Abstract
We have used computational methods to improve the affinity of a foldamer ligand for its target protein. The effort began with a previously reported α/β-peptide based on the BH3 domain of the pro-apoptotic protein Puma; this foldamer binds tightly to Bcl-xL but weakly to Mcl-1. The crystal structure of the Puma-derived α/β-peptide complexed to Bcl-xL was used as the basis for computational design of variants intended to display improved binding to Mcl-1. Molecular modelling suggested modification of three α residues within the original α/β backbone. Individually, each substitution caused only a modest (4- to 15-fold) gain in affinity; however, together the three substitutions led to a 250-fold increase in binding to Mcl-1. These modifications had very little effect on affinity for Bcl-xL. Crystal structures of a number of the new α/β-peptides bound to either Mcl-1 or Bcl-xL validated the selection of each substitution. Overall, our findings demonstrate that structure-guided rational design can be used to improve affinity and alter partner selectivity of peptidic ligands with unnatural backbones that bind to specific protein partners.
Keywords: apoptosis, BH3 domain, Mcl-1, foldamer, peptides, peptidomimetics, peptide design
INTRODUCTION
Medium-length peptides often bind tightly and specifically to partner proteins, which enables these peptides to serve as agonists or antagonists of biological signalling pathways that can be difficult to modulate with small molecules. The clinical application of such peptides, however, is impeded by the susceptibility of oligo-α-amino acid backbones to proteolytic destruction. Numerous strategies have been employed to enhance the metabolic stability of peptides while retaining their protein-binding profiles. These include modifications to the amino acid side-chains such as insertion of intramolecular bridges or “staples” [1], and incorporation of non-natural subunits including d-amino acids [2]. Another approach to enhance peptide stability involves alterations to the α-peptide backbone including backbone amide methylation [3] and incorporation β-amino acids [4].
We have been using α-helical BH3 domains derived from pro-apoptotic BH3-only proteins as a model system for exploring the effects of incorporating β-amino acid residues into synthetic peptidic oligomers [4b, 4c, 5]. BH3 domains are short segments (approximately 15 α-amino acid residues) that engage a large hydrophobic groove on pro-survival Bcl-2 family proteins [5b, 6]. There are eight BH3-only proteins in mammals, and these display a variety of binding preferences among the five pro-survival proteins (Bcl-2, Bcl-xL, Bcl-w, Mcl-1 and Bfl-1), ranging from promiscuity to high selectivity [7].
Incorporation of a β-amino acid residue in place of an α residue extends the backbone by one carbon atom; therefore, multiple α→β replacements can modulate overall peptide shape and potentially have significant consequences in terms of affinity for a binding partner. Nevertheless, our initial reports utilising α/β BH3 domain peptides with a 1:1 alternation of α and cyclic β substitutions demonstrated that key side-chain interactions required for engaging anti-apoptotic binding partners could be accurately mimicked despite the unnatural backbone [5b, 5d, 5e]. Subsequent studies showed that replacement of approximately one α residue per α-helical turn with a homologous β3 residue (same side chain; Figure 1) could more efficiently deliver foldamers with high affinity for some pro-survival proteins [4b, 4c]. Surprisingly, these α/β-peptides manifested different pro-survival protein binding profiles relative to the BH3 sequences from which they were derived, even though the α/β-peptides retain the side chain sequence of the natural BH3 domain. Associated structural studies revealed subtle alterations in the α/β-peptide helix (e.g., slight helix radius expansion), compared to a canonical α-helix, that may be required to accommodate the extra backbone carbon atom associated with each α→β substitution [4b, 5b, 5c]. These changes likely also influence binding specificity. Therefore, a central challenge in the development of α/β-peptide antagonists is to recover affinity that may be lost upon replacement of some of the original α residues with β residues.
Figure 1. Puma-mimetic α/β-peptide variants display increased affinity for Mcl-1 and elicit cytochrome c release.
A. Peptides used in this study; β3–residues are highlighted in blue. NL: norleucine, HL: homonorleucine (n-pentyl side-chain). h1-h4: Conserved hydrophobic residues in all BH3 domains. B. Binding curves for competition assays with α/β-peptides 1–7 and Mcl-1 (left) or Bcl-xL (right) (1:blue, 2:purple, 3:red, 4:orange, 5:green, 6:cyan, 7:black). Curves show the combined data from 6 independent experiments. Error bars indicate standard deviation. C. IC50 values for peptides with Mcl-1 and Bcl-xL D. Consistent with their uniformly high affinity for Bcl-xL α/β-peptide 1–7 all elicit release of cytochrome c from the mitochondria of permeabilized mcl-1−/− MEFs into the soluble fraction whilst the varying levels of release observed in bcl-x−/− MEF extracts correlate with varying affinities of the α/β-peptides for Mcl-1. P: pellet fraction containing mitochondria, S: soluble fraction. WB: Western blot.
Bcl-2 pro-survival proteins are important targets for anti-cancer drugs as they are often over-expressed in tumours and allow rogue cancer cells to survive when they should otherwise be eliminated [8]. Indeed, several small molecule drugs (“BH3-mimetics”) targeting pro-survival proteins have now entered clinical trials and are showing significant promise [9]. Potent small molecules to antagonise Mcl-1 and/or Bfl-1, however, have not yet been developed. These two anti-apoptotic proteins represent important drug targets due to their role in tumourigenesis and their ability to act as resistance factors for other anti-cancer drugs [10]. As the binding selectivity of BH3 peptides can be manipulated [11], it is possible that BH3 foldamers could ultimately prove to have some clinical applications where suitable small molecule compound target profiles cannot be generated. Indeed we have recently shown that viral delivery of a peptide-based ligand targeting just Mcl-1 can kill acute myeloid leukaemia cell lines as well as primary cells derived from AML patients [12].
Previously we have used the BH3 domain from the BH3-only protein Puma as a basis for exploring different α/β-peptide designs in the context of binding to pro-survival proteins [4c, 5c]. These studies resulted in the crystal structure of a Puma-based foldamer bound to Bcl-xL[5c], providing key insights into how the α/β-peptide engages this target. In addition, the structure provided clues regarding the difference in Bcl-xL versus Mcl-1 selectivity between the α/β-peptide (selective for Bcl-xL) and the Puma BH3 α-peptide (binds all anti-apopotic proteins with high affinity). In this report we extend these studies by using the α/β-peptide+Bcl-xL complex to explore the feasibility of structure-guided modification of BH3-derived α/β-peptides to improve affinity for Mcl-1. Our studies demonstrate new strategies for manipulating α/β-peptide specificity through modification of side chains and/or configuration of α residues.
RESULTS
Modelling α/β-Puma:Mcl-1 interactions
Our previous studies using α/β-peptides based on the Puma BH3 domain involved an ααβαααβ backbone pattern. Upon adoption of an α-helix-like conformation, this pattern gives rise to a “stripe” of β residues along the helix axis [4c]. There are seven ways in which this pattern can be imposed on a given helical amino acid sequence, and we found that the placement of the β residues within the Puma sequence strongly influences pro-survival protein binding [4c]. Comparable trends were subsequently observed with Bim BH3-based foldamers [4b]. The Puma-based foldamers that displayed high affinity for pro-survival proteins bound selectively (>100-fold) to Bcl-xL over Mcl-1. The best of these molecules, 1 (Fig. 1A), was shown to bind tightly to Bcl-2 and Bcl-w as well; however, 1 exhibited only weak affinity for Mcl-1.
Using the structure of the 1:Bcl-xL complex (PDB: 2YJ1), we created a model of 1 bound to Mcl-1 with the aim of designing Puma-based α/β-peptides that display increased affinity for Mcl-1. This model complex was generated by superimposing the structure of Bcl-xL in complex with 1 with the structure of Mcl-1 in complex with α-Puma (PDB: 2ROC) [6b], removing Bcl-xL and α-Puma, and then minimizing the remaining 1:Mcl-1 complex. Inspection of the model suggested several changes to the α/β-peptide that could potentially increase affinity. 1) Replacement of Arg3 of 1 with Glu. We previously observed that changing of Arg3 of 1 to Ala leads to improved Mcl-1 affinity, probably due to removal of a potential steric clash and/or electrostatic repulsion with the side-chain of His223 [5c]. This putative unfavorable interaction is reflected in the calculated model by a movement of His223 away from the Arg3 side-chain (Supp Fig. 1A). The binding of 1 to Mcl-1 was also improved by changing Arg229 and His233 of Mcl-1 to Ala [5c]. We therefore proposed that replacing Arg3 on 1 with Glu could engage a favourable electrostatic interaction with Arg229, as shown in the model (Supp. Fig. 1B), or alternatively mimic the interaction between 1 and Bcl-xL in this region, forming a hydrogen bond between Arg3 on 1 and Glu129 on Bcl-xL (this residue is analogous to His223 in Mcl-1). 2) Filling a small hydrophobic pocket adjacent to Gly6 of 1. We proposed that this pocket could accommodate a d-alanine residue, resulting in favourable contacts with Mcl-1 (Supp Figs 1C,D). 3) Replacement of Leu9 with a residue bearing a larger side-chain. Our Mcl-1+α/β-peptide model revealed a hydrophobic pocket beneath Leu9, which is also observed in some X-ray crystal structures of BH3 peptides bound to Mcl-1 [13]. Accordingly, we predicted that lengthening this side chain on the α/β-peptide would enhance affinity for Mcl-1. Modeling predicted that a norleucine side-chain (n-butyl) would have minimal impact on affinity (Supp. Fig. 1E), but that extension to an n-pentyl side-chain would completely fill the pocket (Supp. Fig. 1F) and likely impart higher affinity.
Binding affinities of modified α/β-Puma foldamers
Variants of 1 based on the designs described above were synthesised (Fig. 1A) and tested in competition binding assays using surface plasmon resonance (Figs. 1B,C). α/β-Peptide 2, in which Arg3 was replaced with Glu, had a 15-fold lower IC50 for Mcl-1 relative to 1, whilst 3, in which Gly6 was replaced with d-Ala, had a 10-fold gain in affinity compared to 1. Replacing Leu9 with norleucine (4) had no effect on affinity for Mcl-1, while replacing Leu9 with homonorleucine (pentyl side-chain), which we designate HL (5), increased affinity by approximately 4-fold. The behaviour of 4 and 5 is consistent with the model-based predictions. Combinations of the beneficial substitutions resulted in further increases in affinity. The Arg3→Glu plus Gly6→d-Ala combination (6) binds to Mcl-1 55-fold more tightly than does α/β-peptide 1. Combining all three substitutions (7) results in 250-fold higher affinity than the original α/β-peptide 1. Each variant of 1 retained high affinity for Bcl-xL, although very small decreases in binding were observed for each of the three substitutions individually and their combinations (Figs. 1B,C).
We examined whether the increases in affinity for Mcl-1 observed among the new α/β-peptides would be reflected in the ability of these molecules to engage pro-survival proteins in a cellular milieu (Fig. 1D). Since α-peptides and α/β-peptides of the length used in this study cannot cross cellular membranes readily, we used mouse embryonic fibroblasts (MEFs) in which the plasma membrane (but not mitochondrial membranes) was permeabilised using digitonin so that the peptides could gain access to the cellular apoptotic machinery. Induction of apoptotic signalling is detected via cytochrome c release from mitochondria. Both Bcl-xL and Mcl-1 must be antagonised in order to induce apoptotic signaling in MEFs [14]. To establish whether each α/β-peptide could engage either of these proteins, we used MEFs that were genetically deficient in one or the other (i.e., bcl-x−/− or mcl-1−/− MEFs) (Fig. 1D). Following exposure of permeabilized mcl-1−/− MEFs to α/β-peptides 1–7 we observed release of cytochrome c from the pellet fraction (containing mitochondria) into the cytosol (soluble fraction), which indicates that each α/β-peptide is able to engage Bcl-xL with high affinity (Figs. 1B,C). For experiments with bcl-x−/− MEFS, we observed essentially complete release of cytochrome c for α/β-peptide 2 or 7, partial release for 3, and no release for 4, 5 or 1. This trend is consistent with the trend in affinities for Mcl-1. α/β-Peptides 1, 4, and 5 all display IC50 values >2.5 µM, suggesting that they cannot effectively neutralise Mcl-1 in the MEF experiments. In contrast, α/β-peptides 2 and 7 bind with significantly higher affinity to Mcl-1, which allows these compounds to engage the apoptosis signalling network. Overall, our data demonstrate that the computational approach enabled sufficient improvement in Mcl-1 affinity, relative to starting α/β-peptide 1, to allow control of apoptotic signalling.
Crystal structures of α/β-peptides bound to Bcl-xL or Mcl-1
As an incisive test of our computational modelling, we sought crystal structures of the new α/β-peptides bound to Mcl-1 or Bcl-xL. These efforts led to the first two crystal structures of α/β-peptides bound to Mcl-1, involving 2 and 3, and a crystal structure of the 5+Bcl-xL complex. Comparison of these three new structures with the previously reported structure of the 1+Bcl-xL complex provides atomic-level insight on the impact of each of the three α residue modifications we evaluated.
In general, the individual α residue modifications had very little effect on the α/β-peptide binding mode to the BH3-recognition clefts, relative to 1 complexed to Bcl-xL (Supp. Fig 2). Although we lack a structure for the Mcl-1+1 complex, the interactions of α/β-peptides 2 and 3 with this partner can be compared with the interactions documented crystallographically and by nuclear magnetic resonance studies for BH3-derived α/β- with Mcl-1 (Fig. 1A, Supp Fig. 2). In each of the new complex structures, the α/β-peptide adopts an α-helix-like conformation, and the helix occupies the large hydrophobic BH3-recognition groove on the pro-survival proteins, which is formed by helices α2-α4. The β residues of 2, 3 and 5 are aligned as expected along the solvent-exposed surface of the BH3-mimetic helix (Supp. Fig. 2). In all three new structures, each of the key residues on the ligand (i.e., residues corresponding to h1-h4 and the conserved aspartic acid residue found in all BH3 domains; see Fig. 1A) is accurately mimicked by the expected residue of the α/β-peptide (Fig. 2B). Details of X-ray data collection and refinement statistics for all complexes are presented in Table 1. All co-ordinates have been submitted to the Protein Data Bank.
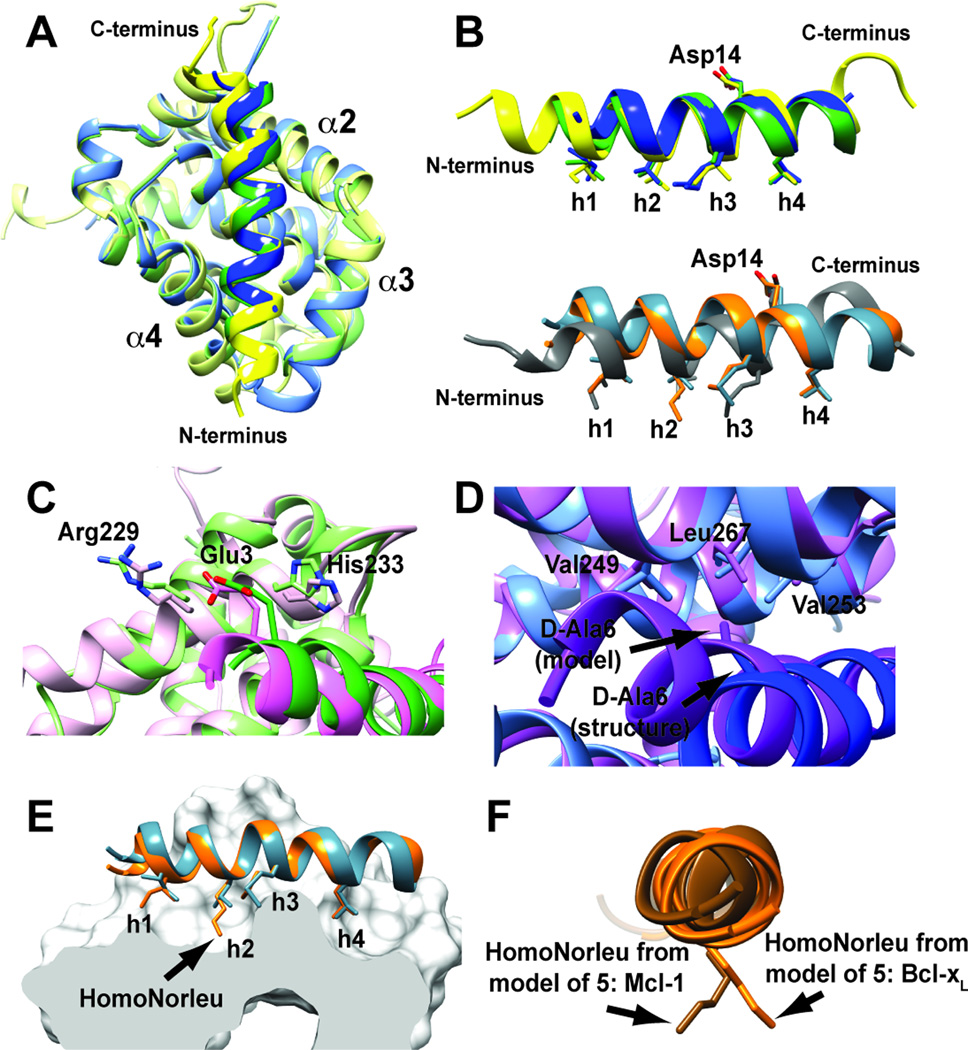
Figure 2. Structural analysis of Mcl-1 and Bcl-xL complexes with α/β-peptide or α-peptide ligands.
A. Overlay of 2+Mcl-1 (dark green:light green), 3+Mcl-1 (dark blue:light blue) and all-α Puma:Mcl-1 (PDB: 2ROC) (dark yellow:light yellow). Overall, the α/β-peptides 2 and 3 bind to Mcl-1 similarly to the all-α Puma BH3 peptide, engaging the hydrophobic groove formed by helices α2-α4 on Mcl-1. B. Overlay of structures of α/β-peptides 2 (blue) and 3 (green) with the all-α Puma BH3 peptide (yellow) (top) or, 1 (teal) and 5 (orange) with 8, the all-α Puma BH3 peptide (PDB:2M04) (grey) from the structure of this α-peptide in complex with Bcl-xL (bottom). The key hydrophobic residues (h1-h4) and the conserved aspartate (Asp14) important for BH3:pro-survival protein interactions are mimicked in the α/β-peptides. C. Overlay of 2+Mcl-1 (dark green:light green) with the model of same complex (dark pink:light pink) showing details in the region where Arg3 in 1 was replaced by Glu. D: Overlay of 3+Mcl-1 (dark blue:light blue) with the model of same complex (dark purple:light purple) showing details in the region where Gly6 in 1 was replaced by D-Ala. E. Overlay of 5 (orange) in complex with Bcl-xL (white surface) showing details of how the HL n-pentyl side-chain engages the h2 binding pocket more deeply than the corresponding Leu residue on 1 (teal).
Table 1.
Data collection and refinement statistics.
| Mcl-1+2 | Mcl-1+3 | Bcl-xL+5 | |
|---|---|---|---|
| PDB | 4BPI | 4BPJ | 4BPK |
| Wavelength (Å) | 1.2651 | 1.2651 | 0.9537 |
| Resolution range (Å) | 18.69- 1.98 (2.05 - 1.98) |
19.69- 1.60 (1.66 - 1.60) |
36.07- 1.76 (1.82 - 1.76) |
| Space group | P 32 2 1 | C 1 2 1 | P 21 21 21 |
| Unit cell (Å, degrees) |
a=b=53.8,c=94.0 α=β=90, γ=120 |
a=78.5, b=60.3, c=44.8 α=90, β=118.5, γ=90 |
a=65.2,b=72.1,c=80.6 α=β=γ=90 |
| Total reflections | 113571 (16052) | 86142 (13257) | 198352 (34392) |
| Unique reflections | 11279 (1026) | 24069 (2302) | 38368 (3678) |
| Multiplicity | 10.1 | 3.6 | 7.8 |
| Completeness (%) | 99.1 (92.9) | 99.2 (96.4) | 99.5 (97.4) |
| Mean I/sigma(I) | 16.9 (3.0) | 15.8 (2.5) | 21.7 (2.4) |
| Wilson B-factor | 37.2 | 21.9 | 28.7 |
| R-sym | 0.108 (0.828) | 0.043 (0.458) | 0.046 (0.709) |
| R-factor | 0.217 (0.207) | 0.199 (0.261) | 0.220 (0.351) |
| R-free | 0.243 (0.278) | 0.230 (0.309) | 0.254(0.370) |
| Number of atoms | 1320 | 1558 | 2991 |
| macromolecules | 1250 | 1381 | 2668 |
| ligands | 53 | 49 | 163 |
| water | 17 | 121 | 152 |
| Protein residues | 141 | 144 | 277 |
| RMS(bonds, Å) | 0.016 | 0.006 | 0.010 |
| RMS(angles, degrees) | 1.2 | 1.1 | 1.1 |
| Ramachandran favored (%) | 97 | 95 | 97 |
| Ramachandran outliers (%) | 0.6 | 2.4 | 0.6 |
| Clashscore | 6.7 | 4.1 | 5.4 |
| Average B-factor | 50.1 | 28.6 | 49.9 |
| macromolecules | 49.8 | 27.8 | 48.7 |
| solvent | 51.3 | 36.9 | 55.6 |
Statistics for the highest-resolution shell are shown in parentheses.
The Mcl-1+2 complex (PDB: 4BPI)
The rationale for replacing Arg3 with glutamic acid was based on both the modelling studies and our previous report showing that the Arg3→Ala substitution increased affinity of a longer variant of 1 for Mcl-1 [5c]. The recent structure of a Puma BH3 α-peptide bound to Bcl-xL (PDB: 2MO4) [15] shows that Arg3 is positioned on the solvent-exposed face of the α-helix and makes no contact with Bcl-xL. Our modelling of the Puma BH3 α-peptide bound to Mcl-1 suggested a similar geometry of Arg3 (Supp Fig. 1A, B). Consistent with our previous mutagenesis studies [5c], the model predicted that Arg3 in α/β-peptide 1 bound to Mcl-1 would extend from the helix in a slightly different direction relative to this side chain in the Bcl-xL+1 complex, approaching His223 on α4 of Mcl-1 and setting up a potential Coulombic or steric repulsion. We implemented an Arg3→Glu substitution as our model suggested that His223 of Mcl-1 could move slightly to overcome the potential steric clash, and the Glu side chain could potentially form a salt-bridge with Arg229 on Mcl-1 (Supp. Fig. 1B). The crystal structure of the Mcl-1+2 complex demonstrates that the predicted movement of His223 occurs, preventing any possible clash with the Glu3 side-chain of α/β-peptide 2, which projects away from His223. However, Arg229 is not close enough to Glu3 to form a salt bridge, as predicted in the model. The unexpected separation between these two side chains, however, might have arisen as a consequence of the crystallization conditions used as we observed coordination of a cadmium ion (from the cadmium sulphate in the crystalization solution) to the side chains of Mcl-1 His223 and β3-hGlu4 of the ligand, an interaction that alters the geometry in this region relative to the model. Hence, it is not possible to fully establish whether the increase in binding affinity observed in 2 versus 1 involves formation of the Arg223-Glu4 salt bridge, or is just associated with the removal of the of the potential steric and Coulombic clash in this region.
The Mcl-1+3 complex (PDB: 4BPJ)
Our modelling studies suggested that the surface of Mcl-1 offered a hydrophobic pocket adjacent to Gly6 that could accommodate a small hydrophobic moiety such as a methyl group, but that proper projection of the methyl group from the α/β-peptide required a d-alanine rather than l-alanine residue (Supp. Fig. 1C,D). The crystal structure of Mcl-1 bound to α/β-peptide 3 shows that the d-Ala side-chain projects as predicted towards the hydrophobic pocket formed by Mcl-1 residues Val249, Leu267 and Val253. Unexpectedly, relative to the Mcl-1+3 model, the helix axis of 3 appears to be displaced slightly away from the Mcl-1 α4 helix and the hydrophobic pocket that it was predicted to engage. As a consequence, the d-Ala side-chain lies in approximately the same position as Cα of Gly6 in the Puma α-peptide bound to Mcl-1 (Supp. Fig. 3). We conclude that the pocket provided by Mcl-1 is not large enough to accommodate the d-Ala methyl group, and that the increased affinity of α/β-peptide 3 for Mcl-1 relative to α/β-peptide 1 is due to additional van der Waals contacts with the nonpolar surface of the α4 region of Mcl-1 that arise from the larger hydrophobic surface of the d-Ala methyl group compared to the Gly6 Cα. This benefit is presumably operative for α/β-peptides 6 and 7 as well.
The Bcl-xL+5 complex (PDB: 4BPK)
We were unable to obtain well-diffracting crystals of Mcl-1 bound to α/β-peptide 5, in which Leu9 of 1 is replaced by a homonorleucine residue (n-pentyl side chain). In the model, this side-chain was predicted to engage a hydrophobic pocket in the ligand-binding groove more effectively than the wild-type leucine side-chain (Supp. Fig. 1F). We did, however, obtain a crystal structure of Bcl-xL with 5, which clearly demonstrates that the longer side-chain does fill this binding pocket in Bcl-xL more fully than does the wild-type leucine side chain of the Puma BH3 α-peptide (Fig. 2E). However, the n-pentyl side-chain in the Bcl-xL+5 complex displays a slightly different conformation relative to that predicted in the model for the Mcl-1+5 complex. Overlaying the structure determined for α/β-peptide 5 in its complex with Bcl-xL with the structure of α/β-peptide 2 bound to Mcl-1 suggests that the n-pentyl side-chain in 5 would more likely adopt the orientation predicted by the model; otherwise, the n-pentyl group would clash with Mcl-1 side-chains at the base of the binding pocket (Supp. Fig. 4A). α/β-Peptides 1 and 5, which differ only in the α residue at positions 9 (leucine vs. homonorleucine), bind to Bcl-xL with the same affinity, which seems puzzling given the larger hydrophobic surface area burial expected for 5 relative to 1. However, the crystal structure of the Bcl-xL+5 complex shows that the side-chain of Phe105, which lines the bottom of the binding pocket in Bcl-xL, moves slightly (rmsd 1.38 Å relative to Phe105 in the Bcl-xL+1 complex) to accommodate the n-pentyl side-chain. This side-chain shift seems to be correlated with a cascade of other small changes in the protein: the Phe105 position in Bcl-xL+5 leads to displacement of the N-terminal region of the Bcl-xL α3 helix, which results in a more efficient burial of the side-chain of Tyr101 (Supp. Fig. 4B). Thus, it is likely that one must look to many contributing factors to understand why the leucine→homonorleucine change (1→5) does not increase the binding affinity of 1 for Bcl-xL as it does for Mcl-1
Protease sensitivity
We have previously shown that analogues of the Puma BH3 sequence containing multiple α→β replacements display significantly increased resistance to proteolysis relative to the Puma BH3 α-peptide (8). Very similar proteolytic resistance would be expected for the new α/β-peptides reported here, since the backbone ααβαααβ pattern has been retained relative to previously studied cases. We tested this prediction by examining the effect of an aggressive protease, proteinase K, on α/β-peptides 1–6 and the analogous α-peptide 8, by reverse-phase HPLC and mass-spectrometry (Fig. 3, Supp. Fig. 5). The Arg3→Glu modification that generates α/β-peptide 2 from 1, and the Gly6→d-Ala modification that generates α/β-peptide 3 had little or no effect on half-life in the presence of proteinase K; these three α/β-peptides are indistinguishable in this regard. Both α/β-peptides with substitution of Leu9 (α/β-peptides 4 and 5) were slightly more susceptible to proteolysis than α/β-peptides 1–3, but 4 and 5 are nevertheless much more resistant to cleavage than is α-peptide 8.
Figure 3. Summary of sensitivity of Puma-derived α/β-peptides compared to the all-α Puma BH3 peptide.

α/β-Peptides 1–6 are significantly more resistant to proteolysis by proteinase K than is all-α Puma BH3 peptide 8. The filled triangles indicate the first cleavage sites observed at 30 sec for 8, and 1.5 hr for 1–6. Subsequent cleavage sites (120 sec for 8, 20 hr for 1–6) are indicated by the white triangles.
To learn which amide bonds are cleaved during proteolysis, we analysed the proteinase K reaction mixture aliquots quenched at different time points by mass spectrometry. The cleavage fragments identified for α/β-peptides 1–6 were largely similar to one another. α-Peptide 8 showed a slightly different cleavage pattern relative to the α/β-peptides, with the cleavages of 8 occurring after Gln8 (a β residue in the α/β-peptides) and Leu9, and the absence of cleavage between residues Ala13 and Asp14. The differences in the observed cleavage pattern for α-peptide 8 compared to the α/β-peptides shows that the susceptibility of individual amide bonds to proteolysis can be influenced by the incorporation and positioning of β residues.
DISCUSSION
The sequence-based design approach previously described for generation of α/β-peptides that mimic natural information-bearing α-helices involves substitution of approximately one α residue per turn of the helix with the homologous β3 residue [4c]. This level of substitution is sufficient to confer significant resistance to proteolysis, a major goal in the development of protein-mimetic foldamers. Sequence-based design can identify high-affinity ligands for a helix-recognizing protein based on evaluation of only a few β residue incorporation patterns [4b, 4c, 4g]. An unexpected consequence of this approach is that the binding specificity of the α/β-peptide can be altered, relative to the prototype α-peptide. This type of specificity alteration is exemplified by α/β-peptide 1, which is based on the Puma BH3 domain: 1 retains the high affinity of the analogous Puma BH3 α-peptide for Bcl-xL, but 1 does not bind tightly to Mcl-1, in contrast to the Puma BH3 α-peptide.
In the present study we have demonstrated the feasibility of rationally altering the selectivity of BH3-inspired α/β-peptides for binding to pro-survival proteins by using information from X-ray crystal structures of related targets, molecular modelling approaches, and side-chain variation studies to overcome some of the detrimental effects arising from α→β3 replacements. The incorporation of just three α residue substitutions into Puma BH3-based 21-mer α/β-peptide 1, to generate 7, leads to a 250-fold gain in affinity for Mcl-1 with only a small decline in affinity for Bcl-xL. The relative increase in binding affinity was largely additive based on the affinity gains for each individual substitution.
Modifications to the original model of Mcl-1+1 were incorporated by modification of individual side-chains followed by minimization. These models were used to assess the compatibility of the modification in the context of the Mcl-1+peptide complex. Modifications were considered compatible provided they did not result in any large-scale structural perturbations from the original model. The X-ray crystal structures we obtained for the Mcl-1+α/β-peptide complexes mostly validated the changes we employed to increase the affinity of 1 for Mcl-1. However, unexpected differences between the model and X-ray structures were observed, and high-resolution structural evidence for some affinity gains is still lacking due to technical issues. In the Mcl-1+2 structure we observed the predicted movement of His223 on Mcl-1 (relative to its location in previously determined Mcl-1+BH3 peptide complexes) [6b] that removes of the potential steric clash with residue 3 on the α/β-peptide. However, we could not have anticipated the effect of the cadmium ion present in the crystallization solution on the conformation of Glu3. Hence, the Mcl-1+2 X-ray structure does not provide the insight we desired regarding the predicted salt bridge interaction between Glu3 and Arg229 on Mcl-1, which might occur in solution even though it is not present in the crystalline state. The incorporation of a d-Ala substitution in 3 was designed to take advantage of a small hydrophobic pocket on the peptide-binding surface of Mcl-1. The X-ray structure of the Mcl-1+3 complex confirms the interaction of the methyl side-chain of the d-Ala with the hydrophobic site; however, the model did not predict the displacement of the α/β-peptide helix relative to the protein. Finally, we were unsuccessful in our attempts to obtain an X-ray crystal structure of 5 in complex with Mcl-1. However, the structure of the Bcl-xL+5 complex helps explain why the leucine-to-homonorleucine substitution did not improve binding to Bcl-xL. The pocket in Mcl-1 into which the n-pentyl side-chain was predicted to bind is not present in Bcl-xL. The absence of this pocket results in the n-pentyl side-chain having to adopt a different conformation relative to that predicted in the model of the Mcl-1+5 complex. This conformational difference results in a rearrangement of the binding site, including movement of Bcl-xL residues Phe105 and Tyr101, to compensate.
Why does α/β-peptide 1 bind Mcl-1 so poorly compared to the analogous Puma BH3 α-peptide? This is a somewhat difficult question to address as there is not yet a structure of Mcl-1 bound to 1 to compare with our Mcl-1+2 and Mcl-1+3 complex structures. Such a comparison, would provide information on any new interactions or conformational changes in Mcl-1 that led to the improvements in affinity observed with α/β-peptides 2, 3 and 5. Part of the answer does lie in different positioning of the Arg3 side-chain relative to the protein surface in the complex formed by 1 versus that formed by the α-peptide. However, substitution of Arg3 by Glu leads to only small changes in affinity for Mcl-1. Further increases in affinity were gained from substitutions at Gly6 and Leu9, but the features of 1 that lead to low affinity for Mcl-1 are not apparent from our new X-ray crystal structures involving closely related α/β-peptides 2 and 3 bound to this protein. These α/β-peptides differ from 1 by just a single α residue side-chain each, possess an almost identical overall structure to 1 in the bound state, and they are relatively weak Mcl-1 binders. In these two new structures of α/β-peptides bound to Mcl-1, the interactions of the ligands with Mcl-1 very accurately mimic the analogous interactions in the native α-Puma peptide with this protein. By extension, we anticipate that 1 would interact similarly.
One partial explanation for the low affinity of 1 for Mcl-1 might be the absence of potentially stabilizing intramolecular interactions in all the structures of the Puma-derived α/β-peptides with either Mcl-1 or Bcl-xL. Such stabilizing interactions are present in the high affinity Mcl-1+αPuma complex (PDB: 2ROC); Glu4 of αPuma forms both a hydrogen bond with Gln8 and a classical intrahelical i to i+7 salt bridge with Arg11 in the peptide. In the context of the Bcl-xL+BimBH3 complex, intramolecular salt-bridge interactions were estimated to contribute 3–7 kJ mol−1 to the total binding affinity (corresponding to a loss in binding affinity of 3–17 fold) [1j]. Hence the loss of potentially stabilizing intramolecular interactions due to incorporation of β-residues at positions 4, 8 and 11 could be a contributing factor to the weaker affinity for Mcl-1 of α/β-peptide 1 relative to the native αPuma BH3 peptide. Critically, in the X-ray crystal structure of a 26mer αPuma peptide in complex with Bcl-xL (PDB: 2M04), none of the side chains are observed to engage in intramolecular interactions; specifically, Glu4, Gln8 and Arg11 do not interact with one another, nor are they engaged in any specific interactions with Bcl-xL. Similarly in the structure of 1 in complex with Bcl-xL (PDB: 2YJ1) these residues also do not form any intramolecular interactions with one another. Thus, there is no loss of intramolecular stabilisation of the complex with Bcl-xL by the introduction of the β amino acids into the Puma peptide, and notably, both the 26-mer versions of 1 and the all-α Puma peptide bind to Bcl-xL with essentially identical affinities [5c].
We acknowledge the intrinsic inadequacy of simple inspection of protein structures to extract the origins of protein-ligand affinity, or the origin of differences in affinity among related ligands. Despite this, the results reported here show that molecular modelling can lead to useful predictions for enhancing the binding of a foldamer ligand to a specific protein target, as manifested by the high-affinity interaction between α/β-peptide 7 and Mcl-1. Critical to our success was the availability of related structural data, for complexes between α-peptides and Mcl-1 and between α/β-peptides and Bcl-xL. Our findings suggest that computational methods will be valuable as the foldamer approach to ligand development is extended to diverse protein targets [16].
Experimental Procedures
Chemicals
Protected α-amino acids, 2-(1H-benzotriazole-1-yl)-1,1,3,3-tetramethyluronium hexafluoro-phosphate (HBTU), and benzotriazole-1-yl-oxy-tris-pyrrolidino-phosphonium hexafluoro-phosphate (PyBOP) were purchased from Novabiochem and Chem-Impex International. Protected β3-amino acids were purchased from Chem-Impex International and PepTech Corporation. Protected homonorleucine, (S)-2-[(9-fluorenylmethoxycarbonyl)amino]-heptanoic acid, was purchased from Watanabe Chemical Industries. NovaPEG Rink Amide resin was purchased from Novabiochem.
Peptide Synthesis and Purification
α-Peptides were synthesized on solid phase using a Symphony automated peptide synthesizer (Protein Technologies), as previously reported [5c]. α/β-peptides were synthesized on NovaPEG Rink Amide resin using microwave-assisted solid-phase conditions based on Fmoc protection of the main chain amino groups, as previously reported [17]. In brief, coupling reactions were carried out by treating the resin with a solution of protected amino acid, activated with either HBTU or PyBOP and 1-hydroxybenzotriazole (HOBt) in the presence of N,N-diisopropylethylamine (DIEA) in 1-methyl-2-pyrrolidinone (NMP). Fmoc deprotection reactions were carried out using a solution of 20% piperidine in N,N-dimethylformamide (DMF). After the final Fmoc deprotection, acetylation was performed using a solution of acetic anhydride/DIEA in DMF. After completion of the synthesis, peptides were cleaved from the resin using a solution of 81.5% trifluoroacetic acid (TFA), 5% thioanisole, 5% phenol, 5% H2O, 2.5% 1,2-ethanedithiol, and 1% triisopropylsilane. Excess TFA was removed under a stream of nitrogen, and the crude peptides were precipitated by the addition of cold diethyl ether. Solutions of crude peptide were purified using preparative scale reverse-phase HPLC on C18 columns. Peptide purity was assessed by analytical HPLC and identity confirmed by MALDI-TOF-MS (Supp. Figs 6a-h).
Molecular modelling
A model of 1 in complex with Mcl-1 was created by taking the structure of Mcl-1 in complex with the all-α Puma peptide (PDB: 2ROC) and overlaying with the structure of α/β-peptide 1 from the crystal structure of 1 in complex with Bcl-xL (PDB: 2YJ1). The resulting complex of Mcl-1 with 1 was then minimized with several rounds of steepest descents and conjugate gradients.. Initially only the hydrogen atoms were allowed to move, keeping all non-hydrogen atoms restrained to their original position; this was followed with mainchain atoms restraints, allowing hydrogen atoms and side-chain atoms to move, and then subsequent unrestrained minimization. Various α residue side-chain modifications were introduced into this structure, and the same minimization protocol was applied to obtain new models. The final models were inspected visually to ensure no large-scale changes had occurred during the minimization procedure. This simple procedure is based on our assumption that the side chain modifications we have selected will not significantly perturb the overall structure of the ligand-protein complex, relative to the original model for 1+Mcl-1. The sites and chemical nature of the α residue side-chain modifications we explored were chosen based on visual inspection of the models. We sought to identify sites at which complementarity between the surfaces of the α/β-peptide and Mcl-1 could be improved, and sites at which potentially repulsive electrostatic interactions could be removed. All calculations were performed using the InsightII package of programs employing the cvff force field and a distance-dependent dielectric (Accelrys Inc.). The cvff force field is sufficiently general to allow simulations of the non-natural amino acid residues. A distance-dependent dielectric (4.0×r) was used to mimic solvent effects and moderate electrostatic interactions.
Surface plasmon resonance solution competition assay
Solution competition assays were performed using a Biacore 3000 instrument as described previously [5b, 11d, 11e, 18]. Briefly, pro-survival proteins (10 nM) were incubated with varying concentrations of peptide for at least 2 h in running buffer (10 mM HEPES, 150 mM NaCl, 3.4 mM EDTA, 0.005% (v/v) Tween 20, pH 7.4) prior to injection onto a CM5 sensor chip on which either a wild-type BimBH3 α-peptide or an inert BimBH3 mutant α-peptide (Bim4E) was immobilized. Specific binding of the pro-survival protein to the surface in the presence and absence of competitor α- or α/β-peptides was quantified by subtracting the signal obtained on the Bim mutant channel from that obtained on the wild-type Bim channel. The ability of the α- or α/β-peptides to prevent protein binding to immobilized BimBH3 was expressed as the IC50, calculated by nonlinear curve fitting of the data using GraphPad Prism (GraphPad Software).
Cytochrome c release assay
Mouse embryonic fibroblasts (mcl-1−/−, bcl-x−/−) (~2×106 cells) were permeabilized in 20 mM HEPES pH 7.2, 100 mM KCl, 5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, 250 mM sucrose, 0.05% (w/v) digitonin (Calbiochem) supplemented with protease inhibitors (Roche), for 10 min on ice. The mitochondria-containing crude lysates were incubated with 10 µM α- or α/β-peptide at 30°C for 1 h before pelleting. The supernatant was retained as the soluble fraction while the pellet, which contained intact mitochondria, was solubilized in 1% (v/v) Triton-X-100-containing lysis buffer (20 mM Tris-pH 7.4, 135 mM NaCl, 1.5 mM MgCl2, 1 mM EDTA, 10% (v/v) glycerol) supplemented with protease inhibitors (Roche). Proteins were resolved by SDS-PAGE and transferred onto nitrocellulose membranes. Cytochrome c was detected with anti-cytochrome c antibody (7H8.2C12, BD Pharmingen).
Proteinase K susceptibility assay
Stock solutions of 50 µg/mL proteinase K (Novagen) were prepared in Tris-buffered saline (TBS), pH 7.5. Stock solutions of 100 µM α-peptide or α/β-peptide in TBS were prepared, as determined by UV absorbance (based on the presence of one Trp and one Tyr side-chain in each molecule). For each proteolysis reaction, the peptide stock was diluted with TBS to achieve a final peptide concentration of 50 µM. Proteinase K stock was added to a final concentration of 10 µg/mL, and the reaction was allowed to proceed at room temperature. At each time point, 50 µL of the reaction mixture was removed and quenched by the addition of 100 µL of 1:1 H2O/acetonitrile with 1% TFA. The resulting quenched solution (125 µL) was injected onto an analytical reverse-phase HPLC, and the amount of α- or α/β-peptide remaining was quantified by integration of the peak at 220 nm in a series of HPLC chromatograms. Each reaction was run at least twice. Half-life values were determined by fitting the time course of peptide degradation to an exponential decay model using GraphPad Prism. For each peptide, quenched reaction mixtures were analysed by MALDI-TOF-MS to identify major cleavage sites.
Crystallization
For structures of Mcl-1 bound to α/β-peptides we used a previously-described human/mouse chimeric Mcl-1 construct with N- and C-terminal deletions (hmMcl-1 ΔN170 ΔC23) to remove the long unstructured N-terminal PEST-containing segment plus the hydrophobic membrane anchor, respectively [13]. Structures of the Bcl-xL+α/β-peptide complexes employed a “loop-deleted” form of human Bcl-xL (Δ27–82 and without membrane anchor), which forms an α1 domain-swapped dimer yet retains BH3 domain binding activity [5b, 5c, 11c, 18]. Crystals were obtained by mixing Mcl-1 or Bcl-xL with the α/β-peptide at a molar ratio of 1:1.3 and then concentrating the sample to 10 mg/ml. Crystals were grown by the sitting drop method at room temperature with the following conditions: Mcl-1+2 – 0.1M HEPES, pH 7.5, 1M sodium acetate, 0.05M cadmium sulphate; Mcl-1+3 – 0.2M imidazole, pH 7.0, 0.2M zinc acetate; Bcl-xL+5 – 0.1M HEPES, pH 7.5, 1M sodium acetate, 50 mM cadmium sulphate. Prior to cryo-cooling in liquid N2, crystals were equilibrated into cryoprotectant consisting of reservoir solution containing 15% (v/v) ethylene glycol. Crystals were mounted directly from the drop and plunge-cooled in liquid N2.
Diffraction data collection and structure determination
Diffraction data were collected at the Australian Synchrotron MX2 beamline. The diffraction data were integrated and scaled with XDS [19]. The structure was obtained by molecular replacement with PHASER [20] using the structures of either Mcl-1 from the BimBH3:Mcl-1 complex (PDB: 2NL9) [13] or Bcl-xL from the BimBH3:Bcl-xL complex (PDB: 3FDL) [5b], with the Bim peptide removed in all cases, as a search model. Several rounds of building in COOT [21] and refinement in PHENIX [22] led to the final model.
Supplementary Material
Acknowledgements
Work at the Walter and Eliza Hall Institute and Latrobe University was supported by grants from Australian Research Council (Discovery Project Grant DP1093909 to Peter M. Colman, B.J.S. and W.D.F.), and the NHMRC of Australia (Project Grants 1041936 and 1008329 to W.D.F. and Peter M. Colman). Crystallization trials were performed at the Bio21 Collaborative Crystallisation Centre. Data were collected on the MX2 beamline at the Australian Synchrotron, Victoria, Australia. Infrastructure support from NHMRC IRIISS grant #361646 and the Victorian State Government OIS grant is gratefully acknowledged. Work at UW-Madison was supported by the NIH (GM056414). J.W.C. was supported in part by an NIH Biotechnology Training Grant (T32 GM008349).
REFERENCES
- 1.a) Stewart ML, Fire E, Keating AE, Walensky LD. Nat. Chem. Biol. 2010;6:595–601. doi: 10.1038/nchembio.391. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Walensky LD, Kung AL, Escher I, Malia TJ, Barbuto S, Wright RD, Wagner G, Verdine GL, Korsmeyer SJ. Science. 2004;305:1466–1470. doi: 10.1126/science.1099191. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Wang D, Liao W, Arora PS. Angew Chem Int Ed Engl. 2005;44:6525–6529. doi: 10.1002/anie.200501603. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Felix AM, Heimer EP, Wang CT, Lambros TJ, Fournier A, Mowles TF, Maines S, Campbell RM, Wegrzynski BB, Toome V, et al. Int. J. Pep. Prot. Res. 1988;32:441–454. doi: 10.1111/j.1399-3011.1988.tb01375.x. [DOI] [PubMed] [Google Scholar]; e) Chorev M, Roubini E, McKee RL, Gibbons SW, Goldman ME, Caulfield MP, Rosenblatt M. Biochemistry. 1991;30:5968–5974. doi: 10.1021/bi00238a022. [DOI] [PubMed] [Google Scholar]; f) Judice JK, Tom JY, Huang W, Wrin T, Vennari J, Petropoulos CJ, McDowell RS. Proc Natl Acad Sci U S A. 1997;94:13426–13430. doi: 10.1073/pnas.94.25.13426. [DOI] [PMC free article] [PubMed] [Google Scholar]; g) Trivedi D, Lin Y, Ahn JM, Siegel M, Mollova NN, Schram KH, Hruby VJ. J. Med. Chem. 2000;43:1714–1722. doi: 10.1021/jm990559d. [DOI] [PubMed] [Google Scholar]; h) Miranda LP, Winters KA, Gegg CV, Patel A, Aral J, Long J, Zhang J, Diamond S, Guido M, Stanislaus S, Ma M, Li H, Rose MJ, Poppe L, Veniant MM. J. Med. Chem. 2008;51:2758–2765. doi: 10.1021/jm701522b. [DOI] [PubMed] [Google Scholar]; i) Murage EN, Gao G, Bisello A, Ahn JM. J. Med. Chem. 2010;53:6412–6420. doi: 10.1021/jm100602m. [DOI] [PubMed] [Google Scholar]; j) Okamoto T, Zobel K, Fedorova A, Quan C, Yang H, Fairbrother WJ, Huang DC, Smith BJ, Deshayes K, Czabotar PE. ACS Chem Biol. 2013;8:297–302. doi: 10.1021/cb3005403. [DOI] [PubMed] [Google Scholar]
- 2.Welch BD, Francis JN, Redman JS, Paul S, Weinstock MT, Reeves JD, Lie YS, Whitby FG, Eckert DM, Hill CP, Root MJ, Kay MS. J. Virol. 2010;84:11235–11244. doi: 10.1128/JVI.01339-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Doedens L, Opperer F, Cai M, Beck JG, Dedek M, Palmer E, Hruby VJ, Kessler H. J. Am. Chem. Soc. 2010;132:8115–8128. doi: 10.1021/ja101428m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Bautista AD, Craig CJ, Harker EA, Schepartz A. Curr Opin Chem Biol. 2007;11:685–692. doi: 10.1016/j.cbpa.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Boersma MD, Haase HS, Peterson-Kaufman KJ, Lee EF, Clarke OB, Colman PM, Smith BJ, Horne WS, Fairlie WD, Gellman SH. J. Am. Chem. Soc. 2012;134:315–323. doi: 10.1021/ja207148m. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Horne WS, Boersma MD, Windsor MA, Gellman SH. Angew. Chem. Int. Ed. Engl. 2008;47:2853–2856. doi: 10.1002/anie.200705315. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Kritzer JA, Lear JD, Hodsdon ME, Schepartz A. J. Am. Chem. Soc. 2004;126:9468–9469. doi: 10.1021/ja031625a. [DOI] [PubMed] [Google Scholar]; e) Seebach D, Gardiner J. Acc. Chem. Res. 2008;41:1366–1375. doi: 10.1021/ar700263g. [DOI] [PubMed] [Google Scholar]; f) Steer DL, Lew RA, Perlmutter P, Smith AI, Aguilar MI. Curr. Med. Chem. 2002;9:811–822. doi: 10.2174/0929867024606759. [DOI] [PubMed] [Google Scholar]; g) Horne WS, Johnson LM, Ketas TJ, Klasse PJ, Lu M, Moore JP, Gellman SH. Proc Natl. Acad. Sci. U S A. 2009;106:14751–14756. doi: 10.1073/pnas.0902663106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.a) Boersma MD, Sadowsky JD, Tomita YA, Gellman SH. Protein Sci. 2008;17:1232–1240. doi: 10.1110/ps.032896.107. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Lee EF, Sadowsky JD, Smith BJ, Czabotar PE, Peterson-Kaufman KJ, Colman PM, Gellman SH, Fairlie WD. Angew. Chem. Int. Ed. Engl. 2009;48:4318–4322. doi: 10.1002/anie.200805761. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lee EF, Smith BJ, Horne WS, Mayer KN, Evangelista M, Colman PM, Gellman SH, Fairlie WD. Chembiochem. 2011;12:2025–2032. doi: 10.1002/cbic.201100314. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Sadowsky JD, Fairlie WD, Hadley EB, Lee HS, Umezawa N, Nikolovska-Coleska Z, Wang S, Huang DC, Tomita Y, Gellman SH. J. Am. Chem. Soc. 2007;129:139–154. doi: 10.1021/ja0662523. [DOI] [PubMed] [Google Scholar]; e) Sadowsky JD, Schmitt MA, Lee HS, Umezawa N, Wang S, Tomita Y, Gellman SH. J. Am. Chem. Soc. 2005;127:11966–11968. doi: 10.1021/ja053678t. [DOI] [PubMed] [Google Scholar]
- 6.a) Czabotar PE, Lee EF, Thompson GV, Wardak AZ, Fairlie WD, Colman PM. J. Biol. Chem. 2011 doi: 10.1074/jbc.M110.161281. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Day CL, Smits C, Fan FC, Lee EF, Fairlie WD, Hinds MG. J. Mol. Biol. 2008;380:958–971. doi: 10.1016/j.jmb.2008.05.071. [DOI] [PubMed] [Google Scholar]; c) Petros AM, Nettseheim DG, Wang Y, Olejniczak ET, Meadows RP, Mack J, Swift K, Matayoshi ED, Zhang H, Thompson CB, Fesik SW. Prot. Sci. 2000;9:2528–2534. doi: 10.1110/ps.9.12.2528. [DOI] [PMC free article] [PubMed] [Google Scholar]; d) Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. Science. 1997;275:983–986. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 7.a) Certo M, Del Gaizo Moore V, Nishino M, Wei G, Korsmeyer S, Armstrong SA, Letai A. Cancer Cell. 2006;9:351–365. doi: 10.1016/j.ccr.2006.03.027. [DOI] [PubMed] [Google Scholar]; b) Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. Mol. Cell. 2005;17:393–403. doi: 10.1016/j.molcel.2004.12.030. [DOI] [PubMed] [Google Scholar]
- 8.Adams JM, Cory S. Oncogene. 2007;26:1324–1337. doi: 10.1038/sj.onc.1210220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Lessene G, Czabotar PE, Colman PM. Nat. Rev. Drug Discov. 2008;7:989–1000. doi: 10.1038/nrd2658. [DOI] [PubMed] [Google Scholar]; b) Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O'Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]; c) Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, Dayton BD, Ding H, Enschede SH, Fairbrother WJ, Huang DC, Hymowitz SG, Jin S, Khaw SL, Kovar PJ, Lam LT, Lee J, Maecker HL, Marsh KC, Mason KD, Mitten MJ, Nimmer PM, Oleksijew A, Park CH, Park CM, Phillips DC, Roberts AW, Sampath D, Seymour JF, Smith ML, Sullivan GM, Tahir SK, Tse C, Wendt MD, Xiao Y, Xue JC, Zhang H, Humerickhouse RA, Rosenberg SH, Elmore SW. Nat. Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]; d) Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, Johnson EF, Marsh KC, Mitten MJ, Nimmer P, Roberts L, Tahir SK, Xiao Y, Yang X, Zhang H, Fesik S, Rosenberg SH, Elmore SW. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 10.a) Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima M, Mc Henry KT, Pinchback RM, Ligon AH, Cho YJ, Haery L, Greulich H, Reich M, Winckler W, Lawrence MS, Weir BA, Tanaka KE, Chiang DY, Bass AJ, Loo A, Hoffman C, Prensner J, Liefeld T, Gao Q, Yecies D, Signoretti S, Maher E, Kaye FJ, Sasaki H, Tepper JE, Fletcher JA, Tabernero J, Baselga J, Tsao MS, Demichelis F, Rubin MA, Janne PA, Daly MJ, Nucera C, Levine RL, Ebert BL, Gabriel S, Rustgi AK, Antonescu CR, Ladanyi M, Letai A, Garraway LA, Loda M, Beer DG, True LD, Okamoto A, Pomeroy SL, Singer S, Golub TR, Lander ES, Getz G, Sellers WR, Meyerson M. Nature. 2010;463:899–905. doi: 10.1038/nature08822. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Senft D, Berking C, Graf SA, Kammerbauer C, Ruzicka T, Besch R. PLoS One. 2012;7:e30821. doi: 10.1371/journal.pone.0030821. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Vogler M, Butterworth M, Majid A, Walewska RJ, Sun XM, Dyer MJ, Cohen GM. Blood. 2009;113:4403–4413. doi: 10.1182/blood-2008-08-173310. [DOI] [PubMed] [Google Scholar]; d Yecies D, Carlson NE, Deng J, Letai A. Blood. 2010;115:3304–3313. doi: 10.1182/blood-2009-07-233304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.a) Dutta S, Chen TS, Keating AE. ACS Chem. Biol. 2013;8:778–788. doi: 10.1021/cb300679a. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Dutta S, Gulla S, Chen TS, Fire E, Grant RA, Keating AE. J. Mol. Biol. 2010;398:747–762. doi: 10.1016/j.jmb.2010.03.058. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Lee EF, Czabotar PE, Smith BJ, Deshayes K, Zobel K, Colman PM, Fairlie WD. Cell Death Differ. 2007;14:1711–1713. doi: 10.1038/sj.cdd.4402178. [DOI] [PubMed] [Google Scholar]; d) Lee EF, Czabotar PE, van Delft MF, Michalak EM, Boyle MJ, Willis SN, Puthalakath H, Bouillet P, Colman PM, Huang DC, Fairlie WD. J. Cell Biol. 2008;180:341–355. doi: 10.1083/jcb.200708096. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Lee EF, Fedorova A, Zobel K, Boyle MJ, Yang H, Perugini MA, Colman PM, Huang DC, Deshayes K, Fairlie WD. J. Biol. Chem. 2009;284:31315–31326. doi: 10.1074/jbc.M109.048009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Glaser SP, Lee EF, Trounson E, Bouillet P, Wei A, Fairlie WD, Izon DJ, Zuber J, Rappaport AR, Herold MJ, Alexander WS, Lowe SW, Robb L, Strasser A. Genes Dev. 2012;26:120–125. doi: 10.1101/gad.182980.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Czabotar PE, Lee EF, van Delft MF, Day CL, Smith BJ, Huang DC, Fairlie WD, Hinds MG, Colman PM. Proc. Natl. Acad. Sci. U S A. 2007;104:6217–6222. doi: 10.1073/pnas.0701297104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Willis SN, Chen L, Dewson G, Wei A, Naik E, Fletcher JI, Adams JM, Huang DC. Genes Dev. 2005;19:1294–1305. doi: 10.1101/gad.1304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Follis AV, Chipuk JE, Fisher JC, Yun MK, Grace CR, Nourse A, Baran K, Ou L, Min L, White SW, Green DR, Kriwacki RW. Nat. Chem. Biol. 2013;9:163–168. doi: 10.1038/nchembio.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haase HS, Peterson-Kaufman KJ, Lan Levengood SK, Checco JW, Murphy WL, Gellman SH. J. Am. Chem. Soc. 2012;134:7652–7655. doi: 10.1021/ja302469a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Horne WS, Price JL, Gellman SH. Proc. Natl. Acad. Sci. U S A. 2008;105:9151–9156. doi: 10.1073/pnas.0801135105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee EF, Czabotar PE, Yang H, Sleebs BE, Lessene G, Colman PM, Smith BJ, Fairlie WD. J. Biol. Chem. 2009;284:30508–30517. doi: 10.1074/jbc.M109.040725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kabsch W. Acta Cryst. 2010;66:125–132. doi: 10.1107/S0907444909047337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.a) McCoy AJ, Grosse-Kunstleve RW, Storoni LC, Read RJ. Acta crystallographica. 2005;61:458–464. doi: 10.1107/S0907444905001617. [DOI] [PubMed] [Google Scholar]; b) Read RJ. Acta Cryst. 2001;57:1373–1382. doi: 10.1107/s0907444901012471. [DOI] [PubMed] [Google Scholar]; c Storoni LC, McCoy AJ, Read RJ. Acta Cryst. 2004;60:432–438. doi: 10.1107/S0907444903028956. [DOI] [PubMed] [Google Scholar]
- 21.Emsley P, Cowtan K. Acta Cryst. 2004;60:2126–2132. doi: 10.1107/S0907444904019158. [DOI] [PubMed] [Google Scholar]
- 22.Adams PD, Grosse-Kunstleve RW, Hung LW, Ioerger TR, McCoy AJ, Moriarty NW, Read RJ, Sacchettini JC, Sauter NK, Terwilliger TC. Acta Cryst. 2002;58:1948–1954. doi: 10.1107/s0907444902016657. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.