Abstract
Pathogens have evolved strategies to promote their survival by dramatically modifying the transcriptional profile and protein content of the host cells they infect. Modifications of the host transcriptome and proteome are mediated by pathogen-encoded effector molecules that modulate host cells through a variety of different mechanisms. Recent studies highlight the importance of the host chromatin and other epigenetic regulators as targets of pathogens. Host gene regulatory mechanisms may be targeted through cytoplasmic signaling, directly by pathogen effector proteins, and possibly by pathogen RNA. Although many of these changes are short-lived and persist only during the course of infection, several studies indicate that pathogens are able to induce long-term, heritable changes that are essential to pathogenesis of infectious diseases and persistence of pathogens within their hosts. In this review, we discuss how pathogens modulate the epigenome of host cells, a new and flourishing avenue of host-pathogen interaction studies.
CME Accreditation Statement: This activity (“ASIP 2014 AJP CME Program in Pathogenesis”) has been planned and implemented in accordance with the Essential Areas and policies of the Accreditation Council for Continuing Medical Education (ACCME) through the joint sponsorship of the American Society for Clinical Pathology (ASCP) and the American Society for Investigative Pathology (ASIP). ASCP is accredited by the ACCME to provide continuing medical education for physicians.
The ASCP designates this journal-based CME activity (“ASIP 2014 AJP CME Program in Pathogenesis”) for a maximum of 48 AMA PRA Category 1 Credit(s)™. Physicians should only claim credit commensurate with the extent of their participation in the activity.
CME Disclosures: The authors of this article and the planning committee members and staff have no relevant financial relationships with commercial interests to disclose.
Due to the emergence of drug-resistant strains and newly discovered pathogens, infectious diseases remain a major concern for public health. Host organisms respond to infection by initiating inflammatory and immune responses in an attempt to clear organisms from their systems. Pathogens have adapted to alter host cell functionality to their own advantage, to promote survival, and, in the case of intracellular pathogens, to generate a suitable environment for replication within the host cell. Pathogens use a wide variety of strategies to manipulate host cells to their benefit. In case of Mycobacterium leprae, the causal agent of leprosy, mycobacterial dissemination to different tissues is mediated through the induction of cell differentiation programs in the Schwann cells it infects.1 Shigella flexneri, a Gram-negative bacterium responsible for bacterial dysentery, induces its own uptake by epithelial cells by modifying the host actin cytoskeleton,2 whereas other Gram-negative bacteria, such as Chlamydia spp., hide inside neutrophils and induce nonapoptotic programmed cell death, before being absorbed by macrophages.3 Obligate intracellular parasites of the phylum Apicomplexa, many of which are important clinical and veterinary pathogens, extensively remodel host cells by incorporating parasite proteins into the cell membrane, restructuring the host cytoskeleton, forming transvesicular networks, and even constructing new organelles.4 On the other hand, viruses hijack host transcriptional and translational machinery to promote virus replication, and can induce uncontrolled proliferation and cancer.
Historically, the focus of most host-pathogen studies has been the interactions of pathogenic proteins with proteins on the host cell surface or cytoplasm. The NF-κB, mitogen-activated protein kinase (MAPK), and Janus-activating kinase/signal transducers and activators of transcription family protein (STAT) signaling pathways are all often activated during infection by pathogens5 and are linked to changes in gene expression and post-translational modification on both cytoplasmic and nuclear proteins. Although the effects of viruses on host transcription are well known, it is becoming increasingly clear that the nucleus and, specifically, chromatin are important targets of numerous classes of pathogens. Many studies have reported major transcriptional changes in host cells infected by a variety of pathogens.6 These transcriptional changes modulate a wide range of pathways that pathogens exploit to enhance their own survival.
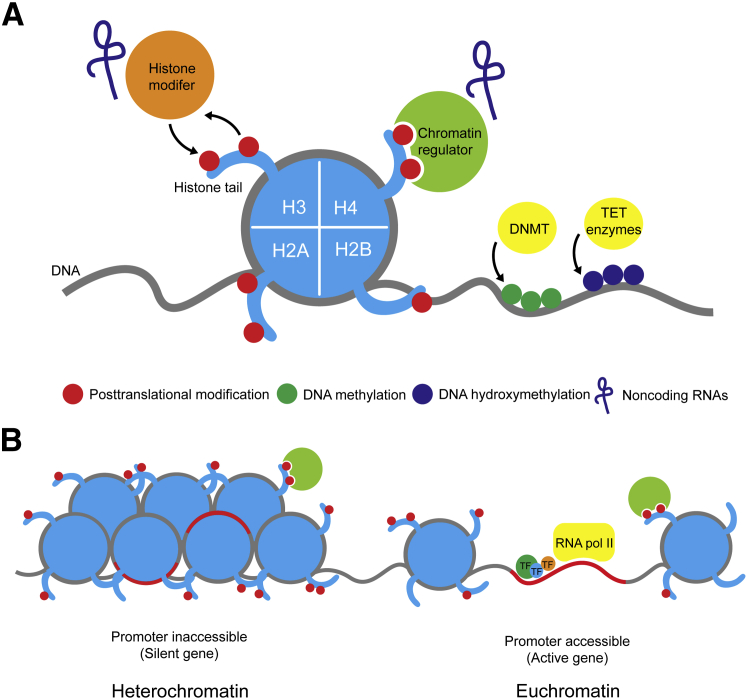
Gene expression is regulated by epigenetic mechanisms that are not directed by DNA sequence (Figure 1). Several types of mechanism are known to occur. First, DNA can be modified by the addition of a methyl group to cytosine or adenosine nucleotides, catalyzed by DNA methyltransferases. Second, DNA methylation predominantly occurs on cytosine residues that are in a CpG dinucleotide context; this modification is associated with transcriptional silencing. Recent studies also show that methylcytosine can be converted to hydroxymethylcytosine by the Ten-eleven translocation proteins, and has been linked to regulation of self-renewal and differentiation in embryonic stem cells.7
Figure 1.
Summary of epigenetics. A: Mechanisms of epigenomic gene regulation. Gene regulation is controlled by multiple epigenetic mechanisms, including DNA methylation, histone post-translational modifications, chromatin remodeling, and ncRNAs. B: Epigenetic modifications regulate chromatin state. Heterochromatin is tightly packed DNA, in which DNA is often methylated and promoters (red lines) are inaccessible to DNA-binding proteins and transcriptional complexes, rendering such genes inactive or silenced. In euchromatin, DNA is unwound by chromatin regulators and accessible to transcriptional machinery, including RNA polymerase II (RNA pol II) and transcription factors (TFs), thus allowing transcription to occur. DNMT, DNA methyltransferase; H2A, H2B, H3, and H4, histone proteins; TET, Ten-eleven translocation proteins.
DNA itself is wrapped around a core complex of four histone proteins, which bind DNA and form a nucleosome. Post-translational modification (PTM) of histones is another major level of epigenetic control, by which combinations of modifications (eg, phosphorylation, acetylation, or methylation) contribute to a histone code, which regulates the accessibility of DNA to transcriptional machinery. Histone modifications are highly dynamic and play an essential role in regulating gene expression during cell cycle, changes in intracellular conditions, or in response to different stimuli. They are added or removed by chromatin-modifying enzymes, which, in turn, are subject to transcriptional and post-translational regulation. PTMs attract chromatin regulators or remodeling complexes, which control changes in chromatin state by altering histone-DNA interactions.
More recently, noncoding RNAs (ncRNAs) and miRNAs were added to the repertoire of epigenetic regulators. ncRNAs appear to play a role in DNA silencing, post-transcriptional regulation, and genome maintenance.8 Furthermore, RNA molecules direct several processes, including DNA methylation, post-translational modification of histones, and binding of chromatin remodeling complexes. Their role is not as well understood as other epigenetic processes previously mentioned.
Epigenomics refers to the study of genome-wide epigenetic modifications. Herein, we discuss the importance and prevalence of epigenomic mechanisms exploited by a variety of different pathogens, speculate on how effector proteins are released into host cells, and look at long-lasting epigenetic changes induced by pathogens. The effects of viruses on the epigenetic and transcriptional machinery of the cells they infect have been studied extensively. Recent studies show that bacterial and eukaryotic microbes also secrete effectors that modify the epigenome of their hosts, having broad impact on host-pathogen interactions.
Dysregulation of Gene Regulation Induced by Pathogens
Transcriptional Dysregulation
Many infections result in the activation of genes central to host cell response, particularly those involved in stress responses or inflammation and immunity. Infection can lead to changes in expression of specific genes, such as those encoding transcription factors and chromatin modifiers. Changes in host gene expression are often organism specific, suggesting that these effects are orchestrated by the organism. Infection of monocyte-derived dendritic cells and macrophages with several phylogenetically distinct organisms results in organism-specific changes in gene expression and differences in transcriptional dysregulation in monocyte-derived dendritic cells and macrophages,9 indicating that transcriptional dysregulation is specific to the cell type infected and the infecting organism. Changes in gene expression can also occur depending on the life cycle stage of an organism. For example, the latent, slow-growing bradyzoite forms of Toxoplasma gondii parasites induce dysregulation of fewer host genes compared with their acute, fast-growing counterparts, the tachyzoites.10
Ordered Transcriptional Dysregulation
When an organism enters a host cell, the host cell responses are rapidly activated in an attempt to eradicate the organism. Hence, immediate targeting of the genes regulating those initial responses by the pathogen would be beneficial to intracellular survival. Plasmodium spp. parasites, responsible for malaria, invade and replicate inside liver cells and induce changes in transcription of >1000 hepatocyte genes11; some of these changes in mRNA can be detected as soon as 30 minutes after infection. To investigate how the host transcriptome changes over time, Albuquerque et al11 performed time-lapse studies on malaria-infected hepatoma cells. Intriguingly, although several gene sets are dysregulated at all times during infection, 24 genes were constitutively differentially expressed during infection, including transcripts encoding signaling enzymes and endoplasmic reticulum-stress response proteins, as well as important transcriptional regulators. In the early stages of infection, stress response genes and genes encoding receptor-binding proteins were up-regulated, and it was only later in infection that genes encoding products involved in host metabolism were altered. This study suggested that transcriptional dysregulation is an ordered, sequential process, with different gene sets being altered throughout the infection process. Similar findings have been reported in infections with the apicomplexan parasite, T. gondii,12 infection of Schwann cells with the bacterium, M. leprae,1 and infections with viruses, such as cytomegalovirus.13
Alterations to the Host DNA Methylome
DNA methylation patterns correlate tightly with transcriptional data and can change dramatically when cells encounter a pathogen. DNA methylation was previously thought to be a stable modification, but is now known to be dynamic, changing even within a single cell cycle.14 Jähner and Jaenisch were the first to show that integration of viral DNA into host DNA induces local changes in DNA methylation, resulting in transcriptional silencing which is thought to contribute to viral latency by the maintenance of proviral DNA in silenced regions.15 Hepatitis B viral infection induces changes in DNA methylation16 that correlate with up-regulation of DNA methyltransferase expression.17 DNA methyltransferases are recruited to DNA in response to hepatitis B infection, resulting in the hypermethylation of the urokinase-type plasminogen activator promoter.17 Urokinase-type plasminogen activator is essential for activation of hepatocyte growth factor, which activates regeneration of liver tissue damaged during severe hepatitis infection. Thus, these studies directly link epigenetic modulation to pathogenesis of hepatitis B infection in the liver. Activation of DNA methyltransferases also may play a role in Epstein-Barr virus (EBV) pathogenesis,18 including development of gastric carcinoma associated with EBV.19
Dysregulation of Nonhost Cells
Although host cells infected by pathogens undergo major remodeling, cells that are not invaded also may undergo transcriptional dysregulation and contribute to disease pathogenesis. During cell invasion, T. gondii secretes several proteins into host cells, several of which have been implicated in host cell remodeling.20 Occasionally, parasites undergo abortive invasion and bind to the surface of cells, but they do not invade. During abortive invasion, T. gondii still secretes proteins into the host cells, and this results in phosphorylation of components of the Janus-activating kinase/STAT pathway and their nuclear translocation,21 as occurs in successful invasions.22
The function of regulation of uninfected cells is unclear—parasites could be simply probing for a suitable cell to infect; alternatively, this phenomenon could be relevant to pathogenesis. The observation that uninfected-injected cells are in abundance in the brains of T. gondii–infected mice21 supports the latter, and is an appealing explanation for the changes in behavior observed in mice that are chronically infected with T. gondii.23 Moreover, it presents a potential mechanism by which T. gondii infection could be involved in pathogenesis of some human psychiatric conditions,24 although a direct association between T. gondii and such disorders has not been demonstrated.
Turning to bacterial infections, the facultative intracellular bacteria, Salmonella typhi, S. flexneri, and Listeria monocytogenes, all induce activation of proinflammatory responses in uninfected bystander cells.25 Exposure to noninvasive S. flexneri does not result in activation of NF-κB; this suggests that the response is not due to abortive invasion, as in T. gondii, but does not exclude the possibility that wild-type S. flexneri alters host signaling by directly injecting effector proteins into cells without invading them. The mechanisms governing these phenomena are unknown, but recent work on exosomes (discussed later) may provide some potential clues.
Molecular Mechanisms of Epigenetic Modification
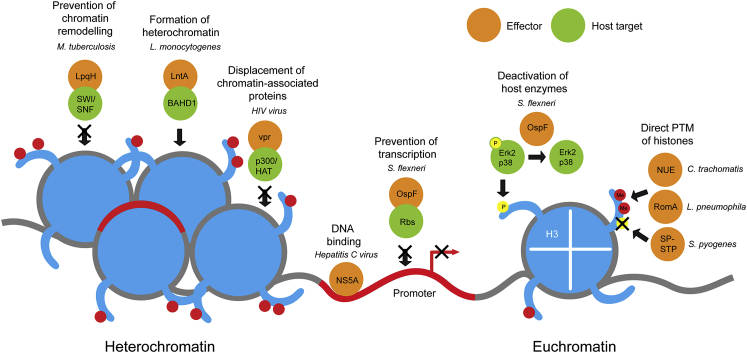
Pathogens manipulate the host epigenome through a diverse set of mechanisms (Table 1 and Figure 2). Recent studies have focused on the concept of hijacking host cell function by direct interaction of pathogen-derived proteins with nuclear components. Such effector proteins have been referred to as nucleomodulins,51 relating to their role in modulating nuclear processes. Bacteria have even been shown to enter the host nucleus themselves (eg, in the case of endobacterium Holospora, which infects Paramecium parasite nuclei and alters gene expression).52 Herein, we focus on proteins that gain access to the nucleus and interfere with nuclear processes, and the implications for studies on host-pathogen interactions.
Table 1.
Strategies Exploited by Pathogens to Modulate the Host Epigenome
| Mechanism | Organism | Effector protein | Target molecule | References |
|---|---|---|---|---|
| Direct interaction with DNA | Anaplasma phagocytophilum | AnkA | DNA | 26 |
| Theileria annulata | Secreted AT hook proteins (eg, SuAT1) | DNA | 27 | |
| Hepatitis C virus | NS5A | DNA | 28 | |
| Hijacking nuclear signaling pathways | Shigella flexneri | OspF | MAPKs | 29 |
| Salmonella spp. | SpvC | MAPKs | 30 | |
| Direct proteolytic degradation | Chlamydia trachomatis | CT441 | p65/ReI | 31 |
| Sequestration or deactivation of transcription factors | Toxoplasma gondii | Unknown | STAT1 | 22 |
| Adenovirus 5 | EB1-55K | DAXX | 32 | |
| Chlamydia spp. | Unknown | ZNF23 | 33 | |
| Post-translational modification by secreted enzymes | Chlamydia trachomatis | NUE methyltransferase | Host chromatin, histones | 34 |
| Streptococcus pyogenes | Ser/Thr phosphatase SP-STP | Host chromatin | 35 | |
| Mycobacterium tuberculosis | Mycobacterial Ser/Thr phosphatase | Histones | 36 | |
| Legionella pneumophila | RomA methyltransferase | Histone H3 K4 | 37 | |
| Paramecium bursaria chorella virus | Chorella virus methyltransferase | Histone H3K27 | 38 | |
| Toxoplasma gondii | Protein phosphatase 2C | Host nuclei | 39 | |
| Association with nuclear proteins | Toxoplasma gondii | GRA16 | HAUSP deubiquitinase and PP2A phosphatase | 20 |
| EBV | EBNA3C | Polycomb, mSin3A, NCoR, histone deacetylases | 40 | |
| Shigella flexneri | OspB, OspF | Rb tumor suppressor proteins | 41 | |
| Anaplasma phagocytophilum | AnkA | SHP-1 | 26 | |
| Listeria monocytogenes | LntA | BAHD1 | 42 | |
| Displacement of chromatin-associated proteins | HIV | Vpr | p300/HAT | |
| Alteration of chromatin structure | Mycobacterium tuberculosis | 19-kDa lipoprotein LpqH | SWI/SNF and C/EBPβ | 44 |
| Toxoplasma gondii | Unknown | NFKB, cJun, CREB | 45 | |
| Varicella zoster virus | Immediate-early 63 protein | ASF1 | 46 | |
| Molecular mimicry | EBV | EBNA1 | Viral/host cell promoters | 47 |
| Poxvirus | A49 | NFKB p65 | 48 | |
| Influenza A virus | NS1 | PAF complex | 49 | |
| Neisseria meningitidis | DMP12 | NHTF | 50 |
A wide variety of mechanisms are exploited by pathogens to modulate nuclear processes in host cells, from effector proteins, which target host DNA to mediate or repress transcription, to post-translational modification of histones by secreted effector proteins. Some examples mentioned herein are summarized.
ASF1, anti-silencing function protein 1; CREB, cAMP response element binding protein; NCoR, nuclear corepressor; NHTF, nitrogen-response transcription factor; RomA, regulator of methylation; SHP-1, SH2 domain containing protein tyrosine phosphatase 1; SpvC, salmonella plasmid virulence C protein.
Figure 2.
Host epigenetic mechanisms affected by pathogens. Pathogens use a wide variety of mechanisms to modulate host chromatin, as discussed further in Molecular Mechanisms of Epigenetic Modification and summarized in Table 1. To prevent chromatin remodeling and, therefore, maintain a silenced state, M. tuberculosis secretes LpqH lipoprotein, which binds to SWI/SWF remodeling complexes and blocks their function. L. monocytogenes regulates chromatin state via the effector protein LntA, which recruits heterochromatin regulator BAHD1 to recruit heterochromatin proteins and induce formation of heterochromatin. HIV, on the other hand, uses vpr protein to target p300/HAT complexes, causing them to dissociate from chromatin. Alternatively, some pathogens express proteins that directly bind DNA to induce transcription or prevent it. Hepatitis C virus expresses NS5A, which binds promoter regions of host genes. S. flexneri prevents transcription by sequestering host transcription factors, such as the Rb tumor-suppressor proteins. Chromatin state is also regulated by histone post-translational modifications, which can be modulated through manipulation of host enzymes or directly through secreted effector enzymes. For example, S. flexneri modulates the phosphorylation of histone H3S10 through the activity of OspF, a secreted phosphothreonine lyase. OspF removes phosphate groups from Erk2 and p38, two members of the MAPK pathway, which prevents MAPK-dependent H3S10 phosphorylation. Gray line, DNA; red line, silenced promoter; red circles, histone PTMs. Me, cytosine methylation.
Modulation of Host Signaling Pathways
Hijacking of Nuclear Signaling Pathways
Signaling pathways in the nucleus orchestrate gene expression and are hijacked by pathogens to control host genes. Like a multitude of pathogens, S. flexneri infection strongly activates the NF-κB signaling pathway; however, in this case, NF-κB is prevented from binding selected promoters by S. flexneri–induced dephosphorylation of histone H3 at serine 10.29 In uninfected cells, H3S10p increases the accessibility of chromatin to transcription factors, such as NF-κB. Blocking H3S10 phosphorylation prevents the activation of NF-κB–regulated genes, some of which encode cytokines. This is achieved through the secretion of a phosphothreonine lyase, outer surface protein F (OspF) which hijacks nuclear MAPK enzymes to catalyze H3S10 dephosphorylation.29 More important, recombinant OspF is unable to directly dephosphorylate H3S10 in vitro, but it does target several MAPKs in the nucleus, causing their irreversible dephosphorylation.53 The ultimate effect of OspF secretion is prevention of leukocyte recruitment to sites of infection,29 which presumably aids survival of S. flexneri because the bacteria are not cleared by the immune system. Furthermore, the studies suggest that OspF is also responsible for an increased transmigration of leukocytes across the epithelial barrier, resulting in increased access to tissue for bacteria to invade. Other histone modifications induced by S. flexneri have not been studied, although it is likely that others play a role in this complex process.
Deactivation of Host Cytoplasmic Signaling by Protein Degradation
Pathogen-induced proteolysis is a major mechanism for deactivation or aberrant activation of host cell effector proteins. Unlike many other pathogens, Chlamydia trachomatis, an intracellular bacterium that causes ocular and sexually transmitted infections, does not induce NF-κB signaling on cell invasion. Rather, it prevents activation of NF-κB by direct proteolytic cleavage of p65/ReI protein,31 a constituent of the NF-κB signaling cascade. A secreted C-tail protease called CT441 specifically cleaves p65/ReI into two fragments, p40 and p22. The p40 fragment is inhibitory to NF-κB activation. Whether p65/ReI is the only substrate for CT441 is unknown. Although the in vivo role of this proteolytic activity is unclear, it could contribute to the ability of C. trachomatis to persist in humans through failure to mount long-lasting, protective immunity.
Direct Targeting of Host Nuclear Proteins by Pathogen Mediator Proteins
Direct Interaction of Pathogen-Derived Proteins with DNA
Some effector proteins interact directly with DNA and may act as eukaryotic transcription factors. The rickettsial bacterium, Anaplasma phagocytophilum, induces transcriptional changes during infection, and down-regulates host defense genes.26 A key molecule is the secreted protein, ankyrin-repeat protein A (AnkA), which translocates to host nuclei and directly binds host DNA and nuclear proteins.26 Transfection of cells with DNA encoding AnkA induces some of the transcriptional changes associated with Anaplasma infection, such as silencing of the cytochrome b-245 gene promoter,26 but not all, suggesting that other bacterial factors come into play.
The apicomplexan parasite, Theileria spp., also secretes several proteins into the host cell, notably including those with high similarity to eukaryotic AT hook domains, which are transported to the host cell nucleus.27 When macrophages are transfected with one of these AT hook proteins, SuAT1, significant changes in cell morphological characteristics and in transcription of cytoskeletal proteins are observed. Whether these proteins play a role in the ability of Theileria spp. to induce continuous cell proliferation (described later) is unclear.
Virally encoded transcription factors have also been described. Hepatitis C non-structural protein 5A (NS5A) was previously shown to be important for viral replication, but recent evidence suggests that it is a multifunctional protein able to regulate host gene expression.28 The C-terminus of NS5A is cleaved in a caspase-dependent manner in the cytoplasm, after which it translocates to the nucleus and binds the promoters of host genes. This study lays the groundwork for future searches for unique, pathogen-encoded transcription factors.
Association of Pathogen Factors with Nuclear Proteins
Pathogens also influence the epigenome through interaction with host nuclear proteins, including enzymes. Toxoplasma gondii secretes several virulence factors, including GRA16, which is released from dense granule organelles into the host cell several hours after invasion.20 GRA16 is essential for virulence in mice and is able to modify the host transcriptome, altering the expression of host metabolism and cell cycle genes. Immunoprecipitation of GRA16 reveals that it interacts with several host nuclear proteins, including herpes virus-associated ubiquitin-specific protease (HAUSP) and protein phosphatase 2A (PP2A), with which it forms a high-molecular-weight complex. GRA16 appears to induce the translocation of PP2A into host nuclei, where it assembles into the complex with HAUSP. Both PP2A and HAUSP have links to cell proliferation and cell cycle functions. HAUSP is known to stabilize TP53 during EBV infections, leading to immortalization of cells,54 and HAUSP could play a similar prosurvival role in T. gondii infections.
Negative regulation of transcription is achieved, in part, through inhibitory transcription factors called repressors, which can be hijacked by pathogens. One of the Epstein-Barr virus nuclear antigens (EBNA), EBNA3C, acts as a repressor of host transcriptional activity, targeting several different genes, such as the gene-encoding proapoptotic protein, Bim.55 Transcriptional repression seems to be achieved through the association of EBNA3C with polycomb-repressive complexes, histone deacetylases, and corepressor proteins (mSin3A and NCoR).
Host repressor proteins are also exploited by several bacteria. Shigella flexneri secretes two effector proteins, OspB and OspF, which bind members of the retinoblastoma (Rb) group of tumor-suppressor proteins41 and presumably prevent their binding to DNA. Dysregulation of Rb proteins is observed in many cancers, and they are essential for normal cell growth, with roles in cell cycle regulation, recruitment of chromatin remodeling complexes, and chromatin architecture. By binding Rb proteins, S. flexneri may be able to down-regulate the host immune response, dampening the production of IL-8. 41
Listeria monocytogenes, a foodborne pathogen, modulates host gene expression by reversing the formation of heterochromatic regions.42 This is achieved by interfering with the function of bromo adjacent homology domain-containing protein 1 (BAHD1), a repressor protein that promotes the formation of heterochromatin by recruiting proteins involved in heterochromatin assembly.56 Listeria monocytogenes secretes the effector protein, listeria nuclear targeted protein A (LntA), which binds BAHD1 and colocalizes with it at heterochromatic regions, ultimately resulting in impaired binding of BAHD1 to promoters and stimulation of type III interferon (IFN).42 How LntA achieves the exclusion of BAHD1 from promoters is unclear, but its effect mirrors a study showing that depletion of BAHD1 from cells leads to increased expression of prosurvival and proliferation genes.56
Sequestration or Deactivation of Transcription Factors
Some pathogens interfere with transcription by preventing trafficking or deactivation of host transcription factors. Toxoplasma gondii infection induces phosphorylation of STAT1,22 which normally activates STAT1 and results in its translocation to the nucleus. But, during T. gondii infection, transcription of IFN-γ genes regulated by STAT1 is impaired.22 Since STAT1 is phosphorylated and able to bind an STAT1-dependent, IFN-γ–responsive DNA sequence, how transcriptional inhibition occurs has not been determined.22 As trafficking of STAT1 to the nucleus and DNA-binding activity are unaffected, one hypothesis is that a T. gondii effector protein interferes with recruitment of proteins by STAT1 for transcriptional activation. In an alternative mechanism, T. gondii sequesters IκBα, an inhibitor constituent of the NF-κB complex, at the parasitophorous vacuole membrane by phosphorylating it in a host-independent manner.57 In this way, T. gondii reconfigures the host cell signaling pathways to induce transcriptional changes.
Similarly, Chlamydia spp. sequester host nuclear proteins by recruiting them to the site of Chlamydia replication, a type of parasitophorous vacuole termed an inclusion.33,58 One of the proteins recruited to the inclusion is zinc finger nuclear protein 23 (ZNF23),33 a proapoptotic transcription factor and repressor of cell division. Intriguingly, ZNF23 disappears from the host nucleus and cytoplasm and is apparently incorporated into the lumen of the inclusion, along with its binding partner, acetyl-CoA binding protein ACBD6, which usually localizes to the periphery of nuclei. Recruitment of ZNF23 to the inclusion may sequester the protein and prevent activation of apoptotic pathways,33 but further study is needed to determine whether ZNF23 is important for other aspects of inclusion maintenance or if inclusion proteins modulate host apoptosis.
Although sequestration of transcription factors prevents their binding of target genes, other pathogens induce the degradation of host proteins for the same gain. Death domain–associated proteins (DAXXs) are associated with X-linked α-thalassemia retardation syndrome chromatin remodeling complexes, which regulate the deposition of histones onto heterochromatin and act as transcriptional repressors through methylation of viral DNA and epigenetic repression.59 In adenovirus 5 infection, the virus has evolved to restore transcription by targeting DAXX for degradation. The mechanism for this is controversial and may occur by ubiquitin/proteasome-dependent degradation via the viral protein, EB1-55K,60 or through assembly of viral proteins into a ubiquitin ligase complex, which then leads to proteasome-dependent degradation.61
Post-Translational Modification of Host Nuclear Proteins by Enzymes Secreted by Pathogens
Some bacteria secrete methyltransferases that directly catalyze methylation of host histones. These include nuclear effector E (NUE), a secreted histone methyltransferase, one of many proteins secreted by C. trachomatis into the host cell. NUE localizes to host nuclei during infection and binds to host chromatin.34 In vitro methyltransferase activity assays indicate that NUE is able to methylate mammalian histones. The sites of mammalian histone methylation by NUE have yet to be identified, but will provide valuable information about the influence of this enzyme on the host histone code. Another secreted bacterial methyltransferase, Legionella pneumophila RomA, is a member of a group of genes encoding proteins with high similarity to eukaryotic proteins (Legionella eukaryotic-like genes). Like NUE, RomA targets histones for methylation, inducing trimethylation of histone H3K14,37 a mark that had not previously been identified in mammals. Such effectors are not restricted to bacteria: a SET domain-containing protein with methyltransferase activity was identified in Paramecium bursaria chlorella virus, a virus that infects certain types of algae.38 The chorella virus methyltransferase specifically targets histone H3K27 for dimethylation, a histone mark that correlates with gene silencing.
Aside from methyltransferases, a few other candidate secreted epigenetic modifiers are known. Mycobacterium tuberculosis secretes a protein phosphatase that can dephosphorylate histones in vitro,36 although there is no evidence that it performs this function in vivo. The Gram-positive bacterium Streptococcus pyogenes expresses a serine/threonine phosphatase, which is secreted into host cells and targets to host nuclei.62 There, it acts as a proapoptotic factor that induces apoptosis of pharyngeal cells, a hallmark of streptococcal infections, by influencing transcription of apoptotic genes and preventing the transcription of other genes, such as cytochrome p450. Although the enzyme is functional and has a role in bacterial adhesion, its targets in host nuclei remain elusive.
T. gondii also targets a protein phosphatase 2C protein to host nuclei,39 but its effect on the epigenome has not been investigated. The transcriptional and epigenetic machinery of protozoan parasites shares many similarities with that of other eukaryotes,63 and many apicomplexans secrete kinases and phosphatases into the host cell. It is possible that some of these secreted effectors alter chromatin-modifying activity or directly target histones.
Displacement of Chromatin-Associated Proteins from Chromatin
Chromatin-associated proteins can be displaced from chromatin by pathogenic proteins. One of the HIV accessory proteins, viral protein R (vpr), interferes with sister chromatid segregation during mitosis, through its interaction with p300/HAT, a histone acetyltransferase-regulating transcription factor.43 p300/HAT is actively recruited to chromatin, where it appears to displace heterochromatin protein 1, an important factor in centromere cohesion. Cells expressing vpr exhibit aberrant mitosis. Similar findings have been observed in human cytomegalovirus-infected cells,64 suggesting that pathogen-induced changes in chromatin structure may be more common than is appreciated.
Alteration of Chromatin Accessibility, Chromatin Remodeling
The structure of chromatin governs accessibility of DNA to transcription factors; extensive remodeling around promoter regions is required for transcription initiation to occur. Because of this, chromatin structure plays an important role in host transcriptional responses in many infections. During M. tuberculosis infection, inhibition of expression of some IFN-γ–responsive genes is observed65; the same effect is noted when cells are exposed to LpqH, a 19-kDa lipoprotein of M. tuberculosis.66 Mechanistically, LpqH prevents binding of the SWItch/Sucrose NonFermentable (SWI/SNF) chromatin remodeling complex to chromatin at the class II transactivator locus, leading to inactivation of this gene.44,67 Furthermore, LpqH induces binding of transcription factor CCAAT/enhancer-binding protein beta (C/EBPβ) to the promoter of the gene-encoding class II transactivator44 and, thus, contributes to its silencing.
After infection with T. gondii, several host transcription factors are prevented from binding their TNF-α promoter binding sites.45 These findings suggest that either chromatin remodeling is inhibited at that locus or these proteins are actively excluded from DNA by another mechanism. In support of the former hypothesis, infection with T. gondii prevents phosphorylation of histone H3S10 and acetylation of H3K9 and H3K14 at the TNF-α locus on stimulation of cells with lipopolysaccharide (LPS).45 The same effect is observed at the locus encoding the cytokine IL-10, where H3S10 and K3K9/K14 marks also were abolished,68 suggesting that this mechanism of silencing is not solely specific to the TNF-α gene.
Studies in yeast have shown that nucleosomes are extensively repositioned in response to physiological stress.69 Consistently, nucleosome repositioning occurs in response to stimulation with LPSs. A single nucleosome spans the promoter of IL-12, and during LPS stimulation, this nucleosome is displaced, and cytokine IL-12 mRNA can be transcribed.70 This phenomenon has also been observed in response to viral infections, where it is mediated by SWI/SNF complexes71; changes in nucleosome position in CpG island p16 are observed in gastric carcinomas induced by Helicobacter pylori,72 although a lack of genome-wide studies makes it difficult to interpret the relevance of this observation.
In other cases, nucleosomes may be evicted from DNA. For example, the herpes virus Varicella zoster interacts with host nuclear protein ASF1,46 a host nuclear protein involved in histone deposition and eviction of nucleosomes from DNA, a function that may be important for the regulation of viral and cellular transcription.
Examples of Molecular Mimicry of Nuclear Proteins in Infectious Diseases
Molecular mimicry is a mechanism used by pathogens for immune evasion, and recent studies suggest that molecular mimicry extends to interference with nuclear processes. EBV protein, EBNA1, has homology to high-mobility group A transcription factors and is important for tethering viral DNA to cellular DNA during mitosis.73 EBNA1 binds to both viral and host cell promoters, where it promotes chromatin decompaction and regulates transcription.47 Poxviruses evade the NF-κB signaling pathway through protein A49, which contains a conserved IκBα motif and replaces IκBα in a complex with NF-κB p65,48 preventing the nuclear translocation of NF-κB and activation of NF-κB–responsive genes.
Influenza A virus uses mimicry to interfere with host transcriptional elongation. Influenza A non-structural protein 1 (NS1) contains a peptide that shares high similarity with histone H3.49 NS1 has multiple functions in dampening host response to infection, including post-transcriptional blocking of pre-mRNA maturation by prevention of polyadenylation and export of processed mRNAs.74 NS1 specifically interacts with the host cell epigenome by targeting the host RNA polymerase II associated factor 1 (PAF1) transcriptional elongation complex through its histone-like domain, causing PAF1 and RNA polymerase II levels to decrease at specific target genes to alter transcription of antiviral genes.49 Histone mimics have also been identified in many bacterial species, including Mycobacteria spp.75; however, their role in regulating the host epigenome has not been investigated.
Few DNA mimics have been described. Such mimics act by occupying sites that would otherwise be bound by DNA-binding proteins. Neisseria meningitidis expresses a DNA mimic called DNA mimic protein 12 (DMP12), which is able to neutralize repressive effects of another transcription factor, nitrogen-response transcription factor (NHTF),50,76 representing a new mode of gene regulation.
Delivery of Effector Proteins
Both intracellular and extracellular pathogens can deliver effector proteins to the host cell. Most bacteria use some kind of specialized secretion system.77 Intracellular pathogens can use specialized secretion systems, regulated secretory vesicles, and protein export through parasitophorous vacuoles to direct proteins into the host cell.
An emerging concept is that exosomes, late endosome–derived microvesicles, can be used by pathogens to transport effector molecules into the host cell. Exosomes have been shown to be vectors of miRNA, lipid mediators, and various types of protein,78 and have roles in cell-cell communication. In the context of infectious diseases, exosomes can be secreted by either infected host cells or pathogenic organisms to modulate host processes. Exosomes secreted from HIV-infected cells are able to induce apoptosis in bystander CD4+ T-cells.79 Macrophages infected with T. gondii, Salmonella typimurium, M. tuberculosis, or Mycobacterium bovis all release exosomes.80 EBV-induced exosomes contain miRNAs that repress EBV target genes,81 a process that could contribute to viral latency.
Microvesicles purified from Plasmodium-infected erythrocytes activate macrophages in vitro, inducing transcription of proinflammatory cytokines and neutrophil chemotaxis.82,83 Interestingly, these particles are more potent than purified parasitized erythrocytes, suggesting that some component of microvesicles is key to activating immune responses to malaria infection. These studies suggest that malaria-infected erythrocytes exploit exosomes for cell-cell communication and that microvesicles derived from infected erythrocytes increase differentiation of parasites into sexual stages that are essential for transmission of the parasite through mosquitoes.82,83 Plasmodium-derived microvesicles could contain factors that are released into target cells, which the authors propose induce transcriptional programs leading to sexual stage development, thus acting as a form of quorum sensing in parasites.
Evidence supporting the release of exosomes from extracellular pathogens, including bacteria and protozoan parasites, has emerged in recent years. Gram-negative bacteria release outer membrane vesicles, which are similar to exosomes in size. The opportunistic pathogen, Acinetobacter baumannii, uses outer membrane vesicles to deliver a transposase protein able to enter host nuclei and methylate promoters of genes encoding E-cadherin,84 implying that this mode of delivery of proteins by pathogens may be an important mode of delivery of epigenetic regulators.
Eukaryotic pathogens also release exosomes. The cargo of such vesicles varies widely. In a proteomic study of exosomes from the fungus Cryptococcus neoformans, histone proteins H2A and H4 were identified.85 Exosomes derived from Histoplasma capsulatum, another fungal pathogen, contain histones as well as GTP-binding nuclear protein, nuclear transport factors, proteins involved in DNA assembly and DNA binding, and an RNA helicasesome.86 Interestingly, Leishmania spp. vesicles contain an elongation initiation factor 1-α homologue, which could interfere with protein translation if absorbed by a cell, and heat shock proteins.87 In addition to conserved exosomal proteins and parasite-derived proteins, vesicles from the sexually transmitted parasite, Trichomonas vaginalis, contain small RNAs,88 such as mammalian exosomes. Purified T. vaginalis exosomes specifically regulate the production of the proinflammatory cytokines, IL-8 and IL-6.88
Long-Term Consequences of Epigenetic Modulation of Host Cells
Although many epigenetic modifications are dynamic and highly transient, the original definition of an epigenetic mark, by Russo and Russo,89 is that it can be inherited through mitosis, allowing a cell to retain its transcriptional profile and provide long-term memory. Most of the modifications described herein follow a transient pattern, but there are some examples (Figure 3) that strongly support the idea that pathogens can induce long-term, heritable, epigenetic modifications essential to the pathogenesis of chronic diseases.
Figure 3.
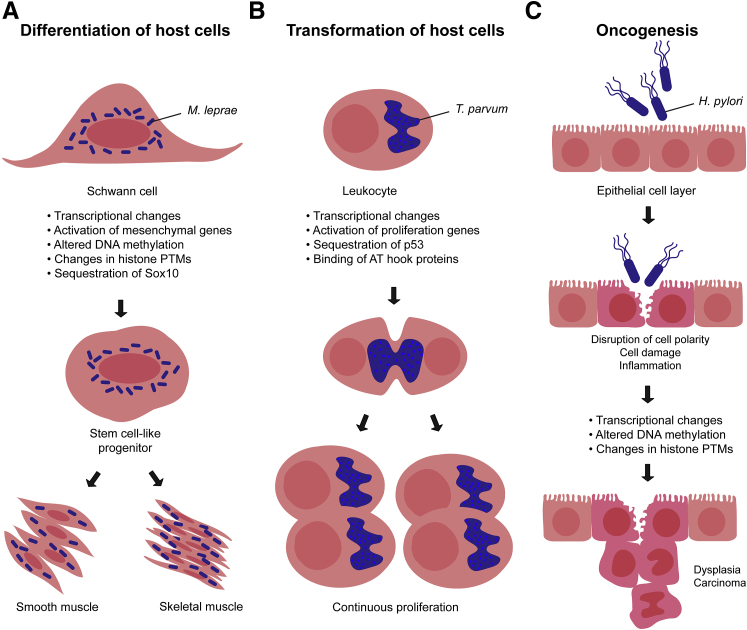
Long-term epigenetic changes mediated by pathogens. A: Reprogramming of host cells by M. leprae. Mycobacterium leprae induces the Schwann cells it infects to differentiate into stem cell–like progenitor cells, which have the capacity to differentiate into multiple cell types, including smooth muscle or skeletal muscle cells. By inducing the reprogramming of Schwann cells, M. leprae regulates its own dissemination throughout different tissues. B: Transformation of host cells by T. parva. Theileria parva is, to date, the only organism known to induce continuous proliferation of the host cells it infects, which is directly tied to the division of this parasite as it hijacks the cell’s division machinery. Parasites induce transcriptional changes that lead to the suppression of apoptosis and up-regulation of proliferation genes. AT hook-binding proteins are also used to influence the transcriptome of host genes to promote survival of T. parva. C: Oncogenesis induced by chronic H. pylori infection. The bacterium H. pylori induces profound changes in transcription in its target tissue, the gastric epithelium. By secreting enzymes and virulence factors onto the surface of the epithelium and into cells, it induces damage to epithelial cells and a loss of cell polarity. Chronic exposure to H. pylori leads to altered transcription and DNA methylation, mirrored by changes in histone PTMs and eventual dysplasia and carcinogenesis.
Differentiation of Host Cells by M. leprae
A fascinating study on M. leprae found that these bacteria regulate their own dissemination in the host by inducing differentiation of the infected host cell by epigenetic reprogramming.1 M. leprae reproduce inside Schwann cells, causing neurological injury and damage to sensorimotor functions. To mediate distribution of bacteria to the body, M. leprae induces the differentiation of Schwann cells into a stem cell–like progenitor state. In infected cells, transcription of genes associated with nuclear functions and, in particular, embryonic development are altered. Reprogrammed cells further develop into mesenchymal, skeletal muscle, or smooth muscle tissue; mycobacteria also induce production of granuloma-like structures able to release macrophages containing bacteria.
Typically, reprogramming of cells into pluripotent stem cells requires major remodeling of chromatin structure. For example, during the early stages of reprogramming, dimethylation of H3K4 is observed at loci associated with pluripotency,90 priming these genes for activation. Although the methylation status of H3K4 was not examined, phosphorylation of H3S28 was observed,1 a mark concurrent with cell cycle stages. Infection with M. leprae is accompanied by alteration in DNA methylation status, with the promoters of several mesodermal and epithelial-mesodermal transition genes being significantly demethylated, indicating that they are epigenetically reprogrammed into a transcriptionally active state during infection.
Reprogramming by M. leprae is likely to occur by multiple mechanisms, including induction of the translocation and removal of the Sry-box transcription factor (SOX) SOX10 from the nucleus.1 SOX10 is a major regulator of Schwann cell homeostasis, gene expression, and myelination, acting through the recruitment of chromatin remodeling complexes. Considering the important role of SOX10 in these cells, removal from nuclei is likely to dramatically influence transcription. Furthermore, the SOX10 locus is strongly methylated in infected Schwann cells, suggesting that M. leprae blocks SOX10 function at both transcriptional and post-translational levels.
Parallels to this study have been observed in many other organisms. Infection of circulating immune cells is a common mechanism for primary infection by pathogens. For example, T. gondii hijacks neutrophils and dendritic cells, altering host cell signaling, morphological features, and motility,91 events that are implicated in spreading of parasites. Salmonella enterica serovar typhimurium also hijacks intestinal neutrophils,92 presumably to traverse the intestinal mucosa and reach the lumen. Effects on the epigenome and transcriptome of cells used as vehicles of dissemination have yet to be investigated.
Transformation by Theileria spp. Parasites: Immortalization
Theileria parva and Theileria annulata are tickborne parasites of the phylum Apicomplexa that cause significant disease and death in cattle, particularly in Africa and Asia. They have the unique capacity of transforming the host cells they infect into continuously proliferating cells that are resistant to apoptosis.93 Infected cells then disseminate to a wide range of tissues, slowly resulting in the destruction of the lymphatic system, and pulmonary edema, resulting from infected cells migrating to the lungs. After the elimination of parasites from cultures, unparasitized leukocytes also continue to proliferate for several days,94 indicating that this phenotype is inherited by daughter cells and that bystander cells are also targeted. The mechanism of continued proliferation is unclear and appears to be multifaceted, involving massive changes in transcription.95 Many transcription factors are induced to be constitutively active,96 as are signaling pathways, such as the NF-κB pathway.97 This results in continuous activation of genes that suppress apoptosis and enhance cell cycle progression, and a lack of responsiveness to LPS stimulation.95 Moreover, Theileria spp. modulates several signaling pathways, including apoptotic pathways through the cell cycle regulator TP53, which Theileria spp. sequesters in the host cytoplasm, leading to inhibition of apoptosis and promotion of host cell replication.98 Major up-regulation and activation of transcription factors and proinflammatory molecules can, however, be detrimental to cells, and only a few infected cells survive and go on to proliferate. The rest undergo apoptosis,99 indicating that there is a delicate balance between survival and death.
Oncogenesis Caused by H. pylori
Transcriptional changes can have various effects at the subcellular level, but also dramatically affect the tissue microenvironment. The extracellular bacterium H. pylori is a major factor in gastric carcinomas, in which it infects the lower stomach and induces excessive acid production, which can lead to ulceration, tissue damage, and eventual transformation into malignant tissue. Chronic infection with H. pylori induces changes in DNA methylation, particularly in promoter regions of genes encoding tumor-suppressor proteins and oncogenes.100 Some of these changes persist even after eradication of H. pylori from the gut with antimicrobial drugs,101 suggesting that H. pylori induces long-lasting changes to the epigenome. Supporting this idea, clearance of H. pylori does not guarantee that cancer does not develop. 102
Whether epigenetic changes are maintained after H. pylori eradication is unknown, but modifications of the epigenome induced by H. pylori are linked to oncogenesis. For example, the forkhead transcription factor, FOXD3, is normally responsible for the transcription of proapoptotic factors and plays a key role in activating tumor apoptosis. After H. pylori infection, the FOXD3 promoter is hypermethylated in mice and human gastric cancers,103 and FOXD3 cannot be activated. Histone post-translational modifications, such as dephosphorylation of H3S10, are also altered in H. pylori infection, and NF-κB–responsive genes are not induced.104 The change in phosphorylation status of H3 is thought to be caused by H. pylori–induced premitotic arrest in cell cycle,105 which may be responsible for prevention of epithelial cell renewal in the stomach. A wide range of histone post-translational modifications is altered in response to H. pylori, and more important, many differences occur on genes encoding tumor-suppressor proteins and oncogenes,106 reflecting the changes in DNA methylation previously described.
The mechanistic link between the virulence factors of H. pylori and host chromatin has yet to be established. Other gram-negative bacteria secrete cytolethal distending toxins (CDTs), genotoxins that target the nucleus, inducing double-stranded breaks in DNA that lead to DNA damage,51 which may contribute to H. pylori–related carcinogenesis. Studies on a mouse model of Helicobacter hepaticus, a related bacterium that causes liver cancer and inflammatory bowel disease, revealed that CDTs appear to be responsible for promoting development of dysplasia; H. hepaticus lacking CDT activity does not induce dysplasia in mice.107 In addition, in comparison to wild-type bacteria, CDT mutants do not induce the transcription of proinflammatory cytokines, suggesting that CDT proteins influence these transcriptional pathways, preceding the development of dysplasia.
Remaining Questions
Modulation of the host epigenome by pathogen-derived effector molecules is emerging as a key mechanism for pathogenesis, although several pieces of the puzzle are missing. First, how do these pathogen effector proteins get into the nucleus? Many lack classic nuclear localization signals. They may have unconventional trafficking signals or perhaps interact with host proteins to hitch a ride into the nucleus. Either way, it is likely that pathogens exploit host cell trafficking mechanisms to target proteins to the correct subcellular location.
Second, which pathogen effector proteins influence the host epigenome? Studies characterizing the secreted proteome or secretome of infectious agents have provided many potential targets for studies on nuclear modulation of host cells. Characterization of the M. tuberculosis secretome has revealed the presence of a diverse range of proteins in the culture filtrate,108 several of which could be epigenetic modifiers. These include a putative single-stranded binding protein, which is predicted to bind single-stranded DNA to prevent degradation by nucleases; other examples are putative transcriptional repressor and regulator proteins, a transcription elongation factor protein, and a secreted DNA-directed RNA polymerase. M. tuberculosis secretes a group of interrelated proteins termed mammalian cell entry proteins, which are essential for survival of the bacterium inside macrophages. The function and mechanism of action of these proteins remain elusive; however, a recent study indicated that mammalian cell entry protein 1 is important for activating transcription of a specific group of genes in macrophages.109
Finally, are ncRNAs key epigenetic regulators of the host-pathogen interaction? Although there has been substantial speculation about the role of ncRNAs in infectious disease biological features, ncRNAs have not been shown to be a vehicle of epigenetic dysregulation by any pathogen. Replication of many viruses, such as EBV, requires noncoding and small RNAs for the maintenance and propagation of viral genomes in the host cell.110 Polyomaviruses use a single miRNA to evade natural killer cell responses through the down-regulation of cell surface ligand ULBP3,111 which is usually recognized by natural killer cells and T-cell subsets.
Further investigation of the role of ncRNAs in host-pathogen interactions is likely, given promising results with hepatitis C virus. In 2005, Jopling et al112 demonstrated that replication of hepatitis C viral RNA is prevented in the absence of miRNA miR-122. Since then, studies have shown that targeting miR-122 with a synthetic antisense oligonucleotide (SPC3649, miravirsen) effectively prevents viral replication in chimpanzees.113 This molecule shows promise in clinical trials,114 in which treatment induced a decrease in hepatitis C viral RNA levels. More important, this study did not identify any escape mutants, suggesting that this treatment does not select for mutant, drug-resistant hepatitis C virus. Miravirsen may be the first of many miRNA-targeted treatments for infectious diseases.
Although host miRNAs are dysregulated during several different types of infection, research is only beginning to uncover the relevance of these molecules in infectious diseases. In a unique mechanism, Cryptosporidium parvum, a waterborne apicomplexan parasite, suppresses host miRNAs by hijacking histone deacetylases and the NF-κB signaling pathway, whereas it up-regulates other miRNA.115 This includes miR-27b, which was shown to cause translational repression of splicing factor KH-type splicing-regulatory protein. KH-type splicing-regulatory protein is a regulator of mRNA stability; on translational repression induced by C. parvum, increased stability of inducible nitric oxide synthase, a key molecule in epithelial cell immunity and anti-C. parvum defense, is observed.
Future Directions
Infectious diseases are a scourge of humankind, and represent major causes of morbidity and mortality globally.116 Much infectious disease research focuses on the unique nature of pathogens, in a quest to identify enzymes or proteins that represent novel drug targets. Despite many successes, over time, there has been an increase in antibiotic resistance, and resurgence of contained diseases. Multidrug-resistant strains and extensively resistant M. tuberculosis are prevalent in many regions of the world,117 and treatment of these infections is particularly complicated in patients co-infected with HIV. Despite a reduction in malaria cases worldwide, resistance to antimalarial drugs is also widespread, with resistance to artemesinin, the frontline treatment for malaria, now appearing.118
By studying host-pathogen interactions, it may be possible to combine experimental and computational approaches to identify host pathways that are commonly targeted by pathogens.119 Targeting epigenetic changes induced by pathogenic organisms could be an approach to therapeutic development that is less likely to select for drug resistance. Host cells also act as reservoirs for latent pathogens, including HIV, and manipulating the chromatin state of the host has been proposed as a strategy to render latent pathogens more accessible to active drugs.120 Questions remain about the nature of reported epigenetic changes; many could be transient, whereas others may be long-term changes that will be performed on daughter cells. Furthermore, although several long-term effects of epigenetic modulation by pathogens have been identified, there may be other, as yet unexplained, mechanisms, which have an epigenetic basis.
Acknowledgments
We thank Patricia Johnson for communicating results before publication. We apologize to those authors whose work could not be cited because of space limitations.
Footnotes
Supported by NIH grants R01AI087625 and RC4AI092801 (K.K.) and Einstein-Montefiore Center for AIDS Research grant P30AI051519.
Disclosures: None declared.
References
- 1.Masaki T., Qu J., Cholewa-Waclaw J., Burr K., Raaum R., Rambukkana A. Reprogramming adult Schwann cells to stem cell-like cells by leprosy bacilli promotes dissemination of infection. Cell. 2013;152:51–67. doi: 10.1016/j.cell.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adam T., Arpin M., Prevost M.C., Gounon P., Sansonetti P.J. Cytoskeletal rearrangements and the functional role of T-plastin during entry of Shigella flexneri into HeLa cells. J Cell Biol. 1995;129:367–381. doi: 10.1083/jcb.129.2.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rupp J., Pfleiderer L., Jugert C., Moeller S., Klinger M., Dalhoff K., Solbach W., Stenger S., Laskay T., van Zandbergen G. Chlamydia pneumoniae hides inside apoptotic neutrophils to silently infect and propagate in macrophages. PLoS One. 2009;4:e6020. doi: 10.1371/journal.pone.0006020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Delorme-Walker V., Abrivard M., Lagal V., Anderson K., Perazzi A., Gonzalez V., Page C., Chauvet J., Ochoa W., Volkmann N., Hanein D., Tardieux I. Toxofilin upregulates the host cortical actin cytoskeleton dynamics, facilitating Toxoplasma invasion. J Cell Sci. 2012;125:4333–4342. doi: 10.1242/jcs.103648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brodsky I.E., Medzhitov R. Targeting of immune signalling networks by bacterial pathogens. Nat Cell Biol. 2009;11:521–526. doi: 10.1038/ncb0509-521. [DOI] [PubMed] [Google Scholar]
- 6.Baxt L.A., Garza-Mayers A.C., Goldberg M.B. Bacterial subversion of host innate immune pathways. Science. 2013;340:697–701. doi: 10.1126/science.1235771. [DOI] [PubMed] [Google Scholar]
- 7.Ito S., D’Alessio A.C., Taranova O.V., Hong K., Sowers L.C., Zhang Y. Role of Tet proteins in 5mC to 5hmC conversion, ES-cell self-renewal and inner cell mass specification. Nature. 2010;466:1129–1133. doi: 10.1038/nature09303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sabin L.R., Delas M.J., Hannon G.J. Dogma derailed: the many influences of RNA on the genome. Mol Cell. 2013;49:783–794. doi: 10.1016/j.molcel.2013.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chaussabel D., Semnani R.T., McDowell M.A., Sacks D., Sher A., Nutman T.B. Unique gene expression profiles of human macrophages and dendritic cells to phylogenetically distinct parasites. Blood. 2003;102:672–681. doi: 10.1182/blood-2002-10-3232. [DOI] [PubMed] [Google Scholar]
- 10.Fouts A.E., Boothroyd J.C. Infection with Toxoplasma gondii bradyzoites has a diminished impact on host transcript levels relative to tachyzoite infection. Infect Immun. 2007;75:634–642. doi: 10.1128/IAI.01228-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Albuquerque S.S., Carret C., Grosso A.R., Tarun A.S., Peng X., Kappe S.H., Prudencio M., Mota M.M. Host cell transcriptional profiling during malaria liver stage infection reveals a coordinated and sequential set of biological events. BMC Genomics. 2009;10:270. doi: 10.1186/1471-2164-10-270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Blader I.J., Saeij J.P. Communication between Toxoplasma gondii and its host: impact on parasite growth, development, immune evasion, and virulence. APMIS. 2009;117:458–476. doi: 10.1111/j.1600-0463.2009.02453.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marcinowski L., Lidschreiber M., Windhager L., Rieder M., Bosse J.B., Radle B., Bonfert T., Gyory I., de Graaf M., Prazeres da Costa O., Rosenstiel P., Friedel C.C., Zimmer R., Ruzsics Z., Dolken L. Real-time transcriptional profiling of cellular and viral gene expression during lytic cytomegalovirus infection. PLoS Pathogens. 2012;8:e1002908. doi: 10.1371/journal.ppat.1002908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brown S.E., Fraga M.F., Weaver I.C., Berdasco M., Szyf M. Variations in DNA methylation patterns during the cell cycle of HeLa cells. Epigenetics. 2007;2:54–65. doi: 10.4161/epi.2.1.3880. [DOI] [PubMed] [Google Scholar]
- 15.Jähner D., Jaenisch R. Retrovirus-induced de novo methylation of flanking host sequences correlates with gene inactivity. Nature. 1985;315:594–597. doi: 10.1038/315594a0. [DOI] [PubMed] [Google Scholar]
- 16.Benegiamo G., Vinciguerra M., Guarnieri V., Niro G.A., Andriulli A., Pazienza V. Hepatitis delta virus induces specific DNA methylation processes in Huh-7 liver cancer cells. FEBS Lett. 2013;587:1424–1428. doi: 10.1016/j.febslet.2013.03.021. [DOI] [PubMed] [Google Scholar]
- 17.Park E.S., Park Y.K., Shin C.Y., Park S.H., Ahn S.H., Kim D.H., Lim K.H., Kwon S.Y., Kim K.P., Yang S.I., Seong B.L., Kim K.H. Hepatitis B virus inhibits liver regeneration via epigenetic regulation of urokinase-type plasminogen activator. Hepatology. 2013;58:762–776. doi: 10.1002/hep.26379. [DOI] [PubMed] [Google Scholar]
- 18.Hino R., Uozaki H., Murakami N., Ushiku T., Shinozaki A., Ishikawa S., Morikawa T., Nakaya T., Sakatani T., Takada K., Fukayama M. Activation of DNA methyltransferase 1 by EBV latent membrane protein 2A leads to promoter hypermethylation of PTEN gene in gastric carcinoma. Cancer Res. 2009;69:2766–2774. doi: 10.1158/0008-5472.CAN-08-3070. [DOI] [PubMed] [Google Scholar]
- 19.Liu X., Wang Y., Wang X., Sun Z., Li L., Tao Q., Luo B. Epigenetic silencing of WNT5A in Epstein-Barr virus-associated gastric carcinoma. Arch Virol Arch Virol. 2013;158:123–132. doi: 10.1007/s00705-012-1481-x. [DOI] [PubMed] [Google Scholar]
- 20.Bougdour A., Durandau E., Brenier-Pinchart M.P., Ortet P., Barakat M., Kieffer S., Curt-Varesano A., Curt-Bertini R.L., Bastien O., Coute Y., Pelloux H., Hakimi M.A. Host cell subversion by Toxoplasma GRA16, an exported dense granule protein that targets the host cell nucleus and alters gene expression. Cell Host Microbe. 2013;13:489–500. doi: 10.1016/j.chom.2013.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Koshy A.A., Dietrich H.K., Christian D.A., Melehani J.H., Shastri A.J., Hunter C.A., Boothroyd J.C. Toxoplasma co-opts host cells it does not invade. PLoS Pathogens. 2012;8:e1002825. doi: 10.1371/journal.ppat.1002825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schneider A.G., Abi Abdallah D.S., Butcher B.A., Denkers E.Y. Toxoplasma gondii triggers phosphorylation and nuclear translocation of dendritic cell STAT1 while simultaneously blocking IFNgamma-induced STAT1 transcriptional activity. PLoS One. 2013;8:e60215. doi: 10.1371/journal.pone.0060215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vyas A., Kim S.K., Giacomini N., Boothroyd J.C., Sapolsky R.M. Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors. Proc Natl Acad Sci U S A. 2007;104:6442–6447. doi: 10.1073/pnas.0608310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yolken R.H., Dickerson F.B., Fuller Torrey E. Toxoplasma and schizophrenia. Parasite Immunol. 2009;31:706–715. doi: 10.1111/j.1365-3024.2009.01131.x. [DOI] [PubMed] [Google Scholar]
- 25.Kasper C.A., Sorg I., Schmutz C., Tschon T., Wischnewski H., Kim M.L., Arrieumerlou C. Cell-cell propagation of NF-kappaB transcription factor and MAP kinase activation amplifies innate immunity against bacterial infection. Immunity. 2010;33:804–816. doi: 10.1016/j.immuni.2010.10.015. [DOI] [PubMed] [Google Scholar]
- 26.Garcia-Garcia J.C., Rennoll-Bankert K.E., Pelly S., Milstone A.M., Dumler J.S. Silencing of host cell CYBB gene expression by the nuclear effector AnkA of the intracellular pathogen Anaplasma phagocytophilum. Infect Immun. 2009;77:2385–2391. doi: 10.1128/IAI.00023-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shiels B.R., McKellar S., Katzer F., Lyons K., Kinnaird J., Ward C., Wastling J.M., Swan D. A Theileria annulata DNA binding protein localized to the host cell nucleus alters the phenotype of a bovine macrophage cell line. Eukaryot Cell. 2004;3:495–505. doi: 10.1128/EC.3.2.495-505.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maqbool M.A., Imache M.R., Higgs M.R., Carmouse S., Pawlotsky J.M., Lerat H. Regulation of hepatitis C virus replication by nuclear translocation of nonstructural 5A protein and transcriptional activation of host genes. J Virol. 2013;87:5523–5539. doi: 10.1128/JVI.00585-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arbibe L., Kim D.W., Batsche E., Pedron T., Mateescu B., Muchardt C., Parsot C., Sansonetti P.J. An injected bacterial effector targets chromatin access for transcription factor NF-kappaB to alter transcription of host genes involved in immune responses. Nat Immunol. 2007;8:47–56. doi: 10.1038/ni1423. [DOI] [PubMed] [Google Scholar]
- 30.Mazurkiewicz P., Thomas J., Thompson J.A., Liu M., Arbibe L., Sansonetti P., Holden D.W. SpvC is a Salmonella effector with phosphothreonine lyase activity on host mitogen-activated protein kinases. Mol Microbiol. 2008;67:1371–1383. doi: 10.1111/j.1365-2958.2008.06134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lad S.P., Li J., da Silva Correia J., Pan Q., Gadwal S., Ulevitch R.J., Li E. Cleavage of p65/RelA of the NF-kappaB pathway by Chlamydia. Proc Natl Acad Sci U S A. 2007;104:2933–2938. doi: 10.1073/pnas.0608393104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schreiner S., Wimmer P., Sirma H., Everett R.D., Blanchette P., Groitl P., Dobner T. Proteasome-dependent degradation of Daxx by the viral E1B-55K protein in human adenovirus-infected cells. J Virol. 2010;84:7029–7038. doi: 10.1128/JVI.00074-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Soupene E., Rothschild J., Kuypers F.A., Dean D. Eukaryotic protein recruitment into the Chlamydia inclusion: implications for survival and growth. PLoS One. 2012;7:e36843. doi: 10.1371/journal.pone.0036843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pennini M.E., Perrinet S., Dautry-Varsat A., Subtil A. Histone methylation by NUE, a novel nuclear effector of the intracellular pathogen Chlamydia trachomatis. PLoS Pathogens. 2010;6:e1000995. doi: 10.1371/journal.ppat.1000995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Agarwal S., Agarwal S., Jin H., Pancholi P., Pancholi V. Serine/threonine phosphatase (SP-STP), secreted from Streptococcus pyogenes, is a pro-apoptotic protein. J Biol Chem. 2012;287:9147–9167. doi: 10.1074/jbc.M111.316554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chopra P., Singh B., Singh R., Vohra R., Koul A., Meena L.S., Koduri H., Ghildiyal M., Deol P., Das T.K., Tyagi A.K., Singh Y. Phosphoprotein phosphatase of Mycobacterium tuberculosis dephosphorylates serine-threonine kinases PknA and PknB. Biochem Biophys Res Commun. 2003;311:112–120. doi: 10.1016/j.bbrc.2003.09.173. [DOI] [PubMed] [Google Scholar]
- 37.Rolando M., Sanulli S., Rusniok C., Gomez-Valero L., Bertholet C., Sahr T., Margueron R., Buchrieser C. Legionella pneumophila effector RomA uniquely modifies host chromatin to repress gene expression and promote intracellular bacterial replication. Cell Host Microbe. 2013;13:395–405. doi: 10.1016/j.chom.2013.03.004. [DOI] [PubMed] [Google Scholar]
- 38.Manzur K.L., Farooq A., Zeng L., Plotnikova O., Koch A.W., Sachchidanand, Zhou M.M. A dimeric viral SET domain methyltransferase specific to Lys27 of histone H3. Nat Struct Biol. 2003;10:187–196. doi: 10.1038/nsb898. [DOI] [PubMed] [Google Scholar]
- 39.Gilbert L.A., Ravindran S., Turetzky J.M., Boothroyd J.C., Bradley P.J. Toxoplasma gondii targets a protein phosphatase 2C to the nuclei of infected host cells. Eukaryotic Cell. 2007;6:73–83. doi: 10.1128/EC.00309-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Knight J.S., Lan K., Subramanian C., Robertson E.S. Epstein-Barr virus nuclear antigen 3C recruits histone deacetylase activity and associates with the corepressors mSin3A and NCoR in human B-cell lines. J Virol. 2003;77:4261–4272. doi: 10.1128/JVI.77.7.4261-4272.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zurawski D.V., Mumy K.L., Faherty C.S., McCormick B.A., Maurelli A.T. Shigella flexneri type III secretion system effectors OspB and OspF target the nucleus to downregulate the host inflammatory response via interactions with retinoblastoma protein. Mol Microbiol. 2009;71:350–368. doi: 10.1111/j.1365-2958.2008.06524.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lebreton A., Lakisic G., Job V., Fritsch L., Tham T.N., Camejo A., Mattei P.J., Regnault B., Nahori M.A., Cabanes D., Gautreau A., Ait-Si-Ali S., Dessen A., Cossart P., Bierne H. A bacterial protein targets the BAHD1 chromatin complex to stimulate type III interferon response. Science. 2011;331:1319–1321. doi: 10.1126/science.1200120. [DOI] [PubMed] [Google Scholar]
- 43.Shimura M., Toyoda Y., Iijima K., Kinomoto M., Tokunaga K., Yoda K., Yanagida M., Sata T., Ishizaka Y. Epigenetic displacement of HP1 from heterochromatin by HIV-1 Vpr causes premature sister chromatid separation. J Cell Biol. 2011;194:721–735. doi: 10.1083/jcb.201010118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Pennini M.E., Liu Y., Yang J., Croniger C.M., Boom W.H., Harding C.V. CCAAT/enhancer-binding protein beta and delta binding to CIITA promoters is associated with the inhibition of CIITA expression in response to Mycobacterium tuberculosis 19-kDa lipoprotein. J Immunol. 2007;179:6910–6918. doi: 10.4049/jimmunol.179.10.6910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Leng J., Butcher B.A., Egan C.E., Abi Abdallah D.S., Denkers E.Y. Toxoplasma gondii prevents chromatin remodeling initiated by TLR-triggered macrophage activation. J Immunol. 2009;182:489–497. doi: 10.4049/jimmunol.182.1.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ambagala A.P., Bosma T., Ali M.A., Poustovoitov M., Chen J.J., Gershon M.D., Adams P.D., Cohen J.I. Varicella-zoster virus immediate-early 63 protein interacts with human antisilencing function 1 protein and alters its ability to bind histones h3.1 and h3.3. J Virol. 2009;83:200–209. doi: 10.1128/JVI.00645-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Coppotelli G., Mughal N., Callegari S., Sompallae R., Caja L., Luijsterburg M.S., Dantuma N.P., Moustakas A., Masucci M.G. The Epstein-Barr virus nuclear antigen-1 reprograms transcription by mimicry of high mobility group A proteins. Nucleic Acids Res. 2013;41:2950–2962. doi: 10.1093/nar/gkt032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mansur D.S., Maluquer de Motes C., Unterholzner L., Sumner R.P., Ferguson B.J., Ren H., Strnadova P., Bowie A.G., Smith G.L. Poxvirus targeting of E3 ligase beta-TrCP by molecular mimicry: a mechanism to inhibit NF-kappaB activation and promote immune evasion and virulence. PLoS Pathog. 2013;9:e1003183. doi: 10.1371/journal.ppat.1003183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Marazzi I., Ho J.S., Kim J., Manicassamy B., Dewell S., Albrecht R.A., Seibert C.W., Schaefer U., Jeffrey K.L., Prinjha R.K., Lee K., Garcia-Sastre A., Roeder R.G., Tarakhovsky A. Suppression of the antiviral response by an influenza histone mimic. Nature. 2012;483:428–433. doi: 10.1038/nature10892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang H.C., Wu M.L., Ko T.P., Wang A.H. Neisseria conserved hypothetical protein DMP12 is a DNA mimic that binds to histone-like HU protein. Nucleic Acids Res. 2013;41:5127–5138. doi: 10.1093/nar/gkt201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bierne H., Cossart P. When bacteria target the nucleus: the emerging family of nucleomodulins. Cell Microbiol. 2012;14:622–633. doi: 10.1111/j.1462-5822.2012.01758.x. [DOI] [PubMed] [Google Scholar]
- 52.Hori M., Fujii K., Fujishima M. Micronucleus-specific bacterium Holospora elegans irreversibly enhances stress gene expression of the host Paramecium caudatum. J Eukaryot Microbiol. 2008;55:515–521. doi: 10.1111/j.1550-7408.2008.00352.x. [DOI] [PubMed] [Google Scholar]
- 53.Li H., Xu H., Zhou Y., Zhang J., Long C., Li S., Chen S., Zhou J.M., Shao F. The phosphothreonine lyase activity of a bacterial type III effector family. Science. 2007;315:1000–1003. doi: 10.1126/science.1138960. [DOI] [PubMed] [Google Scholar]
- 54.Frappier L. Contributions of Epstein-Barr nuclear antigen 1 (EBNA1) to cell immortalization and survival. Viruses. 2012;4:1537–1547. doi: 10.3390/v4091537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paschos K., Parker G.A., Watanatanasup E., White R.E., Allday M.J. BIM promoter directly targeted by EBNA3C in polycomb-mediated repression by EBV. Nucleic Acids Res. 2012;40:7233–7246. doi: 10.1093/nar/gks391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bierne H., Tham T.N., Batsche E., Dumay A., Leguillou M., Kerneis-Golsteyn S., Regnault B., Seeler J.S., Muchardt C., Feunteun J., Cossart P. Human BAHD1 promotes heterochromatic gene silencing. Proc Natl Acad Sci U S A. 2009;106:13826–13831. doi: 10.1073/pnas.0901259106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Molestina R.E., Sinai A.P. Host and parasite-derived IKK activities direct distinct temporal phases of NF-kappaB activation and target gene expression following Toxoplasma gondii infection. J Cell Sci. 2005;118:5785–5796. doi: 10.1242/jcs.02709. [DOI] [PubMed] [Google Scholar]
- 58.Moorhead A.M., Jung J.Y., Smirnov A., Kaufer S., Scidmore M.A. Multiple host proteins that function in phosphatidylinositol-4-phosphate metabolism are recruited to the chlamydial inclusion. Infect Immun. 2010;78:1990–2007. doi: 10.1128/IAI.01340-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shalginskikh N., Poleshko A., Skalka A.M., Katz R.A. Retroviral DNA methylation and epigenetic repression are mediated by the antiviral host protein Daxx. J Virol. 2013;87:2137–2150. doi: 10.1128/JVI.02026-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schreiner S., Burck C., Glass M., Groitl P., Wimmer P., Kinkley S., Mund A., Everett R.D., Dobner T. Control of human adenovirus type 5 gene expression by cellular Daxx/ATRX chromatin-associated complexes. Nucleic Acids Res. 2013;41:3532–3550. doi: 10.1093/nar/gkt064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hwang J., Kalejta R.F. Proteasome-dependent, ubiquitin-independent degradation of Daxx by the viral pp71 protein in human cytomegalovirus-infected cells. Virology. 2007;367:334–338. doi: 10.1016/j.virol.2007.05.037. [DOI] [PubMed] [Google Scholar]
- 62.Agarwal S., Agarwal S., Pancholi P., Pancholi V. Strain-specific regulatory role of eukaryote-like serine/threonine phosphatase in pneumococcal adherence. Infect Immun. 2012;80:1361–1372. doi: 10.1128/IAI.06311-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Croken M.M., Nardelli S.C., Kim K. Chromatin modifications, epigenetics, and how protozoan parasites regulate their lives. Trends Parasitol. 2012;28:202–213. doi: 10.1016/j.pt.2012.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lukashchuk V., McFarlane S., Everett R.D., Preston C.M. Human cytomegalovirus protein pp71 displaces the chromatin-associated factor ATRX from nuclear domain 10 at early stages of infection. J Virol. 2008;82:12543–12554. doi: 10.1128/JVI.01215-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kincaid E.Z., Ernst J.D. Mycobacterium tuberculosis exerts gene-selective inhibition of transcriptional responses to IFN-gamma without inhibiting STAT1 function. J Immunol. 2003;171:2042–2049. doi: 10.4049/jimmunol.171.4.2042. [DOI] [PubMed] [Google Scholar]
- 66.Pai R.K., Pennini M.E., Tobian A.A., Canaday D.H., Boom W.H., Harding C.V. Prolonged toll-like receptor signaling by Mycobacterium tuberculosis and its 19-kilodalton lipoprotein inhibits gamma interferon-induced regulation of selected genes in macrophages. Infect Immun. 2004;72:6603–6614. doi: 10.1128/IAI.72.11.6603-6614.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Pennini M.E., Pai R.K., Schultz D.C., Boom W.H., Harding C.V. Mycobacterium tuberculosis 19-kDa lipoprotein inhibits IFN-gamma-induced chromatin remodeling of MHC2TA by TLR2 and MAPK signaling. J Immunol. 2006;176:4323–4330. doi: 10.4049/jimmunol.176.7.4323. [DOI] [PubMed] [Google Scholar]
- 68.Leng J., Denkers E.Y. Toxoplasma gondii inhibits covalent modification of histone H3 at the IL-10 promoter in infected macrophages. PLoS One. 2009;4:e7589. doi: 10.1371/journal.pone.0007589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shivaswamy S., Bhinge A., Zhao Y., Jones S., Hirst M., Iyer V.R. Dynamic remodeling of individual nucleosomes across a eukaryotic genome in response to transcriptional perturbation. PLoS Biol. 2008;6:e65. doi: 10.1371/journal.pbio.0060065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Weinmann A.S., Plevy S.E., Smale S.T. Rapid and selective remodeling of a positioned nucleosome during the induction of IL-12 p40 transcription. Immunity. 1999;11:665–675. doi: 10.1016/s1074-7613(00)80141-7. [DOI] [PubMed] [Google Scholar]
- 71.Lomvardas S., Thanos D. Nucleosome sliding via TBP DNA binding in vivo. Cell. 2001;106:685–696. doi: 10.1016/s0092-8674(01)00490-1. [DOI] [PubMed] [Google Scholar]
- 72.Lu Z.M., Zhou J., Wang X., Guan Z., Bai H., Liu Z.J., Su N., Pan K., Ji J., Deng D. Nucleosomes correlate with in vivo progression pattern of de novo methylation of p16 CpG islands in human gastric carcinogenesis. PLoS One. 2012;7:e35928. doi: 10.1371/journal.pone.0035928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Kanda T., Otter M., Wahl G.M. Coupling of mitotic chromosome tethering and replication competence in Epstein-Barr virus-based plasmids. Mol Cell Biol. 2001;21:3576–3588. doi: 10.1128/MCB.21.10.3576-3588.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Hale B.G., Randall R.E., Ortin J., Jackson D. The multifunctional NS1 protein of influenza A viruses. J Gen Virol. 2008;89:2359–2376. doi: 10.1099/vir.0.2008/004606-0. [DOI] [PubMed] [Google Scholar]
- 75.Eriksen N., Kumar S.B., Fukuchi K., Martin G.M., Benditt E.P. Molecular mimicry: histone H3 and mycobacterial protein epitopes. Proc Natl Acad Sci U S A. 1995;92:2150–2153. doi: 10.1073/pnas.92.6.2150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang H.C., Ko T.P., Wu M.L., Ku S.C., Wu H.J., Wang A.H. Neisseria conserved protein DMP19 is a DNA mimic protein that prevents DNA binding to a hypothetical nitrogen-response transcription factor. Nucleic Acids Res. 2012;40:5718–5730. doi: 10.1093/nar/gks177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tseng T.T., Tyler B.M., Setubal J.C. Protein secretion systems in bacterial-host associations, and their description in the Gene Ontology. BMC Microbiol. 2009;9(Suppl 1):S2. doi: 10.1186/1471-2180-9-S1-S2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ratajczak J., Miekus K., Kucia M., Zhang J., Reca R., Dvorak P., Ratajczak M.Z. Embryonic stem cell-derived microvesicles reprogram hematopoietic progenitors: evidence for horizontal transfer of mRNA and protein delivery. Leukemia. 2006;20:847–856. doi: 10.1038/sj.leu.2404132. [DOI] [PubMed] [Google Scholar]
- 79.Lenassi M., Cagney G., Liao M., Vaupotic T., Bartholomeeusen K., Cheng Y., Krogan N.J., Plemenitas A., Peterlin B.M. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11:110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bhatnagar S., Shinagawa K., Castellino F.J., Schorey J.S. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood. 2007;110:3234–3244. doi: 10.1182/blood-2007-03-079152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Pegtel D.M., Cosmopoulos K., Thorley-Lawson D.A., van Eijndhoven M.A., Hopmans E.S., Lindenberg J.L., de Gruijl T.D., Wurdinger T., Middeldorp J.M. Functional delivery of viral miRNAs via exosomes. Proc Natl Acad Sci U S A. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mantel P.Y., Hoang A.N., Goldowitz I., Potashnikova D., Hamza B., Vorobjev I., Ghiran I., Toner M., Irimia D., Ivanov A.R., Barteneva N., Marti M. Malaria-infected erythrocyte-derived microvesicles mediate cellular communication within the parasite population and with the host immune system. Cell Host Microbe. 2013;13:521–534. doi: 10.1016/j.chom.2013.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Regev-Rudzki N., Wilson D.W., Carvalho T.G., Sisquella X., Coleman B.M., Rug M., Bursac D., Angrisano F., Gee M., Hill A.F., Baum J., Cowman A.F. Cell-cell communication between malaria-infected red blood cells via exosome-like vesicles. Cell. 2013;153:1120–1133. doi: 10.1016/j.cell.2013.04.029. [DOI] [PubMed] [Google Scholar]
- 84.Moon D.C., Choi C.H., Lee S.M., Lee J.H., Kim S.I., Kim D.S., Lee J.C. Nuclear translocation of Acinetobacter baumannii transposase induces DNA methylation of CpG regions in the promoters of E-cadherin gene. PLoS One. 2012;7:e38974. doi: 10.1371/journal.pone.0038974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodrigues M.L., Nakayasu E.S., Oliveira D.L., Nimrichter L., Nosanchuk J.D., Almeida I.C., Casadevall A. Extracellular vesicles produced by Cryptococcus neoformans contain protein components associated with virulence. Eukaryotic Cell. 2008;7:58–67. doi: 10.1128/EC.00370-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Albuquerque P.C., Nakayasu E.S., Rodrigues M.L., Frases S., Casadevall A., Zancope-Oliveira R.M., Almeida I.C., Nosanchuk J.D. Vesicular transport in Histoplasma capsulatum: an effective mechanism for trans-cell wall transfer of proteins and lipids in ascomycetes. Cell Microbiol. 2008;10:1695–1710. doi: 10.1111/j.1462-5822.2008.01160.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Silverman J.M., Reiner N.E. Leishmania exosomes deliver preemptive strikes to create an environment permissive for early infection. Front Cell Infect Microbiol. 2011;1:26. doi: 10.3389/fcimb.2011.00026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Twu O., de Miguel N., Lustig G., Stevens G.C., Vashisht A.A., Wohlschlegel J.A., Johnson P.J. Trichomonas vaginalis exosomes deliver cargo to host cells and mediate host:parasite interactions. PLoS Pathog. 2013;9:e1003482. doi: 10.1371/journal.ppat.1003482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Russo V.E., Russo V.E.A. Cold Spring Harbor Laboratory Press; New York: 1996. Epigenetic Mechanisms of Gene Regulation. [Google Scholar]
- 90.Koche R.P., Smith Z.D., Adli M., Gu H., Ku M., Gnirke A., Bernstein B.E., Meissner A. Reprogramming factor expression initiates widespread targeted chromatin remodeling. Cell Stem Cell. 2011;8:96–105. doi: 10.1016/j.stem.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Weidner J.M., Kanatani S., Hernandez-Castaneda M.A., Fuks J.M., Rethi B., Wallin R.P., Barragan A. Rapid cytoskeleton remodelling in dendritic cells following invasion by Toxoplasma gondii coincides with the onset of a hypermigratory phenotype. Cell Microbiol. 2013;15:1735–1752. doi: 10.1111/cmi.12145. [DOI] [PubMed] [Google Scholar]
- 92.Loetscher Y., Wieser A., Lengefeld J., Kaiser P., Schubert S., Heikenwalder M., Hardt W.D., Stecher B. Salmonella transiently reside in luminal neutrophils in the inflamed gut. PLoS One. 2012;7:e34812. doi: 10.1371/journal.pone.0034812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heussler V.T., Machado J., Jr., Fernandez P.C., Botteron C., Chen C.G., Pearse M.J., Dobbelaere D.A. The intracellular parasite Theileria parva protects infected T cells from apoptosis. Proc Natl Acad Sci U S A. 1999;96:7312–7317. doi: 10.1073/pnas.96.13.7312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Pinder M., Kar S., Withey K.S., Lundin L.B., Roelants G.E. Proliferation and lymphocyte stimulatory capacity of Theileria-infected lymphoblastoid cells before and after the elimination of intracellular parasites. Immunology. 1981;44:51–60. [PMC free article] [PubMed] [Google Scholar]
- 95.Durrani Z., Weir W., Pillai S., Kinnaird J., Shiels B. Modulation of activation-associated host cell gene expression by the apicomplexan parasite Theileria annulata. Cell Microbiol. 2012;14:1434–1454. doi: 10.1111/j.1462-5822.2012.01809.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Dobbelaere D., Heussler V. Transformation of leukocytes by Theileria parva and T. annulata. Annu Rev Microbiol. 1999;53:1–42. doi: 10.1146/annurev.micro.53.1.1. [DOI] [PubMed] [Google Scholar]
- 97.Heussler V.T., Rottenberg S., Schwab R., Kuenzi P., Fernandez P.C., McKellar S., Shiels B., Chen Z.J., Orth K., Wallach D., Dobbelaere D.A. Hijacking of host cell IKK signalosomes by the transforming parasite Theileria. Science. 2002;298:1033–1036. doi: 10.1126/science.1075462. [DOI] [PubMed] [Google Scholar]
- 98.Haller D., Mackiewicz M., Gerber S., Beyer D., Kullmann B., Schneider I., Ahmed J.S., Seitzer U. Cytoplasmic sequestration of p53 promotes survival in leukocytes transformed by Theileria. Oncogene. 2010;29:3079–3086. doi: 10.1038/onc.2010.61. [DOI] [PubMed] [Google Scholar]
- 99.Rocchi M.S., Ballingall K.T., MacHugh N.D., McKeever D.J. The kinetics of Theileria parva infection and lymphocyte transformation in vitro. Int J Parasitol. 2006;36:771–778. doi: 10.1016/j.ijpara.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 100.Alvarez M.C., Ladeira M.S., Scaletsky I.C., Pedrazzoli J., Jr., Ribeiro M.L. Methylation pattern of THBS1, GATA-4, and HIC1 in pediatric and adult patients infected with Helicobacter pylori. Dig Dis Sci. 2013;58:2850–2857. doi: 10.1007/s10620-013-2742-6. [DOI] [PubMed] [Google Scholar]
- 101.Shin C.M., Kim N., Lee H.S., Park J.H., Ahn S., Kang G.H., Kim J.M., Kim J.S., Lee D.H., Jung H.C. Changes in aberrant DNA methylation after Helicobacter pylori eradication: a long-term follow-up study. Int J Cancer. 2013;133:2034–2042. doi: 10.1002/ijc.28219. [DOI] [PubMed] [Google Scholar]
- 102.de Vries A.C., Kuipers E.J., Rauws E.A. Helicobacter pylori eradication and gastric cancer: when is the horse out of the barn? Am J Gastroenterol. 2009;104:1342–1345. doi: 10.1038/ajg.2008.15. [DOI] [PubMed] [Google Scholar]
- 103.Cheng A.S., Li M.S., Kang W., Cheng V.Y., Chou J.L., Lau S.S., Go M.Y., Lee C.C., Ling T.K., Ng E.K., Yu J., Huang T.H., To K.F., Chan M.W., Sung J.J., Chan F.K. Helicobacter pylori causes epigenetic dysregulation of FOXD3 to promote gastric carcinogenesis. Gastroenterology. 2013;144:122–133.e9. doi: 10.1053/j.gastro.2012.10.002. [DOI] [PubMed] [Google Scholar]
- 104.Ding S.Z., Fischer W., Kaparakis-Liaskos M., Liechti G., Merrell D.S., Grant P.A., Ferrero R.L., Crowe S.E., Haas R., Hatakeyama M., Goldberg J.B. Helicobacter pylori-induced histone modification, associated gene expression in gastric epithelial cells, and its implication in pathogenesis. PLoS One. 2010;5:e9875. doi: 10.1371/journal.pone.0009875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Fehri L.F., Rechner C., Janssen S., Mak T.N., Holland C., Bartfeld S., Bruggemann H., Meyer T.F. Helicobacter pylori-induced modification of the histone H3 phosphorylation status in gastric epithelial cells reflects its impact on cell cycle regulation. Epigenetics. 2009;4:577–586. doi: 10.4161/epi.4.8.10217. [DOI] [PubMed] [Google Scholar]
- 106.Byun S.W., Chang Y.J., Chung I.S., Moss S.F., Kim S.S. Helicobacter pylori decreases p27 expression through the delta opioid receptor-mediated inhibition of histone acetylation within the p27 promoter. Cancer Lett. 2012;326:96–104. doi: 10.1016/j.canlet.2012.07.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Ge Z., Rogers A.B., Feng Y., Lee A., Xu S., Taylor N.S., Fox J.G. Bacterial cytolethal distending toxin promotes the development of dysplasia in a model of microbially induced hepatocarcinogenesis. Cell Microbiol. 2007;9:2070–2080. doi: 10.1111/j.1462-5822.2007.00939.x. [DOI] [PubMed] [Google Scholar]
- 108.Zheng J., Ren X., Wei C., Yang J., Hu Y., Liu L., Xu X., Wang J., Jin Q. Analysis of the secretome and identification of novel constituents from culture filtrate of bacillus Calmette-Guerin using high-resolution mass spectrometry. Mol Cell Proteomics. 2013;12:2081–2095. doi: 10.1074/mcp.M113.027318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Stavrum R., Valvatne H., Stavrum A.K., Riley L.W., Ulvestad E., Jonassen I., Doherty T.M., Grewal H.M. Mycobacterium tuberculosis Mce1 protein complex initiates rapid induction of transcription of genes involved in substrate trafficking. Genes Immun. 2012;13:496–502. doi: 10.1038/gene.2012.24. [DOI] [PubMed] [Google Scholar]
- 110.Yeung M.L., Benkirane M., Jeang K.T. Small non-coding RNAs, mammalian cells, and viruses: regulatory interactions? Retrovirology. 2007;4:74. doi: 10.1186/1742-4690-4-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bauman Y., Nachmani D., Vitenshtein A., Tsukerman P., Drayman N., Stern-Ginossar N., Lankry D., Gruda R., Mandelboim O. An identical miRNA of the human JC and BK polyoma viruses targets the stress-induced ligand ULBP3 to escape immune elimination. Cell Host Microbe. 2011;9:93–102. doi: 10.1016/j.chom.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 112.Jopling C.L., Yi M., Lancaster A.M., Lemon S.M., Sarnow P. Modulation of hepatitis C virus RNA abundance by a liver-specific MicroRNA. Science. 2005;309:1577–1581. doi: 10.1126/science.1113329. [DOI] [PubMed] [Google Scholar]
- 113.Lanford R.E., Hildebrandt-Eriksen E.S., Petri A., Persson R., Lindow M., Munk M.E., Kauppinen S., Orum H. Therapeutic silencing of microRNA-122 in primates with chronic hepatitis C virus infection. Science. 2010;327:198–201. doi: 10.1126/science.1178178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Janssen H.L., Reesink H.W., Lawitz E.J., Zeuzem S., Rodriguez-Torres M., Patel K., van der Meer A.J., Patick A.K., Chen A., Zhou Y., Persson R., King B.D., Kauppinen S., Levin A.A., Hodges M.R. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 115.Zhou R., Gong A.Y., Chen D., Miller R.E., Eischeid A.N., Chen X.M. Histone deacetylases and NF-kB signaling coordinate expression of CX3CL1 in epithelial cells in response to microbial challenge by suppressing miR-424 and miR-503. PLoS One. 2013;8:e65153. doi: 10.1371/journal.pone.0065153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Mathers C., Boerma T., Fat M. WHO; Geneva, Switzerland: 2004. Global Burden of Disease: 2004 Update. [Google Scholar]
- 117.Dalton T., Cegielski P., Akksilp S., Asencios L., Campos Caoili J., Cho S.N. Prevalence of and risk factors for resistance to second-line drugs in people with multidrug-resistant tuberculosis in eight countries: a prospective cohort study. Lancet. 2012;380:1406–1417. doi: 10.1016/S0140-6736(12)60734-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.WHO . WHO; Geneva, Switzerland: 2012. World Malaria Report 2012. [Google Scholar]
- 119.Kidane Y.H., Lawrence C., Murali T.M. The landscape of host transcriptional response programs commonly perturbed by bacterial pathogens: towards host-oriented broad-spectrum drug targets. PLoS One. 2013;8:e58553. doi: 10.1371/journal.pone.0058553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jermy A. Viral infection: coaxing HIV out of hiding. Nat Rev Microbiol. 2012;10:596–597. doi: 10.1038/nrmicro2861. [DOI] [PubMed] [Google Scholar]