Hypertension is the second leading cause of end stage renal disease (ESRD) in the United States following diabetes1. In 2009, more than half a million people in the U.S. had ESRD, with nearly 400,000 on dialysis and over 17,000 with kidney transplants2. Unfortunately, dialysis and transplantation are the only available treatment options for ESRD, making this a costly and devastating disease. A prominent pathological feature of hypertensive kidney disease is inflammation and fibrosis, characterized by fibroblast activation and excessive production of extracellular matrix, leading to destruction of renal parenchyma and progressive loss of renal function. There is growing evidence that hypertension is inextricably linked to vascular and renal inflammation. Activated T cells and macrophages have been found to infiltrate the kidneys of hypertensive animals, and this is mediated in part by chemoattractants such as monocyte chemoattractant protein-1 (MCP-1) and inflammatory cytokines such as tumor necrosis factor alpha (TNFα)3–5. Human renal biopsy samples also show increased collections of lymphocyte aggregates and interstitial inflammation in hypertensive compared to normotensive individuals6. Renal cortical and vascular endothelial nuclear factor kappa B (NFκB) has been associated with renal inflammation and hypertension-induced renal damage5, 7. Interestingly, T lymphocytes of the adaptive immune system play a critical role in hypertension and its associated vascular and renal dysfunction. A key question is whether renal inflammation is a cause of hypertension or whether hypertension causes renal inflammation. Data presented in this issue of Hypertension by Xia et al8 suggests the latter. The authors found that the chemokine, CXCL16, is a key mediator of the renal inflammation and damage induced by angiotensin II independent of blood pressure.
Chemokines are a subset of the cytokine family responsible for homing immune cells through interactions with their G-protein coupled receptors. They are categorized into subfamilies, CXC, CC, C, and CX3C, according to the number and spacing of conserved cysteine residues in the amino terminus of the protein9. Chemokines are induced by inflammatory cytokines and result in the exacerbation of the inflammatory reaction by homing of leukocytes to secondary lymphoid organs and other tissues. For example, the chemokine CX3CL1 is increased in psoriatic tissue and leads to the migration of CX3CR1 positive T cells into the psoriatic lesion9.
CXCL16 is a recently discovered cytokine belonging to the CXC chemokine family and is unique in that it combines scavenger receptor functions with properties of an inflammatory chemokine. It exists in a transmembrane as well as a soluble form. The transmembrane form is composed of a CXC chemokine domain, a mucin-like stalk, a transmembrane domain, and a cytoplasmic tail. The soluble form results from cleavage at the cell surface and is composed of the extracellular stalk and chemokine domain. The transmembrane form functions an adhesion molecule for CXCR6 expressing cells and a scavenger receptor for oxidized low density lipoprotein (LDL). The soluble form is a chemoattractant that promotes migration of CXCR6 expressing cells including T cells, monocytes, and myeloid fibroblasts. While CXCL16 has been implicated in other forms of renal disease such as lupus nephritis and anti-glomerular basement membrane nephritis10, 11, its role in hypertension and hypertensive kidney disease was not known.
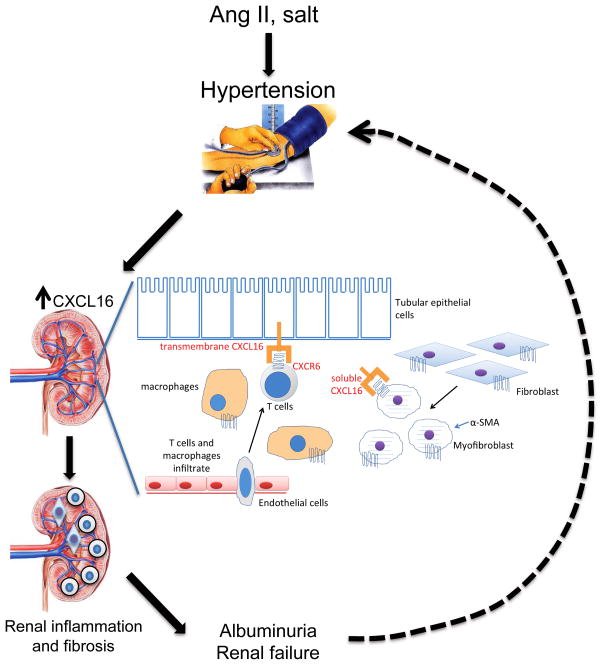
Xia et al8 found that CXCL16 was induced in renal tubular epithelial cells in response to angiotensin II in an NFκB dependent manner. Genetic deletion of CXCL16 did not affect blood pressure but protected against angiotensin II induced renal dysfunction, proteinuria, and fibrosis. CXCL16 deficiency reduced accumulation of bone marrow-derived fibroblasts, myofibroblasts, F4/80+ macrophages, and CD3+ T cells in the kidneys of angiotensin II-treated mice compared to wild type mice. Extracellular matrix proteins and pro-inflammatory cytokines were correspondingly reduced in the CXCL16 deficient mice. Thus, CXCL16 appears to mediate the renal inflammation and fibrosis that accompanies angiotensin II induced hypertension, and targeting CXCL16 may be a novel therapeutic strategy for hypertensive kidney disease. The Figure illustrates a model for the hypertensive kidney damage mediated by CXCL16.
Figure.
Hypothetical model of how CXCL16 aggravates renal inflammation and fibrosis. Hypertensive stimuli such as angiotensin II or high salt causes hypertension and elevation of CXCL16 in kidney tubular epithelial cells. Membrane bound and soluble CXCL16 attracts T cells, macrophages, and myeloid fibroblasts causing renal inflammation, fibrosis, and eventually albuminuria and renal failure. Long standing renal failure could eventually worsen hypertension, leading to a vicious cycle.
Prior work has supported a role of CXCL16 in kidney disease. In humans, elevated serum and urine CXCL16 levels were associated with the development of chronic kidney disease (CKD) and independently associated with glomerular filtration rate12. In a separate study, urine CXCL16 was elevated in several strains of mice and patients with lupus nephritis, correlating well with urine protein levels and systemic lupus erythematosus disease activity index scores10. Based on these results, it is interesting to speculate that serum or urine CXCL16 may serve as a prognostic/diagnostic marker for severity of renal dysfunction and progression to end stage renal disease in hypertensive patients.
In humans, hypertension is both a cause and consequence of long-standing renal dysfunction. In end stage renal disease, hypertension is difficult to control due to diminishing GFR and the accompanying salt and water retention. Xia et al8 found that deletion of CXCL16 had no effect on the hypertensive response to angiotensin II, at least in the short term. It is possible that the mice lacking CXCL16 might demonstrate reduced hypertension in response to a longer term exposure stimulus, given the important role of the kidney in blood pressure control.
Hypertension is intimately linked to vascular inflammation and atherosclerosis. Interestingly, CXCL16 was initially described as a macrophage scavenger receptor for oxidized low density lipoprotein13 and later found to be expressed in human vascular smooth muscle cells14. The primary receptor for CXCL16 is CXCR6. The role of CXCL16/CXCR6 in atherosclerosis is controversial. While CXCR6 deficiency confers atheroprotection, deficiency of CXCL16 in LDL receptor deficient mice was associated with accelerated atherosclerosis and enhanced macrophage recruitment15. This would suggest that CXCL16 is atheroprotective. Recently, Borst and colleagues found that CXCR6 is highly expressed in platelets and that CXCL16 stimulation enhanced platelet adhesion to the endothelium in vitro after high arterial shear stress and to injured vascular wall in vivo after carotid ligation16, suggesting that CXCL16 is prothrombotic. Thus, the role of CXCL16 signaling in atherosclerosis is still unclear and requires further investigation.
In summary the work by Xia et al8 and prior studies emphasize the role of CXCL16 in renal inflammation and injury in several different models of renal disease – lupus, anti-glomerular basement membrane disease, and now hypertensive kidney disease. Novel therapies aimed at inhibiting CXCL16 function or signaling could prove effective in preventing or delaying chronic kidney disease, but the effect of these interventions on atherosclerosis and thrombosis need to be taken into consideration, particularly given the fact that many hypertensive patients have concomitant coronary artery disease.
Acknowledgments
SOURCES OF FUNDING
Supported by a Vanderbilt Physician Scientist Development Award (to MS Madhur) and NIH PO1-HL0580000.
This is a commentary on article Xia Y, Entman ML, Wang Y. Critical role of CXCL16 in hypertensive kidney injury and fibrosis. Hypertension. 2013 Dec;62(6):1129-37.
Footnotes
DISCLOSURES
None
References
- 1.Botdorf J, Chaudhary K, Whaley-Connell A. Hypertension in cardiovascular and kidney disease. Cardiorenal medicine. 2011;1:183–192. doi: 10.1159/000329927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collins AJ, Foley RN, Chavers B, Gilbertson D, Herzog C, Johansen K, Kasiske B, Kutner N, Liu J, St Peter W, Guo H, Gustafson S, Heubner B, Lamb K, Li S, Li S, Peng Y, Qiu Y, Roberts T, Skeans M, Snyder J, Solid C, Thompson B, Wang C, Weinhandl E, Zaun D, Arko C, Chen SC, Daniels F, Ebben J, Frazier E, Hanzlik C, Johnson R, Sheets D, Wang X, Forrest B, Constantini E, Everson S, Eggers P, Agodoa L. United states renal data system 2011 annual data report: Atlas of chronic kidney disease & end-stage renal disease in the united states. American journal of kidney diseases : the official journal of the National Kidney Foundation. 2012;59:A7, e1–420. doi: 10.1053/j.ajkd.2011.11.015. [DOI] [PubMed] [Google Scholar]
- 3.Hilgers KF, Hartner A, Porst M, Mai M, Wittmann M, Hugo C, Ganten D, Geiger H, Veelken R, Mann JF. Monocyte chemoattractant protein-1 and macrophage infiltration in hypertensive kidney injury. Kidney Int. 2000;58:2408–2419. doi: 10.1046/j.1523-1755.2000.00424.x. [DOI] [PubMed] [Google Scholar]
- 4.Rodriguez-Iturbe B, Quiroz Y, Kim CH, Vaziri ND. Hypertension induced by aortic coarctation above the renal arteries is associated with immune cell infiltration of the kidneys. Am J Hypertens. 2005;18:1449–1456. doi: 10.1016/j.amjhyper.2005.05.034. [DOI] [PubMed] [Google Scholar]
- 5.Elmarakby AA, Quigley JE, Imig JD, Pollock JS, Pollock DM. Tnf-alpha inhibition reduces renal injury in doca-salt hypertensive rats. Am J Physiol Regul Integr Comp Physiol. 2008;294:R76–83. doi: 10.1152/ajpregu.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Quiroz Y, Johnson RJ, Rodriguez-Iturbe B. The role of t cells in the pathogenesis of primary hypertension. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27(Suppl 4):iv2–5. doi: 10.1093/ndt/gfs421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Henke N, Schmidt-Ullrich R, Dechend R, Park JK, Qadri F, Wellner M, Obst M, Gross V, Dietz R, Luft FC, Scheidereit C, Muller DN. Vascular endothelial cell-specific nf-kappab suppression attenuates hypertension-induced renal damage. Circ Res. 2007;101:268–276. doi: 10.1161/CIRCRESAHA.107.150474. [DOI] [PubMed] [Google Scholar]
- 8.Xia Y, Entman ML, Wang Y. Critical role of cxcl16 in hypertensive kidney injury and fibrosis. Hypertension. 2013;XX:XXX. doi: 10.1161/HYPERTENSIONAHA.113.01837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Koelink PJ, Overbeek SA, Braber S, de Kruijf P, Folkerts G, Smit MJ, Kraneveld AD. Targeting chemokine receptors in chronic inflammatory diseases: An extensive review. Pharmacol Ther. 2012;133:1–18. doi: 10.1016/j.pharmthera.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 10.Wu T, Xie C, Wang HW, Zhou XJ, Schwartz N, Calixto S, Mackay M, Aranow C, Putterman C, Mohan C. Elevated urinary vcam-1, p-selectin, soluble tnf receptor-1, and cxc chemokine ligand 16 in multiple murine lupus strains and human lupus nephritis. J Immunol. 2007;179:7166–7175. doi: 10.4049/jimmunol.179.10.7166. [DOI] [PubMed] [Google Scholar]
- 11.Garcia GE, Truong LD, Li P, Zhang P, Johnson RJ, Wilson CB, Feng L. Inhibition of cxcl16 attenuates inflammatory and progressive phases of anti-glomerular basement membrane antibody-associated glomerulonephritis. Am J Pathol. 2007;170:1485–1496. doi: 10.2353/ajpath.2007.060065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin Z, Gong Q, Zhou Z, Zhang W, Liao S, Liu Y, Yan X, Pan X, Lin S, Li X. Increased plasma cxcl16 levels in patients with chronic kidney diseases. European journal of clinical investigation. 2011;41:836–845. doi: 10.1111/j.1365-2362.2011.02473.x. [DOI] [PubMed] [Google Scholar]
- 13.Shimaoka T, Kume N, Minami M, Hayashida K, Kataoka H, Kita T, Yonehara S. Molecular cloning of a novel scavenger receptor for oxidized low density lipoprotein, sr-psox, on macrophages. J Biol Chem. 2000;275:40663–40666. doi: 10.1074/jbc.C000761200. [DOI] [PubMed] [Google Scholar]
- 14.Wagsater D, Olofsson PS, Norgren L, Stenberg B, Sirsjo A. The chemokine and scavenger receptor cxcl16/sr-psox is expressed in human vascular smooth muscle cells and is induced by interferon gamma. Biochem Biophys Res Commun. 2004;325:1187–1193. doi: 10.1016/j.bbrc.2004.10.160. [DOI] [PubMed] [Google Scholar]
- 15.Zernecke A, Shagdarsuren E, Weber C. Chemokines in atherosclerosis: An update. Arterioscler Thromb Vasc Biol. 2008;28:1897–1908. doi: 10.1161/ATVBAHA.107.161174. [DOI] [PubMed] [Google Scholar]
- 16.Borst O, Munzer P, Gatidis S, Schmidt EM, Schonberger T, Schmid E, Towhid ST, Stellos K, Seizer P, May AE, Lang F, Gawaz M. The inflammatory chemokine cxc motif ligand 16 triggers platelet activation and adhesion via cxc motif receptor 6-dependent phosphatidylinositide 3-kinase/akt signaling. Circ Res. 2012;111:1297–1307. doi: 10.1161/CIRCRESAHA.112.276444. [DOI] [PubMed] [Google Scholar]