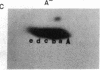
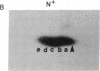
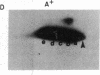
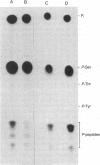
Abstract
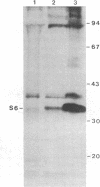
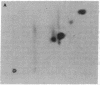
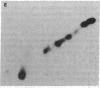
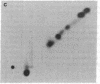
Phosphorylation of ribosomal protein S6 in NIH 3T3 fibroblasts is dependent on the presence of serum, but after transformation of these cells by Abelson murine leukemia virus (Ab-MuLV), S6 remained highly phosphorylated on serine residues either in the absence or the presence of serum. To investigate whether S6 phosphorylation in this system was a consequence of the action of the Ab-MuLV tyrosine-specific protein kinase, purified Ab-MuLV kinase made in Escherichia coli was microinjected into Xenopus oocytes and was observed to cause a 7- to 15-fold increase in the phosphorylation of S6 on serine residues. Two-dimensional phosphopeptide maps of S6 phosphorylated in Ab-MuLV-transformed NIH cells in the absence of serum were identical to those of S6 isolated from normal cells grown in the presence of serum. In addition, S6 from oocytes injected with Ab-MuLV kinase yielded an S6 phosphopeptide map indistinguishable from that of serum-stimulated NIH 3T3 cells, whereas S6 from control oocytes lacked several phosphopeptides. Ab-MuLV kinase did not phosphorylate S6 directly in vitro, and microinjection of a mutant Ab-MuLV protein lacking kinase activity had no effect. These results indicate that the Ab-MuLV kinase interacts with a cellular pathway to enhance S6 phosphorylation by directly or indirectly activating an S6 protein kinase and/or inactivating an S6 protein phosphatase.
Full text
PDF



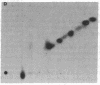
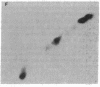
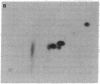
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ballinger D. G., Hunt T. Fertilization of sea urchin eggs is accompanied by 40 S ribosomal subunit phosphorylation. Dev Biol. 1981 Oct 30;87(2):277–285. doi: 10.1016/0012-1606(81)90151-2. [DOI] [PubMed] [Google Scholar]
- Blenis J., Spivack J. G., Erikson R. L. Phorbol ester, serum, and rous sarcoma virus transforming gene product induce similar phosphorylations of ribosomal protein S6. Proc Natl Acad Sci U S A. 1984 Oct;81(20):6408–6412. doi: 10.1073/pnas.81.20.6408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkhard S. J., Traugh J. A. Changes in ribosome function by cAMP-dependent and cAMP-independent phosphorylation of ribosomal protein S6. J Biol Chem. 1983 Nov 25;258(22):14003–14008. [PubMed] [Google Scholar]
- Cobb M. H., Rosen O. M. Description of a protein kinase derived from insulin-treated 3T3-L1 cells that catalyzes the phosphorylation of ribosomal protein S6 and casein. J Biol Chem. 1983 Oct 25;258(20):12472–12481. [PubMed] [Google Scholar]
- Collett M. S., Purchio A. F., Erikson R. L. Avian sarcoma virus-transforming protein, pp60src shows protein kinase activity specific for tyrosine. Nature. 1980 May 15;285(5761):167–169. doi: 10.1038/285167a0. [DOI] [PubMed] [Google Scholar]
- Cooper J. A., Hunter T. Identification and characterization of cellular targets for tyrosine protein kinases. J Biol Chem. 1983 Jan 25;258(2):1108–1115. [PubMed] [Google Scholar]
- Decker S. Phosphorylation of ribosomal protein S6 in avian sarcoma virus-transformed chicken embryo fibroblasts. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4112–4115. doi: 10.1073/pnas.78.7.4112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donahue M. J., Masaracchia R. A. Phosphorylation of ribosomal protein S6 at multiple sites by a cyclic AMP-independent protein kinase from lymphoid cells. J Biol Chem. 1984 Jan 10;259(1):435–440. [PubMed] [Google Scholar]
- Duncan R., McConkey E. H. Preferential utilization of phosphorylated 40-S ribosomal subunits during initiation complex formation. Eur J Biochem. 1982 Apr;123(3):535–538. doi: 10.1111/j.1432-1033.1982.tb06564.x. [DOI] [PubMed] [Google Scholar]
- Ek B., Westermark B., Wasteson A., Heldin C. H. Stimulation of tyrosine-specific phosphorylation by platelet-derived growth factor. Nature. 1982 Feb 4;295(5848):419–420. doi: 10.1038/295419a0. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Hanafusa T., Hanafusa H. Characterization of protein kinase activity associated with the transforming gene product of Fujinami sarcoma virus. Cell. 1980 Dec;22(3):757–765. doi: 10.1016/0092-8674(80)90552-8. [DOI] [PubMed] [Google Scholar]
- Feldman R. A., Wang L. H., Hanafusa H., Balduzzi P. C. Avian sarcoma virus UR2 encodes a transforming protein which is associated with a unique protein kinase activity. J Virol. 1982 Apr;42(1):228–236. doi: 10.1128/jvi.42.1.228-236.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frackelton A. R., Jr, Ross A. H., Eisen H. N. Characterization and use of monoclonal antibodies for isolation of phosphotyrosyl proteins from retrovirus-transformed cells and growth factor-stimulated cells. Mol Cell Biol. 1983 Aug;3(8):1343–1352. doi: 10.1128/mcb.3.8.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover C. V. Heat shock induces rapid dephosphorylation of a ribosomal protein in Drosophila. Proc Natl Acad Sci U S A. 1982 Mar;79(6):1781–1785. doi: 10.1073/pnas.79.6.1781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gressner A. M., Wool I. G. The phosphorylation of liver ribosomal proteins in vivo. Evidence that only a single small subunit protein (S6) is phosphorylated. J Biol Chem. 1974 Nov 10;249(21):6917–6925. [PubMed] [Google Scholar]
- Haselbacher G. K., Humbel R. E., Thomas G. Insulin-like growth factor: insulin or serum increase phosphorylation of ribosomal protein S6 during transition of stationary chick embryo fibroblasts into early G1 phase of the cell cycle. FEBS Lett. 1979 Apr 1;100(1):185–190. doi: 10.1016/0014-5793(79)81160-6. [DOI] [PubMed] [Google Scholar]
- Hofmann F., Bechtel P. J., Krebs E. G. Concentrations of cyclic AMP-dependent protein kinase subunits in various tissues. J Biol Chem. 1977 Feb 25;252(4):1441–1447. [PubMed] [Google Scholar]
- Hunter T., Sefton B. M. Transforming gene product of Rous sarcoma virus phosphorylates tyrosine. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1311–1315. doi: 10.1073/pnas.77.3.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs S., Kull F. C., Jr, Earp H. S., Svoboda M. E., Van Wyk J. J., Cuatrecasas P. Somatomedin-C stimulates the phosphorylation of the beta-subunit of its own receptor. J Biol Chem. 1983 Aug 25;258(16):9581–9584. [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blith D. L., Karlsson F. A., Häring H. U., Kahn C. R. Insulin stimulation of phosphorylation of the beta subunit of the insulin receptor. Formation of both phosphoserine and phosphotyrosine. J Biol Chem. 1982 Sep 10;257(17):9891–9894. [PubMed] [Google Scholar]
- Kasuga M., Zick Y., Blithe D. L., Crettaz M., Kahn C. R. Insulin stimulates tyrosine phosphorylation of the insulin receptor in a cell-free system. Nature. 1982 Aug 12;298(5875):667–669. doi: 10.1038/298667a0. [DOI] [PubMed] [Google Scholar]
- Kawai S., Yoshida M., Segawa K., Sugiyama H., Ishizaki R., Toyoshima K. Characterization of Y73, an avian sarcoma virus: a unique transforming gene and its product, a phosphopolyprotein with protein kinase activity. Proc Natl Acad Sci U S A. 1980 Oct;77(10):6199–6203. doi: 10.1073/pnas.77.10.6199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Peuch C. J., Ballester R., Rosen O. M. Purified rat brain calcium- and phospholipid-dependent protein kinase phosphorylates ribosomal protein S6. Proc Natl Acad Sci U S A. 1983 Nov;80(22):6858–6862. doi: 10.1073/pnas.80.22.6858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maller J. L. Use of microinjection techniques to study protein kinases and protein phosphorylation in amphibian oocytes. Methods Enzymol. 1983;99:219–226. doi: 10.1016/0076-6879(83)99056-0. [DOI] [PubMed] [Google Scholar]
- Martin-Pérez J., Siegmann M., Thomas G. EGF, PGF2 alpha and insulin induce the phosphorylation of identical S6 peptides in swiss mouse 3T3 cells: effect of cAMP on early sites of phosphorylation. Cell. 1984 Feb;36(2):287–294. doi: 10.1016/0092-8674(84)90222-8. [DOI] [PubMed] [Google Scholar]
- Naharro G., Dunn C. Y., Robbins K. C. Analysis of the primary translational product and integrated DNA of a new feline sarcoma virus, GR-FeSV. Virology. 1983 Mar;125(2):502–507. doi: 10.1016/0042-6822(83)90223-4. [DOI] [PubMed] [Google Scholar]
- Neil J. C., Ghysdael J., Vogt P. K. Tyrosine-specific protein kinase activity associated with p105 of avian sarcoma virus PRCII. Virology. 1981 Feb;109(1):223–228. doi: 10.1016/0042-6822(81)90493-1. [DOI] [PubMed] [Google Scholar]
- Nielsen P. J., Thomas G., Maller J. L. Increased phosphorylation of ribosomal protein S6 during meiotic maturation of Xenopus oocytes. Proc Natl Acad Sci U S A. 1982 May;79(9):2937–2941. doi: 10.1073/pnas.79.9.2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishimura J., Deuel T. F. Platelet-derived growth factor stimulates the phosphorylation of ribosomal protein S6. FEBS Lett. 1983 May 30;156(1):130–134. doi: 10.1016/0014-5793(83)80263-4. [DOI] [PubMed] [Google Scholar]
- Nishimura J., Deuel T. F. Stimulation of protein phosphorylation in Swiss mouse 3T3 cells by human platelet derived growth factor. Biochem Biophys Res Commun. 1981 Nov 16;103(1):355–361. doi: 10.1016/0006-291x(81)91700-9. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. The role of protein kinase C in cell surface signal transduction and tumour promotion. Nature. 1984 Apr 19;308(5961):693–698. doi: 10.1038/308693a0. [DOI] [PubMed] [Google Scholar]
- Novak-Hofer I., Thomas G. An activated S6 kinase in extracts from serum- and epidermal growth factor-stimulated Swiss 3T3 cells. J Biol Chem. 1984 May 10;259(9):5995–6000. [PubMed] [Google Scholar]
- Parker P. J., Caudwell F. B., Cohen P. Glycogen synthase from rabbit skeletal muscle; effect of insulin on the state of phosphorylation of the seven phosphoserine residues in vivo. Eur J Biochem. 1983 Jan 17;130(1):227–234. doi: 10.1111/j.1432-1033.1983.tb07140.x. [DOI] [PubMed] [Google Scholar]
- Perisic O., Traugh J. A. Protease-activated kinase II as the potential mediator of insulin-stimulated phosphorylation of ribosomal protein S6. J Biol Chem. 1983 Aug 25;258(16):9589–9592. [PubMed] [Google Scholar]
- Prywes R., Foulkes J. G., Rosenberg N., Baltimore D. Sequences of the A-MuLV protein needed for fibroblast and lymphoid cell transformation. Cell. 1983 Sep;34(2):569–579. doi: 10.1016/0092-8674(83)90389-6. [DOI] [PubMed] [Google Scholar]
- Queen C. A vector that uses phage signals for efficient synthesis of proteins in Escherichia coli. J Mol Appl Genet. 1983;2(1):1–10. [PubMed] [Google Scholar]
- Rubin J. B., Shia M. A., Pilch P. F. Stimulation of tyrosine-specific phosphorylation in vitro by insulin-like growth factor I. 1983 Sep 29-Oct 5Nature. 305(5933):438–440. doi: 10.1038/305438a0. [DOI] [PubMed] [Google Scholar]
- Scott M. P., Pardue M. L. Translational control in lysates of Drosophila melanogaster cells. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3353–3357. doi: 10.1073/pnas.78.6.3353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spivack J. G., Erikson R. L., Maller J. L. Microinjection of pp60v-src into Xenopus oocytes increases phosphorylation of ribosomal protein S6 and accelerates the rate of progesterone-induced meiotic maturation. Mol Cell Biol. 1984 Aug;4(8):1631–1634. doi: 10.1128/mcb.4.8.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stith B. J., Maller J. L. The effect of insulin on intracellular ph and ribosomal protein S6 phosphorylation in oocytes of Xenopus laevis. Dev Biol. 1984 Mar;102(1):79–89. doi: 10.1016/0012-1606(84)90176-3. [DOI] [PubMed] [Google Scholar]
- Swergold G. D., Rosen O. M., Rubin C. S. Hormonal regulation of the phosphorylation of ATP citrate lyase in 3T3-L1 adipocytes. Effects of insulin and isoproterenol. J Biol Chem. 1982 Apr 25;257(8):4207–4215. [PubMed] [Google Scholar]
- Terao K., Ogata K. Proteins of small subunits of rat liver ribosomes that interact with poly(U). II. Cross-links between poly(U) and ribosomal proteins in 40 S subunits induced by UV irradiation. J Biochem. 1979 Sep;86(3):605–617. doi: 10.1093/oxfordjournals.jbchem.a132564. [DOI] [PubMed] [Google Scholar]
- Thomas G., Martin-Pérez J., Siegmann M., Otto A. M. The effect of serum, EGF, PGF2 alpha and insulin on S6 phosphorylation and the initiation of protein and DNA synthesis. Cell. 1982 Aug;30(1):235–242. doi: 10.1016/0092-8674(82)90029-0. [DOI] [PubMed] [Google Scholar]
- Trevillyan J. M., Kulkarni R. K., Byus C. V. Tumor-promoting phorbol esters stimulate the phosphorylation of ribosomal protein S6 in quiescent Reuber H35 hepatoma cells. J Biol Chem. 1984 Jan 25;259(2):897–902. [PubMed] [Google Scholar]
- Ushiro H., Cohen S. Identification of phosphotyrosine as a product of epidermal growth factor-activated protein kinase in A-431 cell membranes. J Biol Chem. 1980 Sep 25;255(18):8363–8365. [PubMed] [Google Scholar]
- Wang J. Y., Queen C., Baltimore D. Expression of an Abelson murine leukemia virus-encoded protein in Escherichia coli causes extensive phosphorylation of tyrosine residues. J Biol Chem. 1982 Nov 25;257(22):13181–13184. [PubMed] [Google Scholar]
- Wettenhall R. E., Cohen P., Caudwell B., Holland R. Differential phosphorylation of ribosomal protein S6 in isolated rat hepatocytes after incubation with insulin and glucagon. FEBS Lett. 1982 Nov 8;148(2):207–213. doi: 10.1016/0014-5793(82)80809-0. [DOI] [PubMed] [Google Scholar]
- Witte O. N., Dasgupta A., Baltimore D. Abelson murine leukaemia virus protein is phosphorylated in vitro to form phosphotyrosine. Nature. 1980 Feb 28;283(5750):826–831. doi: 10.1038/283826a0. [DOI] [PubMed] [Google Scholar]