Abstract
Background and Purpose
In a previous study, 0.3 and 0.45 mg/kg of intravenous recombinant tissue plasminogen activator (rt-PA) were safe when combined with eptifibatide 75 mcg/kg bolus and a 2-hour infusion (0.75 mcg/kg per minute). The Combined Approach to Lysis Utilizing Eptifibatide and rt-PA in Acute Ischemic Stroke–Enhanced Regimen (CLEAR-ER) trial sought to determine the safety of a higher-dose regimen and to establish evidence for a phase III trial.
Methods
CLEAR-ER was a multicenter, double-blind, randomized safety study. Ischemic stroke patients were randomized to 0.6 mg/kg rt-PA plus eptifibatide (135 mcg/kg bolus and a 2-hour infusion at 0.75 mcg/kg per minute) versus standard rt-PA (0.9 mg/kg). The primary safety end point was the incidence of symptomatic intracranial hemorrhage within 36 hours. The primary efficacy outcome measure was the modified Rankin Scale (mRS) score ≤1 or return to baseline mRS at 90 days. Analysis of the safety and efficacy outcomes was done with multiple logistic regression.
Results
Of 126 subjects, 101 received combination therapy, and 25 received standard rt-PA. Two (2%) patients in the combination group and 3 (12%) in the standard group had symptomatic intracranial hemorrhage (odds ratio, 0.15; 95% confidence interval, 0.01–1.40; P=0.053). At 90 days, 49.5% of the combination group had mRS ≤1 or return to baseline mRS versus 36.0% in the standard group (odds ratio, 1.74; 95% confidence interval, 0.70–4.31; P=0.23). After adjusting for age, baseline National Institutes of Health Stroke Scale, time to intravenous rt-PA, and baseline mRS, the odds ratio was 1.38 (95% confidence interval, 0.51–3.76; P=0.52).
Conclusions
The combined regimen of intravenous rt-PA and eptifibatide studied in this trial was safe and provides evidence that a phase III trial is warranted to determine efficacy of the regimen.
Keywords: clinical trial, eptifibatide, ischemic stroke, tissue plasminogen activator
Intravenous (IV) recombinant tissue plasminogen activator (rt-PA) remains the only US Food and Drug Administration–approved and proven therapy for acute ischemic stroke.1 The addition of glycoprotein IIb/IIIa antagonists to fibrinolytic regimens increases both the speed of arterial recanalization and the percentage of patients with open arteries in acute myocardial infarction.2 On the basis of this rationale, we have previously reported that the combination of reduced-dose IV rt-PA plus eptifibatide, a glycoprotein IIb/IIIa antagonist, was safe when administered within 3 hours of symptom onset in acute ischemic stroke.3 Tier 1 of that dose escalation study used 0.3 mg/kg of IV rt-PA, whereas Tier 2 used 0.45 mg/kg of IV rt-PA. In each tier, the eptifibatide dose was a 75 mcg/kg bolus followed by a 2-hour infusion of 0.75 mcg/kg per minute.3 As demonstrated in the Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II), full platelet inhibition at cardiac doses for 12 hours or longer may be unsafe in the setting of stroke.4 As such, our goal was to use the lowest possible combination of doses of rt-PA plus eptifibatide with the highest lytic efficacy and a shorter duration that is likely to affect early reperfusion but limits potential hemorrhagic complications over the first 24 hours. Given the 1.4% symptomatic intracranial hemorrhage (sICH) rate and overall safety of the doses of rt-PA and eptifibatide in the Combined Approach to Lysis Utilizing Eptifibatide and rt-PA in Acute Ischemic Stroke (CLEAR) trial,3 we designed and conducted the Combined Approach to Lysis Utilizing Eptifibatide and rt-PA in Acute Ischemic Stroke–Enhanced Regimen (CLEAR-ER) trial. We hypothesized that this new higher-dose regimen would be safe and provide sufficient evidence that would warrant pursuit of a phase III clinical trial.
Methods
The CLEAR-ER trial was a multicenter, double-blind, randomized safety study designed to provide data concerning the risks and benefits of combining a glycoprotein IIb/IIIa antagonist, eptifibatide, with medium-dose IV rt-PA in 126 acute ischemic stroke patients treated with rt-PA within 3 hours of symptom onset. The institutional review board of each site approved the study protocol, and written informed consent was obtained from each patient or surrogate before study entry. The trial was sponsored by the National Institutes of Health/National Institute of Neurological Disorders and Stroke (NIH/NINDS) as a Specialized Programs of Translational Research in Acute Stroke program project. The trial was conducted at 9 US medical centers comprising 21 hospitals. Study drugs were supplied at no charge by the manufacturers (rt-PA by Genentech and eptifibatide by Merck). The manufacturers had no role in trial design, execution, data analysis, or writing of this article. Eligible patients were 18 to 85 years of age with a clinical diagnosis of acute ischemic stroke and a National Institutes of Health Stroke Scale (NIHSS) score >5. Inclusion and exclusion criteria are listed in Table 1.
Table 1.
Inclusion and Exclusion Criteria
| Inclusion criteria |
| 1. Patients must have had a serious measurable neurological deficit on the NIH Stroke Scale caused by focal brain ischemia |
| 2. An NIH Stroke Scale score >5 at the time the rt-PA was begun |
| 3. Age: 18 to 85 y (ie, candidates must have had their 18th birthday, but not had their 86th birthday) |
| 4. Intravenous rt-PA therapy must have been initiated within 3 h of onset of stroke symptoms |
| Exclusion criteria |
| Clinical |
| 1. History of stroke in the past 3 mo |
| 2. Previous intracranial hemorrhage, neoplasm, subarachnoid hemorrhage, or arterial venous malformation |
| 3. Clinical presentation suggested a subarachnoid hemorrhage, even if initial CT scan was normal |
| 4. Hypertension at time of treatment; systolic BP >185 or diastolic >110 mm Hg, or aggressive measures to lower blood pressure to below these limits were needed |
| 5. Presumed septic embolus |
| 6. Presumed pericarditis, including pericarditis after acute myocardial infarction |
| 7. Recent (within 30 d) surgery or biopsy of parenchymal organ |
| 8. Recent (within 30 d) trauma, with internal injuries or ulcerative wounds |
| 9. Recent (within 90 d) severe head trauma or head trauma with loss of consciousness |
| 10. Any active or recent (within 30 d) serious systemic hemorrhage |
| 11. Known hereditary or acquired hemorrhagic diathesis, coagulation factor deficiency, or oral anticoagulant therapy with INR >1.7 |
| 12. Baseline laboratory values: positive urine pregnancy test, glucose <50 or >400 mg/dL, platelets <100 000/mm3, Hct <25%, or creatinine >4 mg/dL |
| 13. Ongoing renal dialysis, regardless of creatinine |
| 14. If heparin was administered within 48 h from screening, the patient must have had a normal partial thromboplastin time (PTT) |
| 15. Subjects who received low molecular weight heparins (such as dalteparin, enoxaparin, and tinzaparin) as DVT prophylaxis or in full dose within the previous 24 h |
| 16. Subjects who received heparin or a direct thrombin inhibitor (such as bivalirudin, argrtroban, or lepirudin) within 48 h from screening must have had a normal PTT |
| 17. Subjects took Factor Xa inhibitors (such as fondaparinux) within the last 4 d |
| 18. Subjects who received glycoprotein IIb/IIIa inhibitors within the past 2 wk |
| 19. Arterial puncture at a noncompressible site or a lumbar puncture in the previous 7 d |
| 20. Seizure at onset of stroke |
| 21. Preexisting neurological or psychiatric disease which confounded the neurological or functional evaluations |
| 22. Other serious, advanced, or terminal illness or any other condition that the investigator felt would pose a significant hazard to the patient if rt-PA or eptifibatide therapy was initiated |
| 23. Patients whose peripheral venous access was so poor that they were unable to have 2 standard peripheral intravenous lines started |
| 24. Current participation in another research drug treatment protocol. Subjects could not start another experimental agent until after 90 d |
| 25. Informed consent was not or could not be obtained |
| 26. Any known history of amyloid angiopathy |
| CT scan exclusions |
| 1. High-density lesion consistent with hemorrhage of any degree |
| 2. Significant mass effect with midline shift |
| 3. Large (more than one third of the middle cerebral artery) regions of clear hypodensity on the baseline CT scan. Sulcal effacement and loss of gray-white differentiation alone were not contraindications for treatment |
BP indicates blood pressure; CT, computed tomography; DVT, deep venous thrombosis; INR, international normalized ratio; NIH, National institutes of Health; and rt-PA, recombinant tissue plasminogen activator.
Protocol and Randomization Scheme
Using a web-based approach with a telephone backup line using computer-generated number codes, patients were randomized in a 5:1 ratio using minimization techniques to the combination of medium-dose rt-PA (0.6 mg/kg) plus eptifibatide (135 mcg/kg bolus followed by a 2-hour infusion at 0.75 mcg/kg per minute) versus standard-dose rt-PA (0.9 mg/kg). The selected doses were based on a well-established in vitro clot model that suggested similar lytic efficacy may be obtained with reduced-dose rt-PA and eptifibatide compared with full doses of the medications.5 The minimization approach used 2 stratification variables: age (<70 and ≥70 years) and the NIHSS (≤12 and >12) with group allocation based on the selection of the group, with probability of 0.85 that gave the smaller total treatment imbalance. Total treatment imbalance was defined as the sum of the weighted imbalance for age, NIHSS, center, and overall study balance. The weighting factors were chosen before the start of the study according to the importance of achieving balance for that variable.
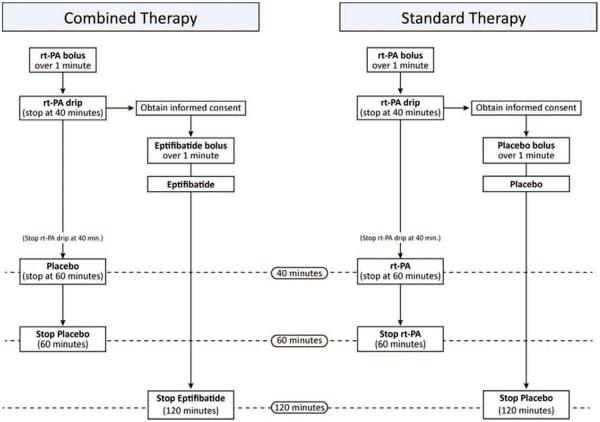
To avoid delaying timely administration of rt-PA, the protocol required that open-label rt-PA be initiated with a planned dose of 0.9 mg/kg as standard of care as soon as clinically indicated; informed consent was then obtained for participation in the trial. Once randomization was accomplished, the investigational pharmacy supplied the emergency department with an infusion bag of rt-PA or placebo and a bolus and infusion bag of eptifibatide or placebo. The eptifibatide or placebo bolus and infusion were started as soon as possible. The open-label rt-PA was then taken down completely (to the saline-lock) and removed at the 40-minute point of the infusion. At that point, patients would have received 0.6 mg/kg of rt-PA. The infusion bag containing either rt-PA or placebo with a primed line was then hung and started. Thus, the patient received either study combination or standard rt-PA in a double-blinded fashion (Figure 1). All study drugs were colorless and compatible in infusion lines, hence there was no potential unblinding caused by mixing of agents in the IV lines.
Figure 1.
Schematic of blinded administration of recombinant tissue plasminogen activator (rt-PA) plus eptifibatide vs standard rt-PA.
Subject Monitoring
Patients were monitored clinically throughout the infusion by study personnel and then admitted to an intensive care unit for continued monitoring for ≥24 hours. Patients had standardized clinical evaluations at 2 hours, 24 hours, and 5 days or at discharge, followed by a telephone follow-up at 7 days and a final in-person standardized evaluation at 90 days. Outcomes measured were the modified Rankin Scale score, NIHSS, Glasgow Outcome Scale, and Barthel Index. Radiological outcome measures included a 24-hour safety head computed tomography scan to evaluate for ICH.
Safety Evaluations
The primary safety end point was the incidence of sICH within 36 hours of treatment. sICH was defined per the NINDS rt-PA Stroke Trial definition1 as any ICH related to a decline in neurological status or the development of new neurological symptoms that, in the judgment of the clinical investigator, was related to the ICH. The final judgment for what constituted a significant neurological decline was made by the local site clinical investigator. A blinded, independent safety monitor reviewed all ICHs. Stopping rules for sICH in the rt-PA+eptifibitide arm were created on the basis of an underlying rate of 8.1%, which was the sICH rate of patients from the NINDS rt-PA Stroke Trial, rt-PA treated arm, with an NIHSS >5, and age between 18 and 85 years. The lower 90% confidence interval (CI) was calculated for each potential observed event, assuming a binomial distribution. The stopping decision was based on whether the assumed true event rate, 8.1%, fell below the lower 90% CI for the observed rate, dependent on the number of subjects accrued. All hemorrhages observed on computed tomography were classified by the independent study neuroradiologist as hemorrhagic infarct 1, hemorrhagic infarct 2, parenchymal hemorrhage 1, and parenchymal hemorrhage 26 regardless of symptomatology.
Additional safety end points evaluated within 7 days of treatment onset included the incidence of life-threatening systemic bleeding (defined as requiring transfusion of ≥3 U of packed red blood cells), mild or moderate systemic bleeding, sICH, asymptomatic ICH, death of any cause, and death because of stroke. Ninety-day safety end points were death of any cause, death caused by stroke, adverse events (AE), serious AE, subjects with ≥1 AE, and subjects with ≥1 serious AE.
Outcome Measures
The primary efficacy outcome measure was the modified Rankin Scale (mRS) score ≤1 or return to baseline mRS at 90 days. The prespecified end point that would suggest that the combination therapy should not be explored further in a phase III trial (no-go) was a proportion of good outcome <0.33. This was based on the 39% good outcome rate in the NINDS trial of rt-PA with an estimated SE of 3.8%, yielding 95% CIs of 32% to 46%.1 A proportion of good outcome no larger than the lower bound of this CI would suggest futility of the combination therapy and represent a no-go for pursuit of a phase III trial.
Additional end points included measures of very early improvement: NIHSS ≤5 at 2 hours; early improvement: NIHSS ≤2 at 24 hours; and late improvement: NIHSS ≤2 at 90 days. The NINDS investigators proposed these end points as excellent end points for future phase II studies of recanalization therapies in acute ischemic stroke on the basis of exploratory analyses of the NINDS rt-PA Trial.7 Secondary clinical end points at 90 days included mRS score ≤1, Barthel Index of ≥95, Glasgow Outcome Scale of 1, and occurrence of new stroke.
The 90-day primary clinical outcome assessments were performed by blinded study investigators who were not directly involved with acute treatment of the patient. All investigators were certified in the NIHSS and received standardized training on the Rankin, Barthel, and Glasgow assessments.
Data Management
Data were managed and analyzed with SAS version 9.3 (SAS Institute Inc; Cary, NC). The case report forms were double-entered into a custom web-based Sharepoint database run using InfoPath, with monthly computer-generated monitoring. In addition, 100% monitoring was completed at the end of the study.
Statistical Analyses
Univariate analysis included range checking and examination of distributional properties of the variables of interest. Bivariate analysis to compare baseline descriptors between the experimental and control groups consisted of Wilcoxon rank sum and Fisher exact tests, as appropriate, on the basis of the nature of the data being analyzed. Analysis of the safety and efficacy outcome variables was done with multiple logistic regression both unadjusted and adjusted for appropriate covariates. When low frequency was encountered (≤5), an exact logistic regression was used. Variables considered a priori for inclusion as potential covariates were age, baseline NIHSS score, and time to treatment. These variables were chosen on the basis of findings from the NINDS rt-PA Stroke Trials.1 Information was available for all subjects on the primary safety and efficacy end points.
Results
The study enrolled 126 subjects from July 2009 to October 2012; 101 received the combination therapy and 25 received standard-dose rt-PA. The demographic and medical history of subjects in the 2 treatment groups are shown in Table 2.
Table 2.
Demographics
| rt-PA+Eptifibatide | rt-PA | P Value | |
|---|---|---|---|
| N | 101 | 25 | |
| Age, y, median (IQR) | 71.6 (58.1–81.5) | 75.5 (60.5–81.4) | 0.63 |
| Women, n (%) | 48 (47.5) | 12 (48.0) | 0.97 |
| Black, n (%) | 14 (13.9) | 2 (8.0) | 0.74 |
| Baseline NIHSS, median (IQR) | 12.0 (9.0–20.0) | 17.0 (11.0–22.0) | 0.11 |
| Baseline mRS (0–1) | 85 (84.2%) | 18 (72.0%) | 0.16 |
| Baseline systolic BP, median (IQR) | 155 (135–175) | 143 (136–159) | 0.22 |
| Baseline diastolic BP, median (IQR) | 84 (70–99) | 83 (74–90) | 0.64 |
| Prior stroke, n (%) | 14 (14.0) | 2 (8.0) | 0.52 |
| Diabetes mellitus, n (%) | 31 (30.7) | 9 (36.0) | 0.61 |
| Hypertension, n (%) | 84 (83.2) | 19 (76.0) | 0.41 |
| Coronary artery disease, n (%) | 12 (12.4) | 4 (16.0) | 0.74 |
| Atrial fibrillation, n (%), (history or initial EKG) | 36 (35.6) | 9 (36.0) | 0.97 |
| Antiplatelet use before stroke, n (%) | 43 (42.6) | 7 (28.0) | 0.18 |
| Anticoagulant use before stroke, n (%) | 7 (6.9) | 1 (4.0) | 1.00 |
| Current smoker, n (%) | 31 (31.0) | 7 (28.0 | 0.93 |
| Symptom onset to IV rt-PA, min, median (IQR) | 113 (99–135) | 129 (90–141) | 0.69 |
| IV rt-PA to eptifibatide or placebo, min, median (IQR) | 40 (33–44) | 43 (38–48) | 0.04 |
| Symptom onset to IV rt-PA, n (%) | 0.50 | ||
| <1 h | 1 (1.0) | 0 | |
| 1–2 h | 56 (55.4) | 11 (44.0) | |
| >2–3 h | 42 (41.6) | 13 (52.0) | |
| >3 h* | 2 (2.0) | 1 (4.0) |
Data presented as median (25th percentile–75th percentile). Wilcoxon rank sum or t test used to test difference between groups for interval variables, χ2, or Fisher exact test, as appropriate, for proportions.
BP indicates blood pressure; EKG, electrocardiogram; IQR, interquartile range; IV, intravenous; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; and rt-PA, recombinant tissue plasminogen activator.
All 3 subjects received rt-PA <3 h and 10 min from symptom onset.
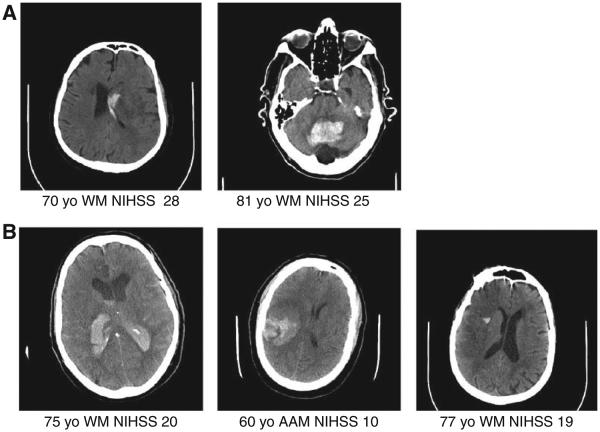
The primary safety outcome of sICH occurred in 2 (2%) of 101 patients in the combination treatment group and 3 (12%) of 25 patients in the standard treatment arm (odds ratio, 0.15; 95% CI, 0.01–1.40; P=0.053). Representative head computed tomography images for all sICH observed are shown in Figure 2. Of the 19 asymptomatic ICHs in both groups, 7 were classified as parenchymal hemorrhage 1 or parenchymal hemorrhage 2 by the neuroradiologist. A figure of these hemorrhages is in the online-only Data Supplement. Safety end points are presented in Table 3. At 7 days after treatment, mild and moderate systemic bleeding was more common in the combination therapy group, but there was no difference in life-threatening systemic bleeding. At 90 days, there were no differences between groups in death of any cause or death caused by stroke (Table 3). Causes of death are shown in the online-only Data Supplement.
Figure 2.
Symptomatic intracranial hemorrhage (sICH) in the Combined Approach to Lysis Utilizing Eptifibatide and rt-PA in Acute Ischemic Stroke–Enhanced Regimen (CLEAR-ER) trial. NIHSS indicates National Institutes of Health Stroke Scale.
Table 3.
Safety End Points and Other SAEs*
| rt-PA+Eptifibatide (n=101) | rt-PA (n=25) | Odds Ratio (95% CI) | P Value | |
|---|---|---|---|---|
| Prespecified primary safety end point | ||||
| Symptomatic ICH in 36 h, n (%) | 2 (2.0) | 3 (12.0) | 0.15 (0.01–1.40)† | 0.053 |
| Other end points | ||||
| Asymptomatic ICH in 36 h, n (%) | 14 (13.9) | 3 (12.0) | 1.18 (0.29–6.95)† | 1.00 |
| Any ICH in 36 h, n (%) | 16 (15.8) | 6 (24.0) | 0.60 (0.21–1.72) | 0.34 |
| Fatal ICH in 36 h, n (%) | 1 (1.0) | 1 (4.0) | 0.24 (0.003–19.62)† | 0.36 |
| Safety end points in 7 d | ||||
| Symptomatic ICH, n (%) | 2 (2.0) | 3 (12.0) | 0.15 (0.01–1.40)† | 0.053 |
| Asymptomatic ICH, n (%) | 16 (15.8) | 3 (12.0) | 1.38 (0.35–8.02) | 0.76 |
| PH-1, n(%) | 6 (5.9) | 3 (12.0) | 0.47 (0.09–3.10)† | 0.38 |
| PH-2, n (%) | 1 (1.0) | 2 (8.0) | 0.12 (0.002–2.35)† | 0.10 |
| Life-threatening systemic bleed (≥2 U packed red blood cells required) |
1 (1.0%) | 0 | not estimable | 1.00 |
| Moderate bleeding* | 4 (4.0%) | 0 | not estimable | 0.58 |
| Mild bleeding* | 12 (11.9%) | 0 | not estimable | 0.12 |
| Death of any cause, n (%) | 12 (11.9) | 3 (12.0) | 0.99 (0.24–5.92)† | 1.00 |
| Death caused by stroke, n (%) | 12 (11.9) | 3 (12.0) | 0.99 (0.24–5.92)† | 1.00 |
| Safety end points in 90 d | ||||
| Death of any cause, n (%) | 20 (19.8) | 4 (16.0) | 1.29 (0.38–5.76)† | 0.78 |
| Death caused by stroke, n (%) | 15 (14.8) | 4 (16.0) | 0.92 (0.26–4.18)† | 1.00 |
| AE rate | 2.59 (2.01–3.33) [223 events] | 2.82 (1.75–4.56) [62 events] | 0.76 | |
| Subjects with ≥1 AE, n (%) | 83 (82.2) | 19 (76.0) | 1.46 (0.51–4.16) | 0.48 |
| SAE rate | 0.40 (0.26–0.60) [34 events] | 0.36 (0.15–0.86) [8 events] | 0.87 | |
| Subjects with ≥1 SAE, n (%) | 26 (25.7) | 7 (28.0) | 0.89 (0.33–2.38) | 0.82 |
AE indicates adverse events; CI, confidence interval; ICH, intracranial hemorrhage; rt-PA, recombinant tissue plasminogen activator; and SAE, serious AE.
Events are not mutually exclusive.
Exact logistic and CI.
At 90 days after treatment, 49.5% of the combination treatment group patients had an mRS ≤1 or return to baseline mRS compared with 36.0% in the standard treatment group (unadjusted odds ratio, 1.74; 95% CI, 0.70–4.31; P=0.23). After adjusting for age, baseline NIHSS score, time to IV rt-PA and baseline mRS, the odds ratio was 1.38 (95% CI, 0.51–3.76; P=0.52). We adjusted for baseline mRS because of the higher proportion of combination therapy patients with minimal or no disability (Table 2). Table 4 shows the odds ratios for clinical outcome at 90 days after adjusting for the a priori covariates age, NIHSS, and time to treatment (ie, not adjusted for baseline mRS). A post hoc analysis using matched controls (n=63 per group) from the rt-PA arm of the NINDS trial showed similar results for the primary outcome (odds ratio, 1.30; 95% CI, 0.57–2.96), suggesting that the estimates from these historical controls were valid. Subjects from the combination treatment group were matched for sex, race, baseline mRS, age (±6 years), baseline NIHSS (±4), and time to rt-PA (±30 minutes). The tables in the online-only Data Supplement show very early improvement, early improvement, late improvement, and change in NIHSS from baseline to 24 hours in both treatment groups.
Table 4.
Ninety-Day Outcomes
| rt-PA+Eptifibatide (n=101) |
rt-PA (n=25) | Unadjusted Odds Ratio (95% CI) |
Adjusted* Odds Ratio (95% CI) |
P Value Unadjusted/ Adjusted |
|
|---|---|---|---|---|---|
| Prespecified primary efficacy outcome measure | |||||
| mRS 0–1 or return to baseline, n (%) | 50 (49.5) | 9 (36.0) | 1.74 (0.70–4.31) | 1.37 (0.51–3.71) | 0.23/0.53 |
| Other efficacy end points | |||||
| mRS 0–1 only, n (%) | 44 (43.6) | 6 (24.0) | 2.44 (0.90–6.64) | 1.98 (0.67–5.88) | 0.07/0.22 |
| Barthel Index of ≥95, n (%) | 55 (54.5) | 11 (44.0) | 1.52 (0.63–3.67) | 1.10 (0.40–3.02) | 0.35/0.85 |
| Glasgow Outcome Scale of 1, n (%) | 52 (51.5) | 10 (40.0) | 1.59 (0.65–3.88) | 1.19 (0.44–3.24) | 0.30/0.73 |
| New stroke within 90 d, n (%) | 1 (1.0) | 0 (0.0) | 1.00 |
CI indicates confidence interval; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; and rt-PA, recombinant tissue plasminogen activator.
Adjusted for age, baseline NIHSS and time to IV rt-PA.
Discussion
The enhanced dosing regimen of medium-dose IV rt-PA combined with a bolus followed by a short infusion of eptifibatide studied in this trial proved to be safe compared with standard-dose IV rt-PA. The 2% sICH rate observed was much lower than the maximum expected 8% sICH rate. The direction of effect with regards to a good clinical outcome also favored the eptifibatide group, although this study was not powered to detect differences in clinical efficacy and the results were not statistically significant. The 49.5% proportion of good outcome in the combination group was well above the prespecified decision point that would justify proceeding to a phase III trial.
There were imbalances in pretreatment factors between the combination and standard therapy groups that generally favored the combination group (Table 2). This is reflected in the decrease in odds ratio favoring the eptifibatide group after adjusting for baseline NIHSS, age, pretreatment Rankin, and time to start of IV t-PA. However, inclusion of control subjects was not for making direct comparisons with the interventional arm. Rather, the small group of controls was used as a calibration group to ascertain the validity of the estimate from the historical control group, as has been suggested for phase II clinical trials.8,9 The control group also ensured that treating physicians remained blinded because there was a 1 in 6 chance that subjects received standard-dose rt-PA.
To our knowledge, excluding our previous dose-escalation study, only 3 other prospective randomized clinical trials of IV glycoprotein IIb/IIIa antagonist for patients with acute ischemic stroke have been published.4,10,11 The Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II) was a placebo-controlled randomized phase III trial. The trial was terminated after 808 of a planned 1800 patients were enrolled because of a greater risk of sICH (5.5% versus 0.5%; P=0.002) with no obvious clinical benefit in the primary cohort of patients.4 The difference in safety between the AbESTT and the CLEAR-ER trials is readily explained by differences in study design and enrolled patients. Most patients enrolled in AbESST were treated in the 3- to 6-hour window and also included wake-up strokes. None of the patients in the abciximab or control group received t-PA. Further, the AbESTT intervention comprised a bolus followed by a 12-hour infusion of a full-dose regimen of abciximab used in cardiac trials. Thus, the amount and duration of platelet inhibition in that trial was greater than our enhanced regimen.
The Safety of Tirofiban in Acute Ischemic Stroke (SaTIS) trial randomized 260 patients to tirofiban versus placebo within 3 to 22 hours from symptom onset. The duration of treatment was 48 hours.10 sICH and total hemorrhage rates were similar between groups, and treatment with tirofiban was associated with lower mortality at 5 months (2.3% versus 8.7%; P=0.03).10 These findings require confirmation in a larger trial. A similar trial of 150 patients randomized to tirofiban versus aspirin found identical mortality at 3 months between groups with no difference in the proportion of patients with minimal or no disability.11 The time to treatment onset was substantially longer in both of these trials than in our current study.
In the CLEAR-ER trial, systemic bleeding was more common in the combination therapy group than in the standard group (Table 3). However, it primarily comprised mild bleeding for which no intervention was warranted. There were no differences in all deaths, death caused by stroke, and the frequency of AEs and serious AEs between groups at 90 days.
Our study design allowed rapid administration of IV rt-PA but allowed only 40 minutes for obtaining consent, randomization, and initiation of study drug. All patients in the study had the open-label rt-PA infusion stopped at the 40-minute mark. In the rt-PA group, the median time (interquartile range) to restarting the infusion was 43 (38–48) minutes (Table 2). It is unknown what impact this interruption may have had on the lytic efficacy of rt-PA in that group. In addition, our study did not include pretreatment imaging of intracranial vessels to determine rates of recanalization of occluded arteries in the 2 treatment groups. Finally, the sICH rate in the rt-PA group was higher than expected. A similar group of patients in the NINDS trial as our controls would be expected to have an sICH rate of 4.8% (95% CI, 0.1–24.8). However, a difference in 1 patient with sICH would have changed the observed rate from 12% (95% CI, 2.5–31.2) to 8% (95% CI, 1.0–26.0). These wide CIs indicate that a larger trial would be required to adequately address the true underlying differences in sICH between IV rt-PA and rt-PA plus eptifibatide.
Overall, this phase II clinical trial demonstrated the safety of the dosing regimen, the feasibility of enrolling patients, and delivery of study drug in a double-blinded fashion and provides evidence that a phase III clinical trial is warranted to determine the efficacy of this dosing regimen in improving clinical outcomes after acute ischemic stroke.
Supplementary Material
Acknowledgments
Sources of Funding
This work was supported by National Institutes of Health grant P50 NS044283.
Footnotes
Disclosures
Dr Pancioli is supported with study drugs provided by Merck and Genentech for the CLEAR-ER trial. Dr Adeoye is a member of Genentech Speakers’ Bureau, modest. Dr Levine received grant support from Genentech, Advisory Board (travel as unpaid consultant); a Medlink Associate Editor; and expert witness in medicolegal cases. Dr Crocco is a consultant for Genentech, Speakers’ Bureau for Medical Dialogues Group. Dr Hemmen is a consultant/Advisory Board: Boehringer Ingelheim, Genentech; and received grant support from NIH 5P50NS044148. Dr Kleindorfer is a member of Genentech, Speaker’s Bureau, modest level; and Genentech, consulting, modest level. Dr Knight is a member of Genentech, Speaker’s Bureau; Roche (Advisory Board). Dr Meyer is a member of Genentech, Speaker’s Bureau; UCSD SPOTRIAS PI. Dr Starkman is an employee of the University of California, Regents, which holds a patent on retriever devices for stroke. He is an investigator in the NIH MR and Recanalization of Stroke Clots Using Embolectomy (MR RESCUE) and International Management of Stroke (IMS) 3 multicenter clinical trials for which the UC Regents receive payments on the basis of clinical trial performance, has served as an unpaid site investigator in multicenter trials run by ev3 for which the UC Regents received payments on the basis of clinical trial contracts for the number of subjects enrolled, and was an unpaid site investigator in a multicenter registry run by Concentric for which the UC Regents received payments on the basis of clinical trial contracts for the number of subjects enrolled. Dr Broderick is a principal investigator of NINDS-funded IMS III Trial, UC SPOTRIAS Center (includes NINDS-funded CLEAR-ER and STOP-IT Clinical Trials); and Genentech Inc (supplier of alteplase for NINDS-funded CLEAR-ER, IMS III trials). The other authors have no conflicts to report.
Clinical Trial Registration
URL: http://www.clinicaltrials.gov. Unique identifier: NCT00894803
References
- 1.Tissue Plasminogen Activator for Acute Ischemic Stroke. The National Institute of Neurological Disorders and Stroke rt-pa Stroke Study Group The N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- 2.Topol EJ, GUSTO V Investigators Reperfusion therapy for acute myocardial infarction with fibrinolytic therapy or combination reduced fibrinolytic therapy and platelet glycoprotein IIb/IIIa inhibition: the GUSTO V randomised trial. Lancet. 2001;357:1905–1914. doi: 10.1016/s0140-6736(00)05059-5. [DOI] [PubMed] [Google Scholar]
- 3.Pancioli AM, Broderick J, Brott T, Tomsick T, Khoury J, Bean J, et al. CLEAR Trial Investigators The combined approach to lysis utilizing eptifibatide and rt-PA in acute ischemic stroke: the CLEAR stroke trial. Stroke. 2008;39:3268–3276. doi: 10.1161/STROKEAHA.108.517656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adams HP, Jr, Effron MB, Torner J, Dávalos A, Frayne J, Teal P, et al. AbESTT-II Investigators Emergency administration of abciximab for treatment of patients with acute ischemic stroke: results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II) Stroke. 2008;39:87–99. doi: 10.1161/STROKEAHA.106.476648. [DOI] [PubMed] [Google Scholar]
- 5.Shaw GJ, Meunier JM, Lindsell CJ, Pancioli AM, Holland CK. Making the right choice: optimizing rt-PA and eptifibatide lysis, an in vitro study. Thromb Res. 2010;126:e305–e311. doi: 10.1016/j.thromres.2010.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Berger C, Fiorelli M, Steiner T, Schäbitz WR, Bozzao L, Bluhmki E, et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? Stroke. 2001;32:1330–1335. doi: 10.1161/01.str.32.6.1330. [DOI] [PubMed] [Google Scholar]
- 7.Broderick JP, Lu M, Kothari R, Levine SR, Lyden PD, Haley EC, et al. Finding the most powerful measures of the effectiveness of tissue plasminogen activator in the NINDS tPA stroke trial. Stroke. 2000;31:2335–2341. doi: 10.1161/01.str.31.10.2335. [DOI] [PubMed] [Google Scholar]
- 8.Tilley BC, Palesch YY, Kieburtz K, Ravina B, Huang P, Elm JJ, et al. NET-PD Investigators Optimizing the ongoing search for new treatments for Parkinson disease: using futility designs. Neurology. 2006;66:628–633. doi: 10.1212/01.wnl.0000201251.33253.fb. [DOI] [PubMed] [Google Scholar]
- 9.Herson J, Carter SK. Calibrated phase II clinical trials in oncology. Stat Med. 1986;5:441–447. doi: 10.1002/sim.4780050508. [DOI] [PubMed] [Google Scholar]
- 10.Siebler M, Hennerici MG, Schneider D, von Reutern GM, Seitz RJ, Röther J, et al. Safety of Tirofiban in acute Ischemic Stroke: the SaTIS trial. Stroke. 2011;42:2388–2392. doi: 10.1161/STROKEAHA.110.599662. [DOI] [PubMed] [Google Scholar]
- 11.Torgano G, Zecca B, Monzani V, Maestroni A, Rossi P, Cazzaniga M, et al. Effect of intravenous tirofiban and aspirin in reducing short-term and long-term neurologic deficit in patients with ischemic stroke: a double-blind randomized trial. Cerebrovasc Dis. 2010;29:275–281. doi: 10.1159/000275503. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.