Abstract
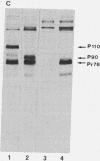
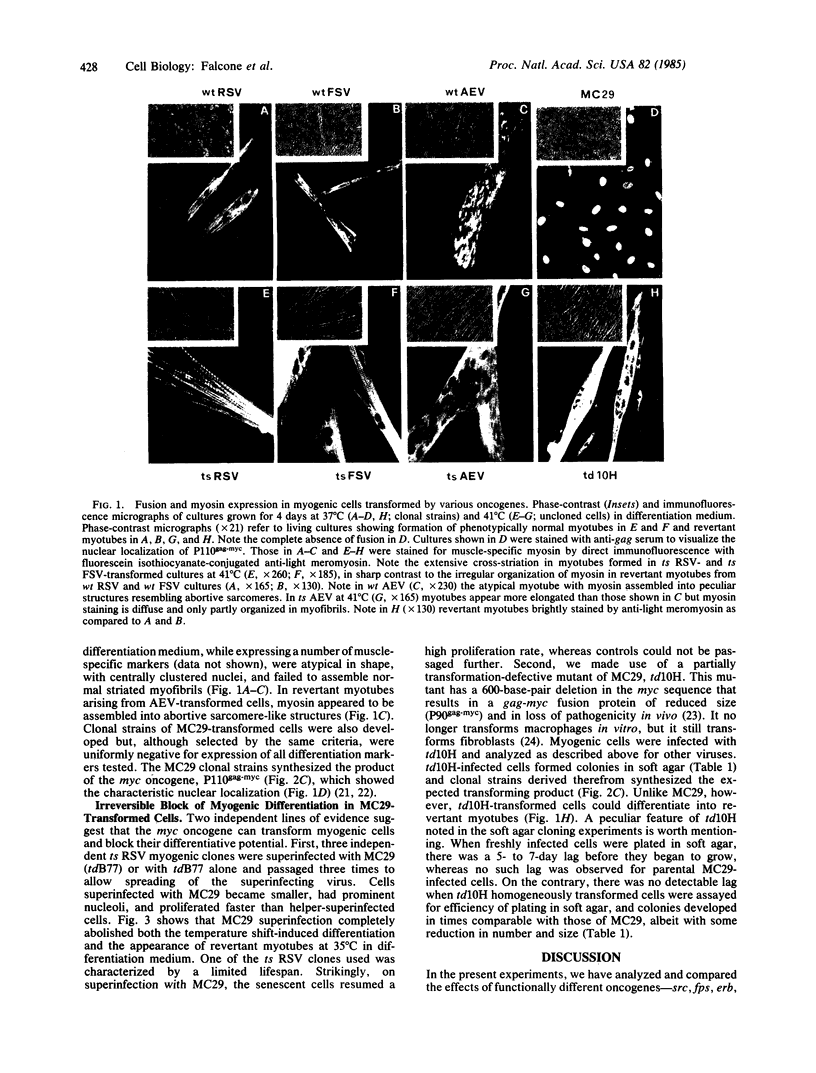
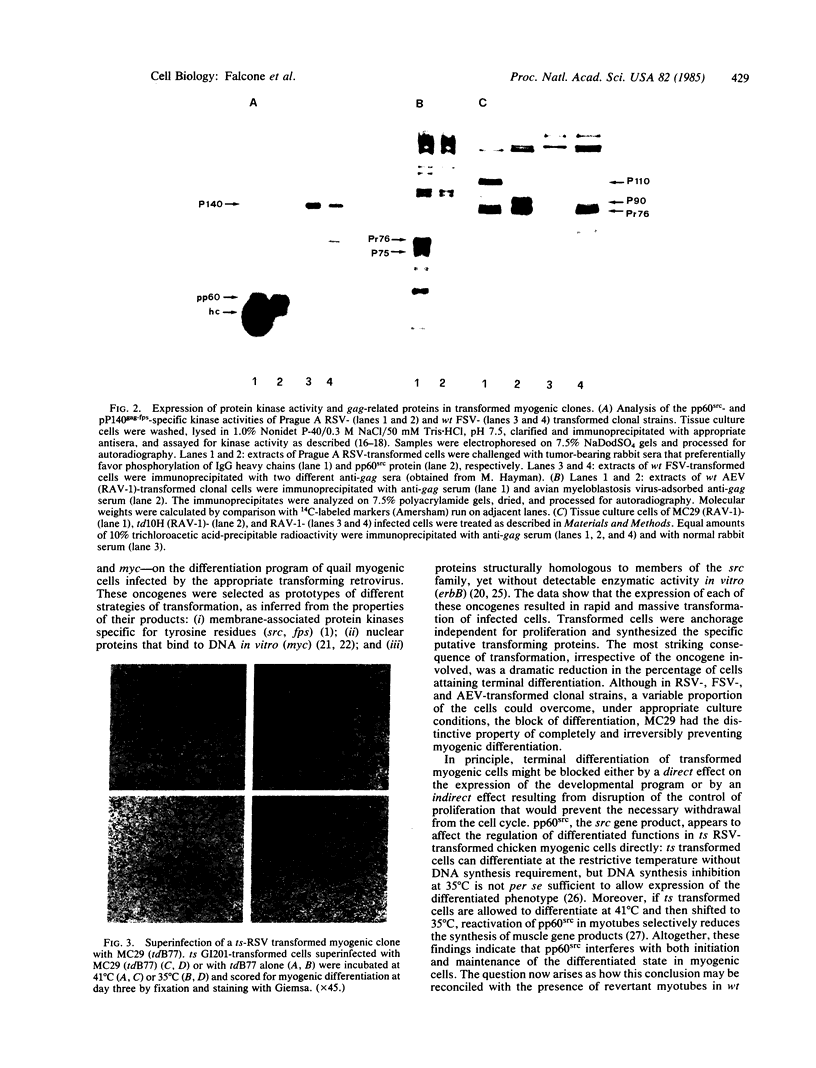
The relationship between susceptibility to transformation in vitro by different oncogenes and terminal differentiation was analyzed in embryonic quail myogenic cells. Infection with Rous sarcoma virus (RSV), Fujinami sarcoma virus (FSV), avian erythroblastosis virus (AEV), and the avian myelocytomatosis virus MC29 led to rapid and massive transformation. Transformed cells had distinctive morphological alterations, increased proliferation rates, and the ability to grow in agar suspension. Furthermore, homogeneously transformed cultures failed to fuse into multinucleated myotubes and to express muscle-specific genes. However, cloned populations of RSV-, FSV-, and AEV-transformed myogenic cells could, under appropriate culture conditions, partially differentiate into atypical "revertant" myotubes. In contrast, competence for terminal differentiation was completely and irreversibly suppressed on transformation by MC29. The specificity of action of a given oncogenic sequence on the inhibition of differentiation was further studied by using conditional and nonconditional transformation mutants. Myogenic cells infected with temperature-sensitive (ts) mutants of RSV and FSV exhibited a fully reversible block of differentiation after shift to restrictive temperature, while cells infected with ts34 AEV were not temperature sensitive for differentiation. Cultures infected with the partially transformation-defective mutant of MC29 td10H were morphologically transformed and acquired anchorage independence for proliferation but maintained a residual competence for terminal differentiation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abrams H. D., Rohrschneider L. R., Eisenman R. N. Nuclear location of the putative transforming protein of avian myelocytomatosis virus. Cell. 1982 Jun;29(2):427–439. doi: 10.1016/0092-8674(82)90159-3. [DOI] [PubMed] [Google Scholar]
- Boettiger D., Roby K., Brumbaugh J., Biehl J., Holtzer H. Transformation of chicken embryo retinal melanoblasts by a temperature-sensitive mutant of Rous sarcoma virus. Cell. 1977 Aug;11(4):881–890. doi: 10.1016/0092-8674(77)90299-9. [DOI] [PubMed] [Google Scholar]
- Brugge J. S., Erikson R. L. Identification of a transformation-specific antigen induced by an avian sarcoma virus. Nature. 1977 Sep 22;269(5626):346–348. doi: 10.1038/269346a0. [DOI] [PubMed] [Google Scholar]
- Collett M. S., Erikson R. L. Protein kinase activity associated with the avian sarcoma virus src gene product. Proc Natl Acad Sci U S A. 1978 Apr;75(4):2021–2024. doi: 10.1073/pnas.75.4.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donner P., Greiser-Wilke I., Moelling K. Nuclear localization and DNA binding of the transforming gene product of avian myelocytomatosis virus. Nature. 1982 Mar 18;296(5854):262–269. doi: 10.1038/296262a0. [DOI] [PubMed] [Google Scholar]
- Duesberg P. H. Retroviral transforming genes in normal cells? Nature. 1983 Jul 21;304(5923):219–226. doi: 10.1038/304219a0. [DOI] [PubMed] [Google Scholar]
- Durban E. M., Boettiger D. Differential effects of transforming avian RNA tumor viruses on avian macrophages. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3600–3604. doi: 10.1073/pnas.78.6.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dyson P. J., Quade K., Wyke J. A. Expression of the ASV src gene in hybrids between normal and virally transformed cells: specific suppression occurs in some hybrids but not others. Cell. 1982 Sep;30(2):491–498. doi: 10.1016/0092-8674(82)90246-x. [DOI] [PubMed] [Google Scholar]
- Enrietto P. J., Hayman M. J., Ramsay G. M., Wyke J. A., Payne L. N. Altered pathogenicity of avian myelocytomatosis (MC29) viruses with mutations in the v-myc gene. Virology. 1983 Jan 15;124(1):164–172. doi: 10.1016/0042-6822(83)90300-8. [DOI] [PubMed] [Google Scholar]
- Falcone G., Boettiger D., Alemà S., Tatò F. Role of cell division in differentiation of myoblasts infected with a temperature-sensitive mutant of Rous sarcoma virus. EMBO J. 1984 Jun;3(6):1327–1331. doi: 10.1002/j.1460-2075.1984.tb01971.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiszman M. Y., Fuchs P. Temperature-sensitive expression of differentiation in transformed myoblasts. Nature. 1975 Apr 3;254(5499):429–431. doi: 10.1038/254429a0. [DOI] [PubMed] [Google Scholar]
- Frykberg L., Palmieri S., Beug H., Graf T., Hayman M. J., Vennström B. Transforming capacities of avian erythroblastosis virus mutants deleted in the erbA or erbB oncogenes. Cell. 1983 Jan;32(1):227–238. doi: 10.1016/0092-8674(83)90513-5. [DOI] [PubMed] [Google Scholar]
- Graf T., Ade N., Beug H. Temperature-sensitive mutant of avian erythroblastosis virus suggests a block of differentiation as mechanism of leukaemogenesis. Nature. 1978 Oct 12;275(5680):496–501. doi: 10.1038/275496a0. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H. Avian leukemia viruses: interaction with their target cells in vivo and in vitro. Biochim Biophys Acta. 1978 Nov 17;516(3):269–299. doi: 10.1016/0304-419x(78)90011-2. [DOI] [PubMed] [Google Scholar]
- Graf T., Beug H., Hayman M. J. Target cell specificity of defective avian leukemia viruses: hematopoietic target cells for a given virus type can be infected but not transformed by strains of a different type. Proc Natl Acad Sci U S A. 1980 Jan;77(1):389–393. doi: 10.1073/pnas.77.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf T., Beug H. Role of the v-erbA and v-erbB oncogenes of avian erythroblastosis virus in erythroid cell transformation. Cell. 1983 Aug;34(1):7–9. doi: 10.1016/0092-8674(83)90130-7. [DOI] [PubMed] [Google Scholar]
- Hayman M. J., Ramsay G. M., Savin K., Kitchener G., Graf T., Beug H. Identification and characterization of the avian erythroblastosis virus erbB gene product as a membrane glycoprotein. Cell. 1983 Feb;32(2):579–588. doi: 10.1016/0092-8674(83)90477-4. [DOI] [PubMed] [Google Scholar]
- Holtzer H., Biehl J., Yeoh G., Meganathan R., Kaji A. Effect of oncogenic virus on muscle differentiation. Proc Natl Acad Sci U S A. 1975 Oct;72(10):4051–4055. doi: 10.1073/pnas.72.10.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly K., Cochran B. H., Stiles C. D., Leder P. Cell-specific regulation of the c-myc gene by lymphocyte mitogens and platelet-derived growth factor. Cell. 1983 Dec;35(3 Pt 2):603–610. doi: 10.1016/0092-8674(83)90092-2. [DOI] [PubMed] [Google Scholar]
- Land H., Parada L. F., Weinberg R. A. Tumorigenic conversion of primary embryo fibroblasts requires at least two cooperating oncogenes. Nature. 1983 Aug 18;304(5927):596–602. doi: 10.1038/304596a0. [DOI] [PubMed] [Google Scholar]
- Mathey-Prevot B., Hanafusa H., Kawai S. A cellular protein is immunologically crossreactive with and functionally homologous to the Fujinami sarcoma virus transforming protein. Cell. 1982 Apr;28(4):897–906. doi: 10.1016/0092-8674(82)90069-1. [DOI] [PubMed] [Google Scholar]
- Pacifici M., Boettiger D., Roby K., Holtzer H. Transformation of chondroblasts by Rous sarcoma virus and synthesis of the sulfated proteoglycan matrix. Cell. 1977 Aug;11(4):891–899. doi: 10.1016/0092-8674(77)90300-2. [DOI] [PubMed] [Google Scholar]
- Palmieri S., Beug H., Graf T. Isolation and characterization of four new temperature-sensitive mutants of avian erythroblastosis virus (AEV). Virology. 1982 Dec;123(2):296–311. doi: 10.1016/0042-6822(82)90263-x. [DOI] [PubMed] [Google Scholar]
- Palmieri S., Kahn P., Graf T. Quail embryo fibroblasts transformed by four v-myc-containing virus isolates show enhanced proliferation but are non tumorigenic. EMBO J. 1983;2(12):2385–2389. doi: 10.1002/j.1460-2075.1983.tb01750.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsay G., Graf T., Hayman M. J. Mutants of avian myelocytomatosis virus with smaller gag gene-related proteins have an altered transforming ability. Nature. 1980 Nov 13;288(5787):170–172. doi: 10.1038/288170a0. [DOI] [PubMed] [Google Scholar]
- STOCKDALE F. E., HOLTZER H. DNA synthesis and myogenesis. Exp Cell Res. 1961 Sep;24:508–520. doi: 10.1016/0014-4827(61)90450-5. [DOI] [PubMed] [Google Scholar]
- Tatò F., Alemà S., Dlugosz A., Boettiger D., Holtzer H., Cossu G., Pacifici M. Development of 'revertant' myotubes in cultures of Rous sarcoma virus transformed avian myogenic cells. Differentiation. 1983;24(2):131–139. doi: 10.1111/j.1432-0436.1983.tb01312.x. [DOI] [PubMed] [Google Scholar]
- Vogel Z., Sytkowski A. J., Nirenberg M. W. Acetylcholine receptors of muscle grown in vitro. Proc Natl Acad Sci U S A. 1972 Nov;69(11):3180–3184. doi: 10.1073/pnas.69.11.3180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West C. M., Boettiger D. Selective effect of Rous sarcoma virus src gene expression on contractile protein synthesis in chick embryo myotubes. Cancer Res. 1983 May;43(5):2042–2046. [PubMed] [Google Scholar]