eur Radiol. 2011;21(4):816–22. doi: 10.1007/s00330-010-1975-4. [DOI] [PubMed] [Google Scholar]
- 22.Liu CY, et al. Fat quantification with IDEAL gradient echo imaging: correction of bias from T(1) and noise. Magn Reson Med. 2007;58(2):354–64. doi: 10.1002/mrm.21301. [DOI] [PubMed] [Google Scholar]
- 23.Hernando D, et al. Addressing phase errors in fat-water imaging using a mixed magnitude/complex fitting method. Magn Reson Med. 2012;67(3):638–44. doi: 10.1002/mrm.23044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thomsen C, et al. Quantification of liver fat using magnetic resonance spectroscopy. Magn Reson Imaging. 1994;12(3):487–95. doi: 10.1016/0730-725x(94)92543-7. [DOI] [PubMed] [Google Scholar]
- 25.Hamilton G, et al. In vivo characterization of the liver fat (1)H MR spectrum. NMR Biomed. 2011;24(7):784–90. doi: 10.1002/nbm.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lindback SM, et al. Pediatric nonalcoholic fatty liver disease: a comprehensive review. Adv Pediatr. 2010;57(1):85–140. doi: 10.1016/j.yapd.2010.08.006. [DOI] [PubMed] [Google Scholar]
- 27.El-Koofy N, et al. Ultrasonography as a non-invasive tool for detection of nonalcoholic fatty liver disease in overweight/obese Egyptian children. Eur J Radiol. 2012;81(11):3120–3. doi: 10.1016/j.ejrad.2012.06.020. [DOI] [PubMed] [Google Scholar]
- 28.Shannon A, et al. Ultrasonographic quantitative estimation of hepatic steatosis in children With NAFLD. J Pediatr Gastroenterol Nutr. 2011;53(2):190–5. doi: 10.1097/MPG.0b013e31821b4b61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pacifico L, et al. MRI and ultrasound for hepatic fat quantification:relationships to clinical and metabolic characteristics of pediatric nonalcoholic fatty liver disease. Acta Paediatr. 2007;96(4):542–7. doi: 10.1111/j.1651-2227.2007.00186.x. [DOI] [PubMed] [Google Scholar]
- 30.Pozzato C, et al. MRI in identifying hepatic steatosis in obese children and relation to ultrasonography and metabolic findings. J Pediatr Gastroenterol Nutr. 2008;47(4):493–9. doi: 10.1097/MPG.0b013e31817b6e10. [DOI] [PubMed] [Google Scholar]
- 31.Bohte AE, et al. US cannot be used to predict the presence or severity of hepatic steatosis in severely obese adolescents. Radiology. 2012;262(1):327–34. doi: 10.1148/radiol.11111094. [DOI] [PubMed] [Google Scholar]
- 32.Pacifico L, et al. T1-weighted dual-echo MRI for fat quantification in pediatric nonalcoholic fatty liver disease. World J Gastroenterol. 2011;17(25):3012–9. doi: 10.3748/wjg.v17.i25.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fishbein MH, et al. Introduction of fast MR imaging in the assessment of hepatic steatosis. Magn Reson Imaging. 1997;15(3):287–93. doi: 10.1016/s0730-725x(96)00224-x. [DOI] [PubMed] [Google Scholar]
- 34.Yu H, et al. Multiecho water-fat separation and simultaneous R2* estimation with multifrequency fat spectrum modeling. Magn Reson Med. 2008;60(5):1122–34. doi: 10.1002/mrm.21737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yu H, et al. Combination of complex-based and magnitude-based multiecho water-fat separation for accurate quantification of fat-fraction. Magn Reson Med. 2011;66(1):199–206. doi: 10.1002/mrm.22840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reeder SB, Hu HH, Sirlin CB. Proton density fat-fraction: a standardized MR-based biomarker of tissue fat concentration. J Magn Reson Imaging. 2012;36(5):1011–4. doi: 10.1002/jmri.23741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kang GH, et al. Reproducibility of MRI-determined proton density fat fraction across two different MR scanner platforms. J Magn Reson Imaging. 2011;34(4):928–34. doi: 10.1002/jmri.22701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mashhood A, et al. Reproducibility of hepatic fat fraction measurement by magnetic resonance imaging. J Magn Reson Imaging. 2013;37(6):1359–70. doi: 10.1002/jmri.23928. [DOI] [PubMed] [Google Scholar]