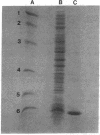
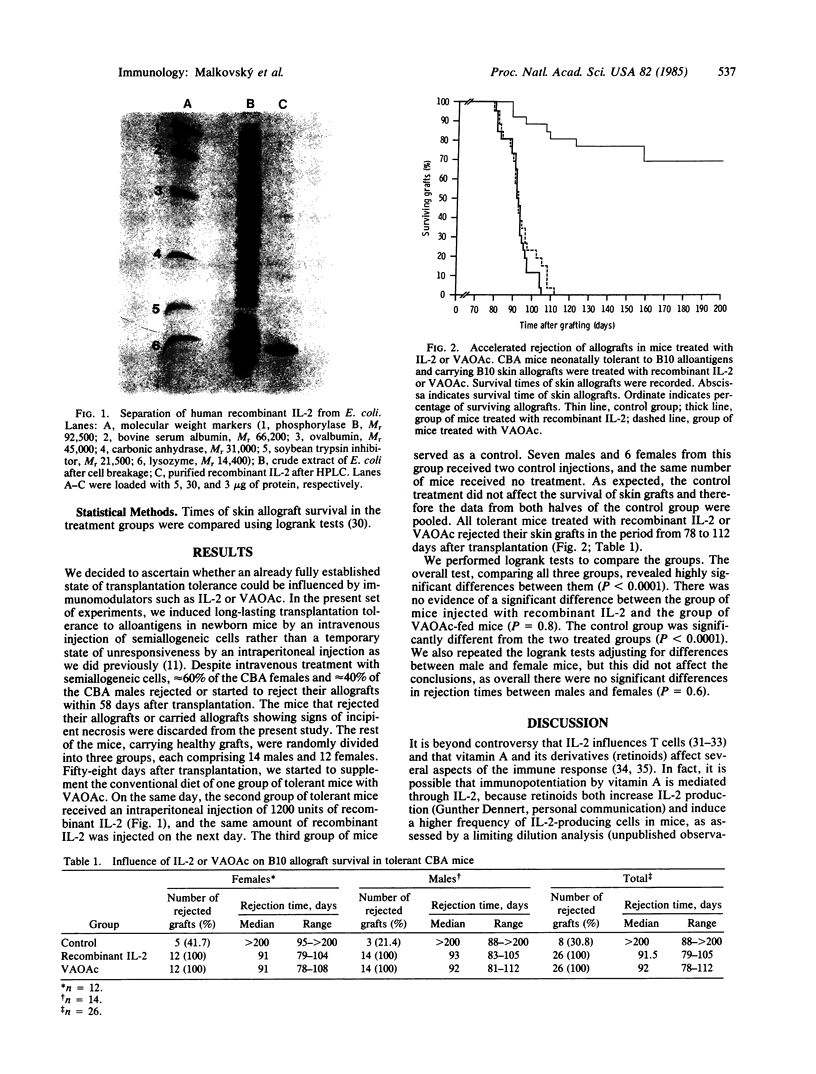
Abstract
The susceptibility of newborn mice to the inception of tolerance after exposure to antigen is associated with their deficiency in the production of endogenous interleukin 2 (IL-2). As further evidence of the complicity of IL-2 in the inception and maintenance of tolerance, it is shown here that a solid and long-lasting state of tolerance induced by the intravenous injection into newborn CBA mice of lymphoid cells from (CBA X C57BL/10ScSn)F1 hybrids can be brought to an end by the administration of exogenous IL-2 or by supplementing an otherwise normal diet with vitamin A acetate, the effect of which is to increase the proportion of the moiety of the T-cell population that produces IL-2. These results indicate that certain nonspecific stimuli can influence whether immunological tolerance is maintained.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BILLINGHAM R. E., BRENT L. A simple method for inducing tolerance of skin homografts in mice. Transplant Bull. 1957 Apr;4(2):67–71. [PubMed] [Google Scholar]
- BILLINGHAM R. E., BRENT L., MEDAWAR P. B. Actively acquired tolerance of foreign cells. Nature. 1953 Oct 3;172(4379):603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- Carnaud C., Ishizaka S. T., Stutman O. Early loss of precursors of CTL and IL 2-producing cells in the development of neonatal tolerance to alloantigens. J Immunol. 1984 Jul;133(1):45–51. [PubMed] [Google Scholar]
- DRESSER D. W. Specific inhibition of antibody production. I. Protein-over loading paralysis. Immunology. 1962 Jan;5:161–168. [PMC free article] [PubMed] [Google Scholar]
- Devos R., Plaetinck G., Cheroutre H., Simons G., Degrave W., Tavernier J., Remaut E., Fiers W. Molecular cloning of human interleukin 2 cDNA and its expression in E. coli. Nucleic Acids Res. 1983 Jul 11;11(13):4307–4323. doi: 10.1093/nar/11.13.4307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRASER D. Bursting bacteria by release of gas pressure. Nature. 1951 Jan 6;167(4236):33–34. doi: 10.1038/167033b0. [DOI] [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Heber-Katz E., Hansburg D., Schwartz R. H. The Ia molecule of the antigen-presenting cell plays a critical role in immune response gene regulation of T cell activation. J Mol Cell Immunol. 1983;1(1):3–18. [PubMed] [Google Scholar]
- Hefeneider S. H., Conlon P. J., Henney C. S., Gillis S. In vivo interleukin 2 administration augments the generation of alloreactive cytolytic T lymphocytes and resident natural killer cells. J Immunol. 1983 Jan;130(1):222–227. [PubMed] [Google Scholar]
- Ishizaka S. T., Stutman O. Analysis by limiting dilution of interleukin 2-producing T cells in murine ontogeny. Eur J Immunol. 1983 Nov;13(11):936–942. doi: 10.1002/eji.1830131113. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Loveland B. E., Hogarth P. M., Ceredig R., McKenzie I. F. Cells mediating graft rejection in the mouse. I. Lyt-1 cells mediate skin graft rejection. J Exp Med. 1981 May 1;153(5):1044–1057. doi: 10.1084/jem.153.5.1044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loveland B. E., McKenzie I. F. Which T cells cause graft rejection? Transplantation. 1982 Mar;33(3):217–221. doi: 10.1097/00007890-198203000-00001. [DOI] [PubMed] [Google Scholar]
- MEDAWAR P. B. Immunological tolerance. Science. 1961 Feb 3;133(3449):303–306. doi: 10.1126/science.133.3449.303. [DOI] [PubMed] [Google Scholar]
- Mahoney W. C., Hermodson M. A. Separation of large denatured peptides by reverse phase high performance liquid chromatography. Trifluoroacetic acid as a peptide solvent. J Biol Chem. 1980 Dec 10;255(23):11199–11203. [PubMed] [Google Scholar]
- Malkovsky M., Asherson G. L., Stockinger B., Watkins M. C. Nonspecific inhibitor released by T acceptor cells reduces the production of interleukin-2. Nature. 1982 Dec 16;300(5893):652–655. doi: 10.1038/300652a0. [DOI] [PubMed] [Google Scholar]
- Malkovský M., Doré C., Hunt R., Palmer L., Chandler P., Medawar P. B. Enhancement of specific antitumor immunity in mice fed a diet enriched in vitamin A acetate. Proc Natl Acad Sci U S A. 1983 Oct;80(20):6322–6326. doi: 10.1073/pnas.80.20.6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malkovský M., Edwards A. J., Hunt R., Palmer L., Medawar P. B. T-cell-mediated enhancement of host-versus-graft reactivity in mice fed a diet enriched in vitamin A acetate. Nature. 1983 Mar 24;302(5906):338–340. doi: 10.1038/302338a0. [DOI] [PubMed] [Google Scholar]
- Malkovský M., Hunt R., Palmer L., Doré C., Medawar P. B. Retinyl acetate-mediated augmentation of resistance to a transplantable 3-methylcholanthrene-induced fibrosarcoma. The dose response and time course. Transplantation. 1984 Aug;38(2):158–161. doi: 10.1097/00007890-198408000-00013. [DOI] [PubMed] [Google Scholar]
- Malkovský M., Medawar P., Hunt R., Palmer L., Doré C. A diet enriched in vitamin A acetate or in vivo administration of interleukin-2 can counteract a tolerogenic stimulus. Proc R Soc Lond B Biol Sci. 1984 Feb 22;220(1221):439–445. doi: 10.1098/rspb.1984.0012. [DOI] [PubMed] [Google Scholar]
- Medawar P. B., Hunt R. Anti-cancer action of retinoids. Immunology. 1981 Feb;42(2):349–353. [PMC free article] [PubMed] [Google Scholar]
- Medawar P., Hunt R., Mertin J. An influence of diet on transplantation immunity. Proc R Soc Lond B Biol Sci. 1979 Dec 31;206(1164):265–280. doi: 10.1098/rspb.1979.0104. [DOI] [PubMed] [Google Scholar]
- Miller K., Maisey J., Malkovský M. Enhancement of contact sensitization in mice fed a diet enriched in vitamin A acetate. Int Arch Allergy Appl Immunol. 1984;75(2):120–125. doi: 10.1159/000233601. [DOI] [PubMed] [Google Scholar]
- Nossal G. J. Cellular mechanisms of immunologic tolerance. Annu Rev Immunol. 1983;1:33–62. doi: 10.1146/annurev.iy.01.040183.000341. [DOI] [PubMed] [Google Scholar]
- Nossal G. J., Pike B. L. Functional clonal deletion in immunological tolerance to major histocompatibility complex antigens. Proc Natl Acad Sci U S A. 1981 Jun;78(6):3844–3847. doi: 10.1073/pnas.78.6.3844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. D. IMMUNOGENETIC CONSEQUENCES OF VASCULAR ANASTOMOSES BETWEEN BOVINE TWINS. Science. 1945 Oct 19;102(2651):400–401. doi: 10.1126/science.102.2651.400. [DOI] [PubMed] [Google Scholar]
- Peto R., Pike M. C., Armitage P., Breslow N. E., Cox D. R., Howard S. V., Mantel N., McPherson K., Peto J., Smith P. G. Design and analysis of randomized clinical trials requiring prolonged observation of each patient. II. analysis and examples. Br J Cancer. 1977 Jan;35(1):1–39. doi: 10.1038/bjc.1977.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfizenmaier K., Scheurich P., Däubener W., Krönke M., Röllinghoff M., Wagner H. Quantitative representation of all T cells committed to develop into cytotoxic effector cells and/or interleukin 2 activity-producing helper cells within murine T lymphocyte subsets. Eur J Immunol. 1984 Jan;14(1):33–39. doi: 10.1002/eji.1830140107. [DOI] [PubMed] [Google Scholar]
- Rosenberg S. A., Grimm E. A., McGrogan M., Doyle M., Kawasaki E., Koths K., Mark D. F. Biological activity of recombinant human interleukin-2 produced in Escherichia coli. Science. 1984 Mar 30;223(4643):1412–1414. doi: 10.1126/science.6367046. [DOI] [PubMed] [Google Scholar]
- Smith K. A. Interleukin 2. Annu Rev Immunol. 1984;2:319–333. doi: 10.1146/annurev.iy.02.040184.001535. [DOI] [PubMed] [Google Scholar]
- Stockinger B. Cytotoxic T-cell precursors revealed in neonatally tolerant mice. Proc Natl Acad Sci U S A. 1984 Jan;81(1):220–223. doi: 10.1073/pnas.81.1.220. [DOI] [PMC free article] [PubMed] [Google Scholar]