Abstract
Proper fibroblast cell migration and differentiation are critical for valve formation and homeostasis, but uncontrolled myofibroblastic activation may precede osteogenic differentiation and calcification. Cadherin-11 (cad-11) is a cell-cell adhesion protein classically expressed at mesenchymal-osteoblast interfaces and it participates in mesenchymal differentiation to osteochondral-lineages. This suggests cad-11 may have an important role in heart valve development and pathogenesis, but its expression patterns in valves are largely unknown. In this study, we profiled the spatial and temporal expression patterns of cad-11 in embryonic chick and mouse heart development. We determined that cad-11 is expressed in both endocardial and mesenchymal cells of the atrioventricular (AV) and outflow tract (OFT) cushions (Pre HH30/E14), but becomes restricted to the valve endocardial/endothelial cells during late fetal remodeling and throughout postnatal life. We then investigated changes in Cad-11 expression in a murine aortic valve disease model (the ApoE−/−). Unlike wildtype mice, cad-11 becomes dramatically re-expressed in the interstitium. Similarly, in calcified human aortic valve leaflets, cad-11 loses endothelial confinement and becomes significantly re-expressed in the valve interstitium. Double labeling identified that 91% of myofibroblastic and 96% of osteoblastic cells in calcified aortic valves were also cad-11 positive. Collectively, our results suggest that cad-11 is important for proper embryonic cushion formation and remodeling, but may also participate in aortic valve pathogenesis if re-expressed in adulthood.
Keywords: cadherin, heart valve development, calcific aortic valve disease
Introduction
Heart valve disease is a serious and growing clinical problem, both in the United States and globally. Congenital abnormalities of heart valves develop in 1-2% of live births [MEMBERS et al., 2009]. Proper growth and maturation of the valve leaflets are essential for long-term postnatal function. The heart valve leaflets originate as amorphous endocardial cushions rich in glycosaminoglycans and proteoglycans [de Lange et al., 2004]. Remodeling and maturation of these cushions into thin fibrous valve leaflets require matrix condensation, extension and eventual trilayer stratification [Ramsdell and Markwald, 1997; Person et al., 2005; Butcher and Markwald, 2007]. However, the mechanisms regulating valve progenitor differentiation into fibroblasts are still poorly understood [de Lange et al., 2004; Kruithof et al., 2007]. Many clinically relevant congenital heart defects arise during later fetal remodeling events, which correlate poorly with specific transcription factor mutations [Mjaatvedt et al., 1998; Camenisch et al., 2000; Kern et al., 2010]. Alternatively, we and other groups have identified extracellular matrix proteins and adhesion ligands, such as periostin and versican, as important regulators of heart valve matrix remodeling [Henderson and Copp, 1998; Butcher et al., 2006; Kruithof et al., 2007; Kern et al., 2010]. These structural and/or matricrine components bind growth factors and provide critical adhesive signals that guide cellular differentiation [Zhang et al., 1998; Horiuchi et al., 1999]. Many of these processes involve interactions between resident cells, but the underlying mechanisms are poorly understood.
Cadherins are calcium dependent membrane glycoproteins that mediate cell-cell interaction. They regulate tissue morphogenesis through endothelial to mesenchymal transition (EMT), mesenchymal-epithelial transition, cell sorting, and cell rearrangement. Their extracellular domain mediates adhesive binding to neighboring cells (homotypic and/or heterotypic), while their cytoplasmic tail interacts with p120, catenins, and other cytoplasmic proteins [Nagafuchi et al., 1987; Friedlander et al., 1989; Levine et al., 1994; Lee and Gumbiner, 1995; Yagi and Takeichi, 2000; Niessen et al., 2011]. Cad-11, also called osteoblastic cadherin, was initially identified in mouse osteoblasts and is a type II classical cadherin [Okazaki et al., 1994]. It is expressed in mesenchymal tissue, maintains bone density, and is essential for formation of the synovial lining [Kimura et al., 1995; Kawaguchi et al., 2001; Lee et al., 2007; Di Benedetto et al., 2010; Kyung Chang et al., 2010; Chang et al., 2011]. Cad-11 mediates synovial fibroblast inflammation by increasing IL-6 production through TNF-α [Chang et al., 2011]. Cad-11 also directly regulates the differentiation of mesenchymal cells into the cells of the osteo-lineage and chondro-lineage [Kii et al., 2004]. Additionally, cad-11 is expressed in several invasive cancer cell lines including breast, prostate, colon, and bone, and it plays a role in tumor invasion and progression [Shibata et al., 1996; Kashima et al., 1999; Pishvaian et al., 1999; Feltes et al., 2002]. We previously discovered that cad-11 is a hemodynamically sensitive protein expressed in adult aortic valve endothelial cells [Butcher et al., 2006]. Yutzey et al. group has also found that the cad-11 gene is expressed in chick endocardium and mitral valves; however these findings were incomplete with respect to cad-11 gene expression patterns throughout the developing heart. [Shelton and Yutzey, 2008]. The localization patterns, such as endothelial-mesenchymal expression differences or the temporal-spatial expression changes in each of the developing valves have not been fully characterized. In addition, cad-11's relationship to the changing valvular shape and resident cell phenotypes is unknown. In this study, we determined the cardiac specific expression pattern of cad-11, at both the gene and protein level across the continuum of development and adulthood. We establish that, in the heart, cad-11 is expressed exclusively in mesenchymal cells of the early embryonic outflow tract (OFT) and atrioventricular (AV) cushions, but becomes restricted to the cardiac valve endothelium postnatally. We then determine that cad-11 expression reverts to an embryonic pattern in diseased aortic valves, and is co-localized with myofibroblastic and osteogenic phenotypes. Together, these suggest that cad-11 participates in important valve developmental and pathogenic mechanisms.
Materials and Methods
Mouse Strains
Wildtype C57BL/6 and ApoE−/− mice (The Jackson Laboratory) were bred according to standard protocols. ApoE−/− mice were fed a high-fat/high-carbohydrate diet for 30 weeks before sacrificing and collecting their hearts. All animal work was conducted according to relevant national and international guidelines. Full details of this study were reviewed and approved by the Cornell IACUC (protocol #2008-0011).
Immunohistochemistry
Embryos were fixed in 4% paraformaldehyde overnight at 4°C, then dehydrated through an ethanol series and paraffin embedded and sectioned at 8 μm thickness. Following dewaxing and rehydration, sections were blocked with 10% goat serum before primary antibodies used were against cad-11 (rabbit anti-Cadherin 11 from Invitrogen; 1:100 dilution), MF20 (Developmental Studies Hybridoma Bank; 1:200 dilution), and PECAM-1 (Invitrogen; 1:200 dilution). Fluorescence-conjugated Alexa Fluor 488 goat anti-mouse and Alexa Fluor 568 goat anti-rabbit secondary antibodies (Invitrogen, 1:300) were used according to the primary antibody species. Sections were nuclei counterstained with DRAQ5 (Abcam, 1:1000 dilution). Healthy human aortic valve sections were used as positive controls for cad-11 (cad-11 is known to be expressed in endothelial cells of porcine aortic valves [Butcher et al., 2006]). Negative controls were incubated with 10% goat serum instead of primary antibodies. Signals were detected and images were collected with Zeiss 710 confocal microscopy (Cornell University Life Sciences Core Laboratories Center).
In situ Hybridization
Chick embryos and hearts were fixed in 4% paraformaldehyde overnight at 4°C, then dehydrated and stored in methanol. Embryos and hearts were rehydrated, digested with proteinase K, washed & refixed, and hybridized using digoxygenin-labeled in vitro transcribed anti-sense cad-11 reboprobes (corresponding to 501-798 of Gallus gallus cad-11 mRNA, NM_001004371.1) and sense reboprobes.
Quantification of Tissue-Specific Gene Expression
Fertilized eggs were incubated at 37°C and 50% humidity. OFT cushion or aortic valve leaflets from E4, E10 and E14 were dissected away from the surrounding myocardium. RNA was isolated from these tissues using the RNeasy Mini Kit (Qiagen) and transcribed into cDNA using the iScript cDNA Synthesis Kit (Bio-Rad, Inc.) according to the manufacturers’ instructions. Cad-11 expression levels were quantified with real-time PCR, using a Mini-Opticon Real-Time PCR Detection System (Bio-Rad, Inc.) and SYBR Green PCR master mix (Bio-Rad, Inc.), normalized to the 18S rRNA housekeeping gene. The following primers were used: cad-11 (F-TGATGGAGATGGCATGGATA, R-TGCCTCTACCTTCAGGCTGT, 79bp), 18S (F-CGGAGAGGGAGCCTGAGAA, R-CGCCAGCTCGATCCCAAGA, 275bp).
Quantitative Analysis of Histological Changes
Immunoreactivity of cad-11 in immunohistochemistry stained tissue sections was measured using ImageJ software version 1.46r (NIH). The area of each cell was selected and the average fluorescence intensity, minus the background, was used for data analysis as previously described [Butcher and Nerem, 2004; Richards et al., 2013]. Statistical significance was determined using Student's t-test (p<0.05).
Results
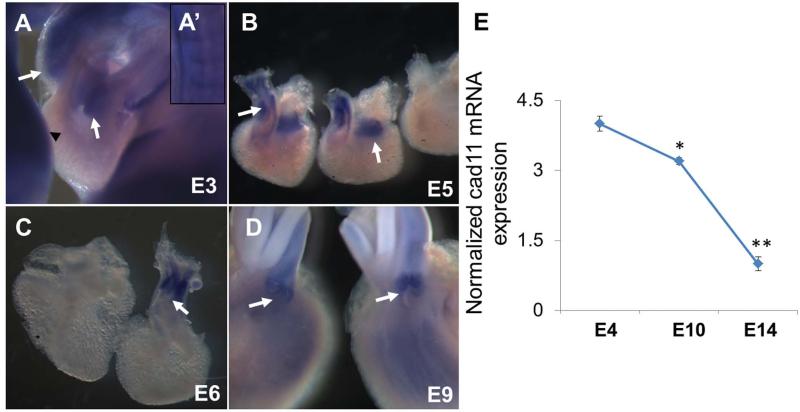
We first profiled the differential expression of cad-11 mRNA in avian hearts. We found that cad-11 mRNA is expressed in the limb bud (Fig. 1A black arrow head), the forming somite (Fig. 1A’), and the pharyngeal arches of early embryos, which is consistent with previous reports [Kimura et al., 1995] and confirmed the specificity of the cad-11 probe. Cad-11 is expressed throughout the cushion tissue of the OFT tract and the AV canal at 3 days of incubation (Fig. 1A white arrows). Cad-11 expression increases with cushion expansion (Day 5), and extends into the atrioventricular sulcus (Fig. 1B white arrows). By day 6, cad-11 mRNA expression is highly concentrated in the OFT, but decreased in the atrioventricular valve primordia (Fig. 1C). By day 9, cad-11 expression becomes restricted to the differentiating aortic and pulmonic valve leaflets and shows reduced expression in the atrioventricular valves (Fig. 1D white arrows). Sense probe controls showed no expression as expected (Fig. 1B far right). Real time PCR quantification of mRNA isolated from stage specific aortic valve progenitor cells/leaflets confirms that cad-11 is elevated during the cushion remodeling period (E4 at 3.2±0.1 fold and E10 at 4.0±0.2 fold, p<0.05), but markedly decreases as the leaflets mature (E14 at 1.0±0.1 fold, p<0.05, Fig. 1E).
Fig. 1.
RNA level expression of cad-11 in different stages of chick hearts. A-D, In situ hybridization of cad-11 at E3 (A), E5 (B), E6 (C), and E9 (D). A’, Cad-11 expression in somites at E3, consistent with previous research. White arrows indicate strong probe binding at the valve progenitor cells and valve cells. E, Real-time PCR quantification of cad-11 expression during aortic valvulogenesis. The values are cad-11 expression normalized to 18S rRNA and are the mean ± SEM (n=3, *p<0.05, between E4 and E10; **p<0.05, between E10 and E14).
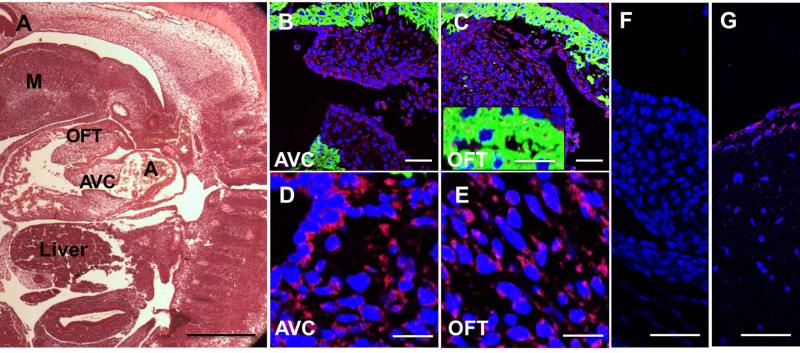
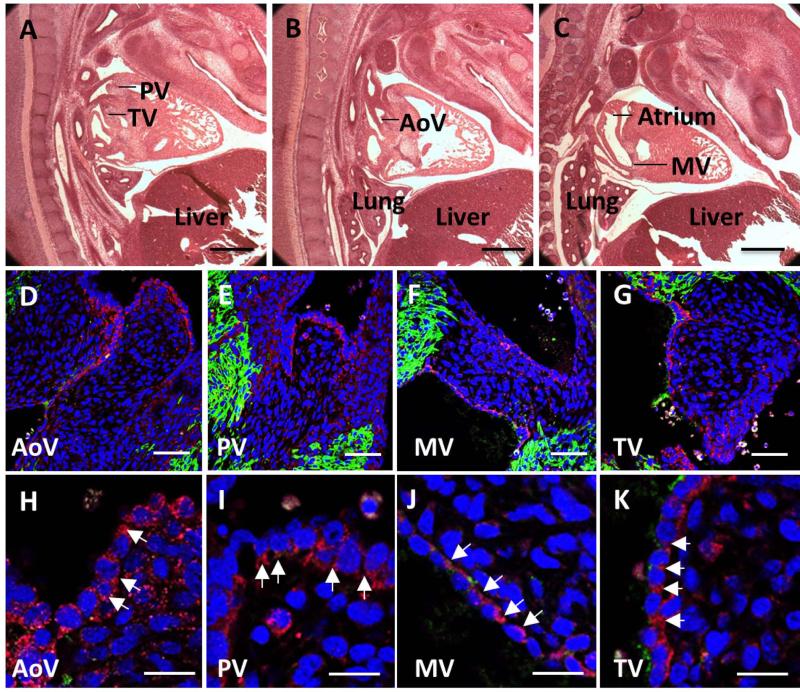
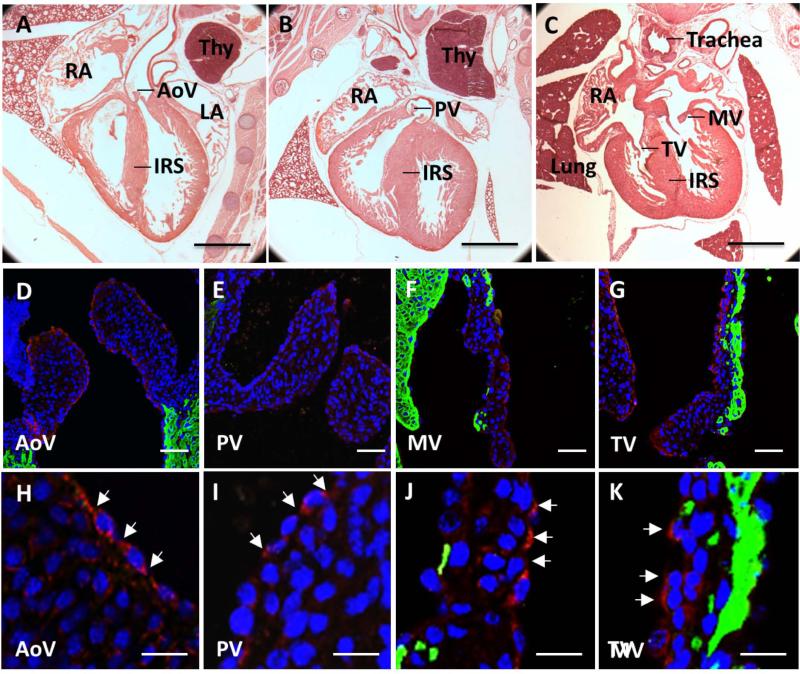
Similarly in mouse, cad-11 expression was confined to the endothelium and mesenchyme of the OFT and AV cushions (E11.5, Fig. 2B-E), but not expressed within the myocardium (Fig. 2C box region). [Butcher et al., 2006]. Negative controls showed a clean background (Fig. 2F). During semilunar valve remodeling (E14.5) however, cad-11 becomes increased in the endothelium but decreased in the mesenchyme of both the semilunar and atrioventricular valves (Fig. 3D-K). Cad-11 is progressively more restricted as valves condense and mature (E18.5, Fig. 4D-K), and virtually exclusively expressed in the valve endothelium postnatally (Fig. 5D, F and G). This expression patterns was largely consistent with previous reports in porcine aortic valve endothelial cells[Butcher et al., 2006], confirming antibody specificity (Fig. 2G).
Fig. 2.
Mesenchymal expression of cad-11 in E11 mice. A, Hematoxylin and eosin staining of the heart valve progenitor region-the OFT and AV cushion of the heart in sagittal sections. B, C, Immunofluorescence staining of cad-11 protein (red) and MF20 (green) in the mesenchymal cells of the OFT (B) and the AV cushion (C). D, E, Higher magnification of immunofluorescence staining of cad-11 protein (red) and MF20 (green) in the mesenchymal cells of the AV cushion (D) and the OFT (E). F, Negative control without primary antibody showed a clean background. G, Positive staining of cad-11 (red) is visible in endothelial cells of healthy human aortic valve leaflets. Abbreviations: A, atrium; OFT, outflow tract; AVC, atrioventricular cushion; M, Mandibular component of first branchial arch. Scale bar = (A) 0.5mm, (B,C, F,G) 50 μm, (D, E) 20 μm.
Fig. 3.
Endothelial expression of cad-11 in E14 mice. A-C, Hematoxylin and eosin staining of the aortic valve, pulmonic valve, mitral valve, and tricuspid valve in sagittal sections. D, H, Immunofluorescence staining of cad-11 protein (red) and MF20 (green) at low (D) and high (H) magnification of the aortic valve. E, I, Immunofluorescence staining of cad-11 protein (red) and MF20 (green) at low (E) and high (J) magnification of the pulmonic valve. F, J, Immunofluorescence staining of cad-11 protein (red) and MF20 (green) at low (F) and high (J) magnification of the mitral valve. G, K, Immunofluorescence staining of cad-11 protein (red) and MF20 (green) at low (G) and high (K) magnification of the tricuspid valve. White arrows indicate endothelial cells expressing cad-11. Abbreviations: AoV, aortic valve; PV, pulmonic valve; MV, mitral valve; TV, tricuspid valve. Scale bar = (A-C) 0.5mm, (D-G) 50 μm, (H-K) 20 μm.
Fig. 4.
Endothelial expression of cad-11 in E18 mice. A-C, Hematoxylin and eosin staining of the aortic valve, pulmonic valve, mitral valve, and tricuspid valve in frontal sections. D, H, Immunofluorescence staining of cad-11 protein (red) and MF20 (green) at low (D) and high (H) magnification of the aortic valve. E, I, Immunofluorescence staining of cad-11 protein (red) and MF20 (green) at low (E) and high (J) magnification of the pulmonic valve. F, J, Immunofluorescence staining of cad-11 protein (red) and MF20 (green) at low (F) and high (J) magnification of the mitral valve. G, K, Immunofluorescence staining of cad-11 protein (red) and MF20 (green) at low (G) and high (K) magnification of the tricuspid valve. White arrows indicate endothelial cells expressing cad-11. Abbreviations: AoV, aortic valve; PV, pulmonic valve; MV, mitral valve; TV, tricuspid valve; LA, left atrium; RA, right atrium; Thy, thymus; IRS, Interventricular septum. Scale bar = (A-C) 1mm, (D-G) 50 μm, (H-K) 20 μm.
Fig. 5.
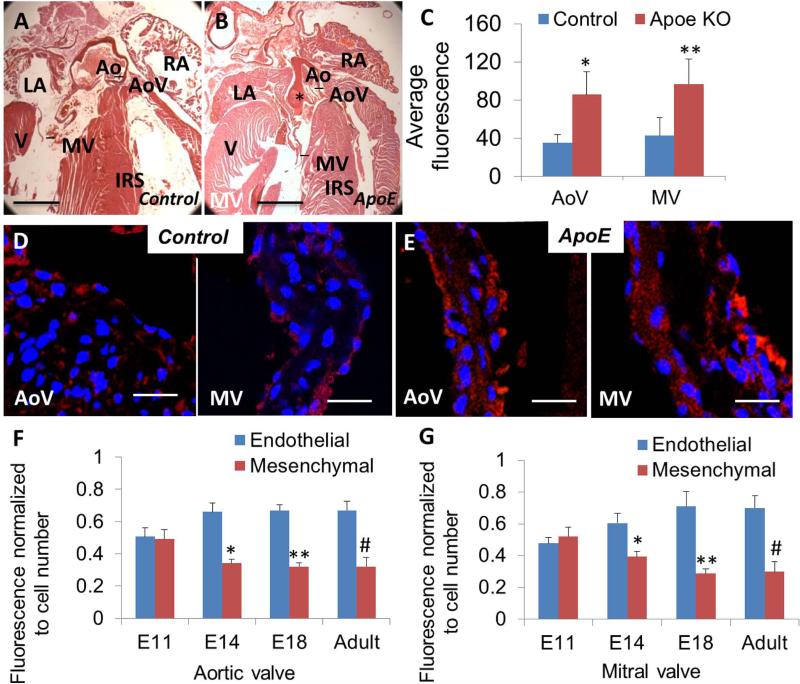
Histological and immunohistochemical analysis of control and ApoE−/− heart valve leaflets. A, B, Hematoxylin and eosin staining of control (A) and ApoE−/− (B) adult mice transverse sections. Notice the ApoE−/− aortic artery exhibits large plaques (indicated by *). C-E, Immunofluorescence staining of cad-11 protein (red) of ApoE−/− aortic and mitral valves (E) showed increased expression (C) compared to control aortic and mitral valves (D). Values are average fluorescence intensity and are means ± SEM (*p<0.05, section number = 3; **p<0.05, section number =3). F, Trends in expression of cad-11 protein from E11 to adult in the aortic valve progenitor cells and leaflets. G, Trends in expression of cad-11 protein from E11 to adult in the mitral valve progenitor cells and valve leaflets. Values are average fluorescence intensity and are means ± SEM (*p<0.05, between E11 and E14 mesenchymal fluorescence; **p<0.05, between E11 and E18 mesenchymal fluorescence; #p<0.05, between E11 and adult mesenchymal fluorescence; E11, n=9 (AoV), 9 (MV); E14, n=9 (AoV), 10 (MV); E18, n=10 (AoV), 11 (MV); Adult, n=10 (AoV), 9 (MV)). Abbreviations: AoV, aortic valve; MV, mitral valve. Scale bar = (A, B) 1mm, (D, E) 20 μm.
Cad-11 expression changed dramatically under diseased heart valve conditions. In the ApoE−/− hyperlipidemic mouse model that presents with aortic valve disease [Plump et al., 1992; Nakashima et al., 1994; Reddick et al., 1994; Hjortnaes et al., 2010], cad-11 expression was markedly increased (2.4±0.2 fold higher in aortic valve and 2.3±0.1 fold higher in mitral valve, p<0.05) compared to wildtype controls (Fig. 5C-E). While still expressed in the endothelium, cad-11 became re-expressed in the interstitial cells of ApoE−/− aortic valves (Fig. 5E).
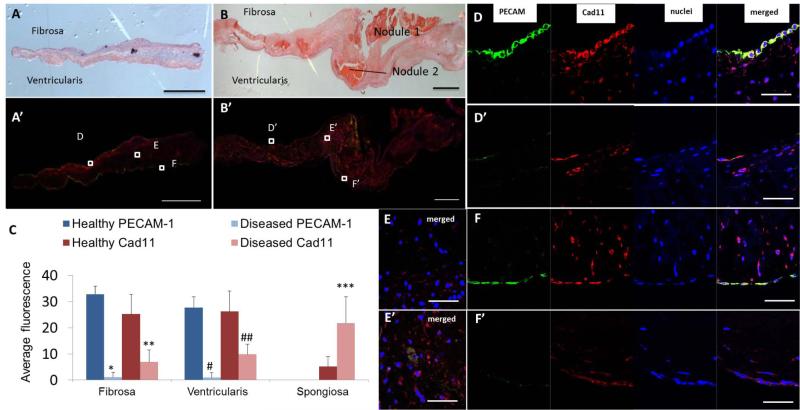
Cad-11 expression was similarly restricted to the endothelium of healthy human aortic valves (Fig. 6A, D, and F). The valve leaflets are composed of three layers: the ventricularis, on the ventricular side of the lealflets, the fibrosa, on the aortic side of the leaflet, and the spongiosa between the two. In calcified human aortic valves, mineralized lesions appear preferentially in the fibrosa (Fig. 6B). Double staining of cad-11 and PECAM-1 confirmed co-localization of the two proteins in endothelial cells on both the fibrosa and ventricularis layers of the healthy valves (Fig 6D and F). Cad-11 expression was statistically lower (27.3±3.4 fold less on the fibrosa, p<0.05, Fig. 6D, D’; 22.7±2.6 fold less on the ventricularis, p<0.05, Fig. 6F, F’, and C) on a per cell basis in the endothelium layer of calcified aortic valves compared to healthy controls. However, significantly fewer valve endothelial cells were present (3.6±0.3 fold less PECAM-1 surface expression on the fibrosa, p<0.05, Fig. 6D, D’ and C, 2.6±0.2 fold less on the ventricularis, p<0.05, Fig. 6F, F’ and C) in endothelial cells on both the fibrosa and ventricularis layers of the calcified valve leaflets. These results suggest that the endothelial expression of cad-11 in healthy aortic valves may be involved in maintaining the integrity of the endothelial monolayer.
Fig. 6.
Histological and immunohistochemical analysis of normal and calcified human aortic valve leaflets. A, B, Hematoxylin and eosin staining of normal (A) and calcified (B) human aortic valve leaflets. Notice the calcified aortic valves exhibit disturbed endothelium layers and several nodules. A’, B’, Double immunofluorescence staining of cad-11 protein (red) and PECAM-1 (green) of normal (A’) and calcified (B’) human aortic valve leaflets. C-F’, Co-localization of cad-11 and PECAM-1 in normal human aortic valve endothelial cells of fibrosa (D) and ventricularis (F). Decreased PECAM-1 and cad-11 expression was found in calcified aortic valve leaflets of fibrosa (D’) and ventricularis (F’). Increased cad-11 expression in calcified aortic valve leaflets (E’), compared to normal leaflets (E). Values are average fluorescence intensity and are means ± SEM. (*p<0.05, between healthy and diseased PECAM-1 fluorescence on the fibrosa, n=10; **p<0.05, between healthy and diseased cad-11 fluorescence on the fibrosa, n=10; #p<0.05, between healthy and diseased PECAM-1 fluorescence on the ventricularis, n=10; ##p<0.05, between healthy and diseased cad-11 fluorescence on the ventricularis, n=10; ***p<0.05, between healthy and diseased cad-11 fluorescence in the intersititium, n=6). Scale bar = (A-B’) 1 mm, (D-F’) 50 μm.
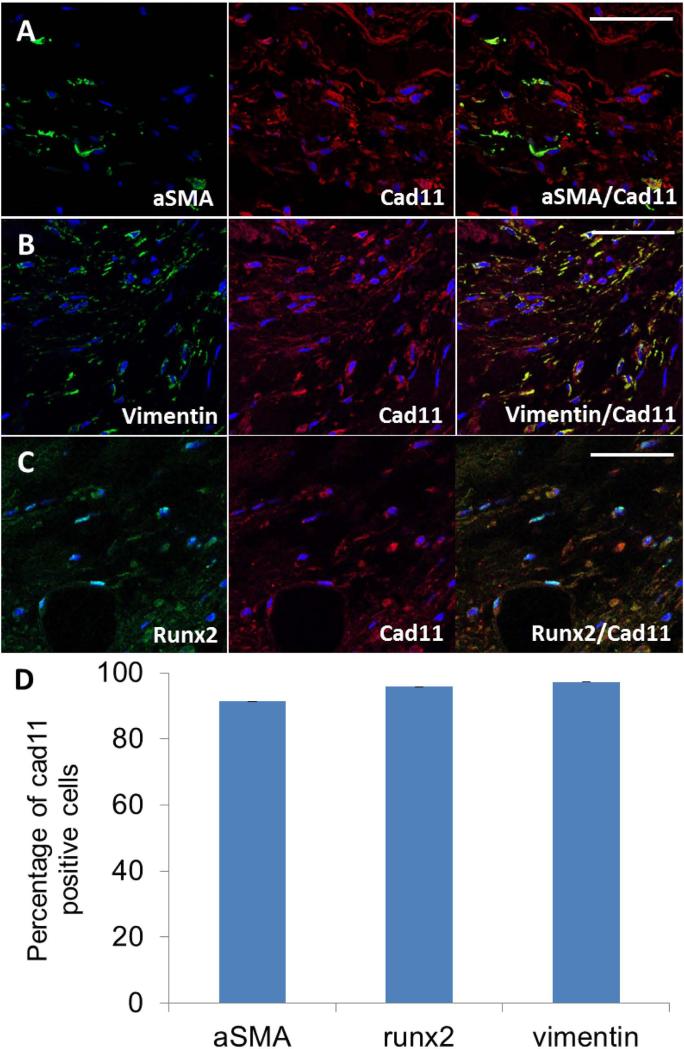
Cad-11 expression was statistically higher (4.2±0.6 fold, p<0.05, Fig. 6E, E’, and C) in the interstitial cells of calcified aortic valves, where the cells are mostly part of the spongiosa. As expected, both myofibroblastic (αSMA positive) and osteoblastic (Runx2 positive) cells were present within the diseased valves, but not in healthy valves (Fig. 7 and Suppl Fig. 1). We identified the phenotypes of the interstitial cells in calcified aortic valves by co-labeling cad-11 with αSMA, vimentin, or Runx2 (Fig. 7A-C). 91.3%±1.4% of myofibroblasts and 96.0%±2.5% of osteoblastic cells were also cad-11 positive (Fig. 7D). These results suggest that cad-11 is associated with myofibroblastic activation and osteoblastic differentiation of the valve interstitial cells (VICs) in diseased valves.
Fig. 7.
Co-staining of cad-11 with αSMA, vimentin, or runx2 in the valve interstitial cells (VICs) of human calcified valve samples. A, Double immunofluorescence staining of cad-11 protein (red) with αSMA protein (green) in the VICs of human calcified valve samples. B, Double immunofluorescence staining of cad-11 protein (red) with vimentin protein (green) in the VICs of human calcified valve samples. C, Double immunofluorescence staining of cad-11 protein (red) with runx2 protein (green) in the VICs of human calcified valve samples. D, Quantification of αSMA, vimentin, and runx2 expressing cells in cad-11 positive VICs. Values are average percentage of cad-11 positive cells and are means ± SEM. (αSMA, section number =3; vimentin, section number =3; runx2, section number =3). Scale bar = 50 μm.
Discussion
Cad-11 is a well-established mesenchymal cadherin involved in skeletal morphogenesis, osteogenic differentiation, and arthritic fibrosis, suggesting a role at the interface between fibrous and osseous tissues. Heart valve formation and disease also involves proper fibroblastic differentiation, and in pathological conditions leads to matrix mineralization, which suggests that cad-11 may be involved. Our results here clarify that cad-11 is expressed in avian and mouse heart valves with unique spatial and temporal characteristics. Cad-11 is found in the endothelium and mesenchyme of embryonic atrioventricular cushions and outflow tract cushions, but it becomes confined to the endothelial layers of semilunar, and to lesser degree, atrioventricular valves as they mature. Mesenchymal cells from different origins proliferate and migrate into the cardiac jelly and become endocardial cushions, valve primordia, and the cardiac septa of the adult heart [Markwald et al., 1975; Markwald et al., 1977]. Considering the highly migratory ability of the mesenchymal cushion cells and of cancer cells, and the up-regulation of cad-11 coinciding with tumor progression in adult tissue [Pishvaian et al., 1999; Tomita et al., 2000], our results suggest cad-11 may play a role in promoting cell migration into the cardiac jelly during early development [Valencia et al., 2004].
Recent studies have highlighted similarities in the transcriptional regulation of embryonic valve maturation and bone-tendon morphogenesis [Chakraborty et al., 2008]. Hutcheson and colleagues [Hutcheson et al., 2013] showed that cad-11 was upregulated in human calcified aortic valves, but cell specific expression was not investigated. Our findings in this study indicate similar results in diseased aortic valves: cad-11 is upregulated in both diseased mouse and human calcified aortic valves on the protein level. We further specified the cell types that over express cad-11 in calcified aortic valves. In ApoE−/− mice, cad-11 is increased in the endothelial and interstitial cells, suggesting that cad-11 may be involved in early inflammation activation of VECs. The first stage of CAVD is an inflammation activation of the valve endothelial cells (VECs), characterized by the recruitment of circulating inflammatory monocytes and T cells [Muller et al., 2000; Guerraty et al., 2010] and by EMT [Mahler et al., 2013]. EMT occurs in postnatal valves and creates a type of progenitor-like cell that maintains the interstitial cell population [Paranya et al., 2001]. We have previously reported that both TNF-α and IL-6 induce EMT in embryonic and adult valve endothelium via an AKT/NFκB-dependent pathway [Mahler et al., 2013]. Schneider and colleagues shown cad-11 knockdown can decrease EMT in A549 epithelial cell lines [Schneider et al., 2012]. Further researches on whether cad-11 is involved in the EMT process in the adult healthy and/or calcified valves will yield interesting results.
The second stage of CAVD is characterized by VICs undergoing myofibroblastic activation, depicted by an increase in αSMA, increased migration, increased proliferation, and increased traction force based matrix compaction [Kaden et al., 2005; Chen et al., 2009; Yip et al., 2009]. Late stage CAVD is portrayed by lesions of large calcified regions with osteogenic differentiating VICs that express a panel of osteoblast-related genes, including osteocalcin, osteonectin, and the transcription factor cbfa1/runx2 [Mohler et al., 1999; Osman et al., 2006]. Cad-11 upregulation is seen in VICs in human calcified aortic valves, with above 90% myofibrolastic-active and 96% osteogenic-active VICs expressing cad-11, suggesting cad-11 may trigger VIC myofibrolastic and osteogenic activation. The VIC family of cells can be divided into several groups according to their functions in normal valve physiology and in pathological processes—embryonic progenitor cells, quiescent VICs, progenitor VICs, and osteoblastic VICs [Liu et al., 2007]. A subset of valve interstitial cells is from hematopoietic stem cell origin [Visconti et al., 2006]. Cad-11 transfected E-cadherin−/− embryonic stem cells directly differentiate into chondrogenic and osteogenic phenotypes [Kii et al., 2004]. It is unclear whether cad-11 exclusively triggers resident valvular interstitial cells or if it triggers other sources, such as hematopoietic stem cells, to differentiate into osteogenic phenotype in diseased valves. Given the high proportion of mesenchymal cell expression of cad-11 in diseased valves in our study, we believe that each cell group has this capacity.
In our previous research, we have demonstrated that cad-11 is a mechanosensitive protein expressed in the aortic valve endothelium that is upregulated by steady shear stress [Butcher et al., 2006]. Cad-11 is also expressed in osteoblast cells [Okazaki et al., 1994] but it is unknown why VECs maintain elevated cad-11 expression during normal valve function. Cad-11 has been shown to function at lower Ca++ concentrations than normal cadherins [Hutcheson et al., 2013] and cad-11 junctions withstand 2-fold higher forces when compared with connections formed with N-cadherin [Pittet et al., 2008]. We did not find side specific differences in cad-11 expression in valve endothelial cells in mice. One explanation could be that mouse valves are thin - there may not be much of a “side” with respect to fibrosa vs. ventricularis (In contrast, in larger mammals/humans, there is a higher likelihood for a side difference). The co-expression of cad-11 and PECAM-1 in healthy aortic valve leaflets suggests that cad-11 may function to maintain the endothelial layer's monolayer integrity. Interestingly, we found co-expression of cad-11 and PECAM-1 inside diseased aortic valves consistent with angiogenic vessels. Hematopoietic stem cells have been reported to promote angiogenesis through angiopoietin-1during embryonic development [Takakura et al., 2000]. Whether cad-11 expressing cells are involved in stem-cell-associated angiogenesis in the calcification process, or if cad-11 expressing cells are involved in the formation of vessels derived from endothelial cells that underwent EMT is still unknown.
In conclusion, our results illustrate the expression pattern of cad-11 on both the gene and protein levels. Our data supports the hypothesis that cad-11 may be involved in mesenchymal cell migration during embryonic heart valve development. Cad-11 may also be involved in myofibroblastic and osteogenic activation during heart valve disease. More research is warranted to better elucidate how cad-11 interacts with other proteins in the promotion of valve cell migration and calcification.
Supplementary Material
Acknowledgements
The authors thank Dr. Sanjay Samy (Guthrie Clinic, Sayre, PA) and Dr. Jonathan Chen (Weill Cornell Medical College, NY) for providing human valve samples. This work was supported by the National Institutes of Health (HL110328, HL118672) and the National Science Foundation (CBET - #0955172). The funding agencies had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Abbreviations
- AVC
atrioventricular cushion
- ApoE
Apolipoprotein E
- CAVD
calcified aortic valve disease
- Cad-11
cadherin-11
- EMT
endothelial to mesenchymal transformation
- OFT
outflow tract
- PECAM-1
platelet endothelial cell adhesion molecule
- TGFβ1
transforming growth factor β1
- VEC
valve endothelial cell
- VIC
valve interstitial cell
References
- Butcher JT, Markwald RR. Valvulogenesis: the moving target. Philosophical transactions of the Royal Society of London Series B. Biological sciences. 2007;362(1484):1489–1503. doi: 10.1098/rstb.2007.2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butcher JT, Nerem RM. Porcine aortic valve interstitial cells in three-dimensional culture: comparison of phenotype with aortic smooth muscle cells. The Journal of heart valve disease. 2004;13(3):478–485. discussion 485-476. [PubMed] [Google Scholar]
- Butcher JT, Tressel S, Johnson T, Turner D, Sorescu G, Jo H, Nerem RM. Transcriptional Profiles of Valvular and Vascular Endothelial Cells Reveal Phenotypic Differences. Arteriosclerosis. Thrombosis, and Vascular Biology. 2006;26(1):69–77. doi: 10.1161/01.ATV.0000196624.70507.0d. [DOI] [PubMed] [Google Scholar]
- Camenisch TD, Spicer AP, Brehm-Gibson T, Biesterfeldt J, Augustine ML, Calabro A, Jr., Kubalak S, Klewer SE, McDonald JA. Disruption of hyaluronan synthase-2 abrogates normal cardiac morphogenesis and hyaluronan-mediated transformation of epithelium to mesenchyme. The Journal of clinical investigation. 2000;106(3):349–360. doi: 10.1172/JCI10272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakraborty S, Cheek J, Sakthivel B, Aronow BJ, Yutzey KE. Shared gene expression profiles in developing heart valves and osteoblast progenitor cells. Physiological genomics. 2008;35(1):75–85. doi: 10.1152/physiolgenomics.90212.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang SK, Noss EH, Chen M, Gu Z, Townsend K, Grenha R, Leon L, Lee SY, Lee DM, Brenner MB. Cadherin-11 regulates fibroblast inflammation. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(20):8402–8407. doi: 10.1073/pnas.1019437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JH, Yip CY, Sone ED, Simmons CA. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. The American journal of pathology. 2009;174(3):1109–1119. doi: 10.2353/ajpath.2009.080750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lange FJ, Moorman AF, Anderson RH, Manner J, Soufan AT, de Gier-de Vries C, Schneider MD, Webb S, van den Hoff MJ, Christoffels VM. Lineage and morphogenetic analysis of the cardiac valves. Circ Res. 2004;95(6):645–654. doi: 10.1161/01.RES.0000141429.13560.cb. [DOI] [PubMed] [Google Scholar]
- Di Benedetto A, Watkins M, Grimston S, Salazar V, Donsante C, Mbalaviele G, Radice GL, Civitelli R. N-cadherin and cadherin 11 modulate postnatal bone growth and osteoblast differentiation by distinct mechanisms. Journal of Cell Science. 2010;123(15):2640–2648. doi: 10.1242/jcs.067777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feltes CM, Kudo A, Blaschuk O, Byers SW. An Alternatively Spliced Cadherin-11 Enhances Human Breast Cancer Cell Invasion. Cancer Research. 2002;62(22):6688–6697. [PubMed] [Google Scholar]
- Friedlander DR, Mege RM, Cunningham BA, Edelman GM. Cell sorting-out is modulated by both the specificity and amount of different cell adhesion molecules (CAMs) expressed on cell surfaces. Proceedings of the National Academy of Sciences of the United States of America. 1989;86(18):7043–7047. doi: 10.1073/pnas.86.18.7043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guerraty MA, Grant GR, Karanian JW, Chiesa OA, Pritchard WF, Davies PF. Hypercholesterolemia induces side-specific phenotypic changes and peroxisome proliferator-activated receptor-gamma pathway activation in swine aortic valve endothelium. Arterioscler Thromb Vasc Biol. 2010;30(2):225–231. doi: 10.1161/ATVBAHA.109.198549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson DJ, Copp AJ. Versican Expression Is Associated With Chamber Specification, Septation, and Valvulogenesis in the Developing Mouse Heart. Circulation Research. 1998;83(5):523–532. doi: 10.1161/01.res.83.5.523. [DOI] [PubMed] [Google Scholar]
- Hjortnaes J, Butcher J, Figueiredo JL, Riccio M, Kohler RH, Kozloff KM, Weissleder R, Aikawa E. Arterial and aortic valve calcification inversely correlates with osteoporotic bone remodelling: a role for inflammation. European heart journal. 2010;31(16):1975–1984. doi: 10.1093/eurheartj/ehq237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiuchi K, Amizuka N, Takeshita S, Takamatsu H, Katsuura M, Ozawa H, Toyama Y, Bonewald LF, Kudo A. Identification and Characterization of a Novel Protein, Periostin, with Restricted Expression to Periosteum and Periodontal Ligament and Increased Expression by Transforming Growth Factor β. Journal of Bone and Mineral Research. 1999;14(7):1239–1249. doi: 10.1359/jbmr.1999.14.7.1239. [DOI] [PubMed] [Google Scholar]
- Hutcheson JD, Chen J, Sewell-Loftin MK, Ryzhova LM, Fisher CI, Su YR, Merryman WD. Cadherin-11 regulates cell-cell tension necessary for calcific nodule formation by valvular myofibroblasts. Arterioscler Thromb Vasc Biol. 2013;33(1):114–120. doi: 10.1161/ATVBAHA.112.300278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaden JJ, Dempfle CE, Grobholz R, Fischer CS, Vocke DC, Kilic R, Sarikoc A, Pinol R, Hagl S, Lang S, Brueckmann M, Borggrefe M. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovascular pathology : the official journal of the Society for Cardiovascular Pathology. 2005;14(2):80–87. doi: 10.1016/j.carpath.2005.01.002. [DOI] [PubMed] [Google Scholar]
- Kashima T, Kawaguchi J, Takeshita S, Kuroda M, Takanashi M, Horiuchi H, Imamura T, Ishikawa Y, Ishida T, Mori S, Machinami R, Kudo A. Anomalous cadherin expression in osteosarcoma. Possible relationships to metastasis and morphogenesis. The American journal of pathology. 1999;155(5):1549–1555. doi: 10.1016/S0002-9440(10)65471-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi J, Azuma Y, Hoshi K, Kii I, Takeshita S, Ohta T, Ozawa H, Takeichi M, Chisaka O, Kudo A. Targeted disruption of cadherin-11 leads to a reduction in bone density in calvaria and long bone metaphyses. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2001;16(7):1265–1271. doi: 10.1359/jbmr.2001.16.7.1265. [DOI] [PubMed] [Google Scholar]
- Kern CB, Wessels A, McGarity J, Dixon LJ, Alston E, Argraves WS, Geeting D, Nelson CM, Menick DR, Apte SS. Reduced versican cleavage due to Adamts9 haploinsufficiency is associated with cardiac and aortic anomalies. Matrix Biology. 2010;29(4):304–316. doi: 10.1016/j.matbio.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kii I, Amizuka N, Shimomura J, Saga Y, Kudo A. Cell-cell interaction mediated by cadherin-11 directly regulates the differentiation of mesenchymal cells into the cells of the osteo-lineage and the chondro-lineage. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2004;19(11):1840–1849. doi: 10.1359/JBMR.040812. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Matsunami H, Inoue T, Shimamura K, Uchida N, Ueno T, Miyazaki T, Takeichi M. Cadherin-11 Expressed in Association with Mesenchymal Morphogenesis in the Head, Somite, and Limb Bud of Early Mouse Embryos. Developmental Biology. 1995;169(1):347–358. doi: 10.1006/dbio.1995.1149. [DOI] [PubMed] [Google Scholar]
- Kruithof BPT, Krawitz SA, Gaussin V. Atrioventricular valve development during late embryonic and postnatal stages involves condensation and extracellular matrix remodeling. Developmental Biology. 2007;302(1):208–217. doi: 10.1016/j.ydbio.2006.09.024. [DOI] [PubMed] [Google Scholar]
- Kyung Chang S, Gu Z, Brenner MB. Fibroblast-like synoviocytes in inflammatory arthritis pathology: the emerging role of cadherin-11. Immunological Reviews. 2010;233(1):256–266. doi: 10.1111/j.0105-2896.2009.00854.x. [DOI] [PubMed] [Google Scholar]
- Lee CH, Gumbiner BM. Disruption of gastrulation movements in Xenopus by a dominant-negative mutant for C-cadherin. Dev Biol. 1995;171(2):363–373. doi: 10.1006/dbio.1995.1288. [DOI] [PubMed] [Google Scholar]
- Lee DM, Kiener HP, Agarwal SK, Noss EH, Watts GFM, Chisaka O, Takeichi M, Brenner MB. Cadherin-11 in Synovial Lining Formation and Pathology in Arthritis. Science. 2007;315(5814):1006–1010. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- Levine E, Lee CH, Kintner C, Gumbiner BM. Selective disruption of E-cadherin function in early Xenopus embryos by a dominant negative mutant. Development. 1994;120(4):901–909. doi: 10.1242/dev.120.4.901. [DOI] [PubMed] [Google Scholar]
- Liu AC, Joag VR, Gotlieb AI. The emerging role of valve interstitial cell phenotypes in regulating heart valve pathobiology. The American journal of pathology. 2007;171(5):1407–1418. doi: 10.2353/ajpath.2007.070251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahler GJ, Farrar EJ, Butcher JT. Inflammatory cytokines promote mesenchymal transformation in embryonic and adult valve endothelial cells. Arterioscler Thromb Vasc Biol. 2013;33(1):121–130. doi: 10.1161/ATVBAHA.112.300504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markwald RR, Fitzharris TP, Manasek FJ. Structural development of endocardial cushions. The American journal of anatomy. 1977;148(1):85–119. doi: 10.1002/aja.1001480108. [DOI] [PubMed] [Google Scholar]
- Markwald RR, Fitzharris TP, Smith WN. Sturctural analysis of endocardial cytodifferentiation. Dev Biol. 1975;42(1):160–180. doi: 10.1016/0012-1606(75)90321-8. [DOI] [PubMed] [Google Scholar]
- MEMBERS WG, Lloyd-Jones D, Adams R, Carnethon M, De Simone G, Ferguson TB, Flegal K, Ford E, Furie K, Go A, Greenlund K, Haase N, Hailpern S, Ho M, Howard V, Kissela B, Kittner S, Lackland D, Lisabeth L, Marelli A, McDermott M, Meigs J, Mozaffarian D, Nichol G, O'Donnell C, Roger V, Rosamond W, Sacco R, Sorlie P, Stafford R, Steinberger J, Thom T, Wasserthiel-Smoller S, Wong N, Wylie-Rosett J, Hong Y, f.t.A.H.A.S. Committee, S.S. Subcommittee Heart Disease and Stroke Statistics—2009 Update: A Report From the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119(3):e21–e181. doi: 10.1161/CIRCULATIONAHA.108.191261. [DOI] [PubMed] [Google Scholar]
- Mjaatvedt CH, Yamamura H, Capehart AA, Turner D, Markwald RR. The Cspg2 gene, disrupted in the hdf mutant, is required for right cardiac chamber and endocardial cushion formation. Dev Biol. 1998;202(1):56–66. doi: 10.1006/dbio.1998.9001. [DOI] [PubMed] [Google Scholar]
- Mohler ER, 3rd, Chawla MK, Chang AW, Vyavahare N, Levy RJ, Graham L, Gannon FH. Identification and characterization of calcifying valve cells from human and canine aortic valves. The Journal of heart valve disease. 1999;8(3):254–260. [PubMed] [Google Scholar]
- Muller AM, Cronen C, Kupferwasser LI, Oelert H, Muller KM, Kirkpatrick CJ. Expression of endothelial cell adhesion molecules on heart valves: up-regulation in degeneration as well as acute endocarditis. The Journal of pathology. 2000;191(1):54–60. doi: 10.1002/(SICI)1096-9896(200005)191:1<54::AID-PATH568>3.0.CO;2-Y. [DOI] [PubMed] [Google Scholar]
- Nagafuchi A, Shirayoshi Y, Okazaki K, Yasuda K, Takeichi M. Transformation of cell adhesion properties by exogenously introduced E-cadherin cDNA. Nature. 1987;329(6137):341–343. doi: 10.1038/329341a0. [DOI] [PubMed] [Google Scholar]
- Nakashima Y, Plump AS, Raines EW, Breslow JL, Ross R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arteriosclerosis and thrombosis : a journal of vascular biology / American Heart Association. 1994;14(1):133–140. doi: 10.1161/01.atv.14.1.133. [DOI] [PubMed] [Google Scholar]
- Niessen CM, Leckband D, Yap AS. Tissue organization by cadherin adhesion molecules: dynamic molecular and cellular mechanisms of morphogenetic regulation. Physiological reviews. 2011;91(2):691–731. doi: 10.1152/physrev.00004.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okazaki M, Takeshita S, Kawai S, Kikuno R, Tsujimura A, Kudo A, Amann E. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. The Journal of biological chemistry. 1994;269(16):12092–12098. [PubMed] [Google Scholar]
- Osman L, Yacoub MH, Latif N, Amrani M, Chester AH. Role of Human Valve Interstitial Cells in Valve Calcification and Their Response to Atorvastatin. Circulation. 2006;114(1 suppl):I–547-I-552. doi: 10.1161/CIRCULATIONAHA.105.001115. [DOI] [PubMed] [Google Scholar]
- Paranya G, Vineberg S, Dvorin E, Kaushal S, Roth SJ, Rabkin E, Schoen FJ, Bischoff J. Aortic Valve Endothelial Cells Undergo Transforming Growth Factor-β-Mediated and Non-Transforming Growth Factor-β-Mediated Transdifferentiation in Vitro. The American journal of pathology. 2001;159(4):1335–1343. doi: 10.1016/s0002-9440(10)62520-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Person AD, Klewer SE, Runyan RB. Cell Biology of Cardiac Cushion Development: International review of cytology. Academic Press. 2005:287–335. doi: 10.1016/S0074-7696(05)43005-3. [DOI] [PubMed] [Google Scholar]
- Pishvaian MJ, Feltes CM, Thompson P, Bussemakers MJ, Schalken JA, Byers SW. Cadherin-11 is expressed in invasive breast cancer cell lines. Cancer Res. 1999;59(4):947–952. [PubMed] [Google Scholar]
- Pittet P, Lee K, Kulik AJ, Meister JJ, Hinz B. Fibrogenic fibroblasts increase intercellular adhesion strength by reinforcing individual OB-cadherin bonds. J Cell Sci. 2008;121(Pt 6):877–886. doi: 10.1242/jcs.024877. [DOI] [PubMed] [Google Scholar]
- Plump AS, Smith JD, Hayek T, Aalto-Setälä K, Walsh A, Verstuyft JG, Rubin EM, Breslow JL. Severe hypercholesterolemia and atherosclerosis in apolipoprotein E-deficient mice created by homologous recombination in ES cells. Cell. 1992;71(2):343–353. doi: 10.1016/0092-8674(92)90362-g. [DOI] [PubMed] [Google Scholar]
- Ramsdell AF, Markwald RR. Induction of Endocardial Cushion Tissue in the Avian Heart is Regulated, in Part, by TGFβ-3-Mediated Autocrine Signaling. Developmental Biology. 1997;188(1):64–74. doi: 10.1006/dbio.1997.8637. [DOI] [PubMed] [Google Scholar]
- Reddick RL, Zhang SH, Maeda N. Atherosclerosis in mice lacking apo E. Evaluation of lesional development and progression. Arteriosclerosis. Thrombosis, and Vascular Biology. 1994;14(1):141–147. doi: 10.1161/01.atv.14.1.141. [DOI] [PubMed] [Google Scholar]
- Richards J, El-Hamamsy I, Chen S, Sarang Z, Sarathchandra P, Yacoub MH, Chester AH, Butcher JT. Side-specific endothelial-dependent regulation of aortic valve calcification: interplay of hemodynamics and nitric oxide signaling. The American journal of pathology. 2013;182(5):1922–1931. doi: 10.1016/j.ajpath.2013.01.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider DJ, Wu M, Le TT, Cho SH, Brenner MB, Blackburn MR, Agarwal SK. Cadherin-11 contributes to pulmonary fibrosis: potential role in TGF-beta production and epithelial to mesenchymal transition. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2012;26(2):503–512. doi: 10.1096/fj.11-186098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelton EL, Yutzey KE. Twist1 function in endocardial cushion cell proliferation, migration, and differentiation during heart valve development. Dev Biol. 2008;317(1):282–295. doi: 10.1016/j.ydbio.2008.02.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibata T, Ochiai A, Gotoh M, Machinami R, Hirohashi S. Simultaneous expression of cadherin-11 in signet-ring cell carcinoma and stromal cells of diffuse-type gastric cancer. Cancer letters. 1996;99(2):147–153. doi: 10.1016/0304-3835(95)04047-1. [DOI] [PubMed] [Google Scholar]
- Takakura N, Watanabe T, Suenobu S, Yamada Y, Noda T, Ito Y, Satake M, Suda T. A role for hematopoietic stem cells in promoting angiogenesis. Cell. 2000;102(2):199–209. doi: 10.1016/s0092-8674(00)00025-8. [DOI] [PubMed] [Google Scholar]
- Tomita K, van Bokhoven A, van Leenders GJ, Ruijter ET, Jansen CF, Bussemakers MJ, Schalken JA. Cadherin switching in human prostate cancer progression. Cancer Res. 2000;60(13):3650–3654. [PubMed] [Google Scholar]
- Valencia X, Higgins JM, Kiener HP, Lee DM, Podrebarac TA, Dascher CC, Watts GF, Mizoguchi E, Simmons B, Patel DD, Bhan AK, Brenner MB. Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes. The Journal of experimental medicine. 2004;200(12):1673–1679. doi: 10.1084/jem.20041545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Visconti RP, Ebihara Y, LaRue AC, Fleming PA, McQuinn TC, Masuya M, Minamiguchi H, Markwald RR, Ogawa M, Drake CJ. An in vivo analysis of hematopoietic stem cell potential: hematopoietic origin of cardiac valve interstitial cells. Circ Res. 2006;98(5):690–696. doi: 10.1161/01.RES.0000207384.81818.d4. [DOI] [PubMed] [Google Scholar]
- Yagi T, Takeichi M. Cadherin superfamily genes: functions, genomic organization, and neurologic diversity. Genes & development. 2000;14(10):1169–1180. [PubMed] [Google Scholar]
- Yip CY, Chen JH, Zhao R, Simmons CA. Calcification by valve interstitial cells is regulated by the stiffness of the extracellular matrix. Arterioscler Thromb Vasc Biol. 2009;29(6):936–942. doi: 10.1161/ATVBAHA.108.182394. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cao L, Yang BL, Yang BB. The G3 Domain of Versican Enhances Cell Proliferation via Epidermial Growth Factor-like Motifs. Journal of Biological Chemistry. 1998;273(33):21342–21351. doi: 10.1074/jbc.273.33.21342. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.