Abstract
Relapse vulnerability in cocaine dependence is rooted in genetic and environmental determinants, and propelled by both impulsivity and the responsivity to cocaine-linked cues (‘cue reactivity'). The serotonin (5-hydroxytryptamine, 5-HT) 5-HT2C receptor (5-HT2CR) within the medial prefrontal cortex (mPFC) is uniquely poised to serve as a strategic nexus to mechanistically control these behaviors. The 5-HT2CR functional capacity is regulated by a number of factors including availability of active membrane receptor pools, the composition of the 5-HT2CR macromolecular protein complex, and editing of the 5-HT2CR pre-mRNA. The one-choice serial reaction time (1-CSRT) task was used to identify impulsive action phenotypes in an outbred rat population before cocaine self-administration and assessment of cue reactivity in the form of lever presses reinforced by the cocaine-associated discrete cue complex during forced abstinence. The 1-CSRT task reliably and reproducibly identified high impulsive (HI) and low impulsive (LI) action phenotypes; HI action predicted high cue reactivity. Lower cortical 5-HT2CR membrane protein levels concomitant with higher levels of 5-HT2CR:postsynaptic density 95 complex distinguished HI rats from LI rats. The frequency of edited 5-HT2CR mRNA variants was elevated with the prediction that the protein population in HI rats favors those isoforms linked to reduced signaling capacity. Genetic loss of the mPFC 5-HT2CR induced aggregate impulsive action/cue reactivity, suggesting that depressed cortical 5-HT2CR tone confers vulnerability to these interlocked behaviors. Thus, impulsive action and cue reactivity appear to neuromechanistically overlap in rodents, with the 5-HT2CR functional status acting as a neural rheostat to regulate, in part, the intersection between these vulnerability behaviors.
Keywords: impulsive action, 5-HT2C receptor, cocaine, cue reactivity, prefrontal cortex, RNA editing
INTRODUCTION
Cocaine dependence continues to extract considerable personal, health and societal tolls in the United States and worldwide. The cycling progressive nature of this chronic brain disorder stymies efforts to stay abstinent with vulnerability to abuse and relapse during abstinence often precipitated by impulsive behavior (Moeller et al, 2001a) and cocaine cue reactivity (Carpenter et al, 2006; Field and Cox, 2008). Impulsivity is a multifaceted construct that has been defined clinically as a predisposition toward rapid unplanned reactions to stimuli without regard to the negative consequences (Moeller et al, 2001b). Cue reactivity refers to the complex phenomenon comprising physiological (eg, heart rate), subjective (eg, craving), appetitive approach behaviors (eg, drug-seeking), and activation of specific corticostriatal subcircuits elicited by conditioned stimuli associated with motivationally salient events (Carter and Tiffany, 1999; Field and Cox, 2008; Koob and Volkow, 2010). We have recently demonstrated that levels of impulsive action correlate positively with cue reactivity as measured by attentional bias toward cocaine-associated cues in human cocaine-dependent subjects (Liu et al, 2011). There is evidence that high impulsivity (Green et al, 2009; Moeller et al, 2007) or cue reactivity (Carpenter et al, 2006) predicts reduced retention in outpatient treatment trials for cocaine dependence, and preclinical studies have established that high impulsive (HI) action predicts escalation of cocaine-taking (Dalley et al, 2007), progression to compulsive cocaine-taking (Belin et al, 2008), and punishment-resistant cocaine-seeking (Economidou et al, 2009). Further explorations are needed to delineate the inter-relationship between impulsive action and cue reactivity, and understand the potential for shared neurobiology.
Corticostriatal connectivity is linked to both impulsive action and cue reactivity with medial prefrontal cortex (mPFC) of particular import (Chen et al, 2013; Dalley et al, 2011). The mPFC is densely innervated by serotonin (5-hydroxytryptamine; 5-HT) fibers in a distinct laminar organization (Linley et al, 2013), and 5-HT neurotransmission in the mPFC has been implicated in the control of impulsive action (Dalley et al, 2002) and cocaine-seeking (Bradberry and Rubino, 2004), particularly through the 5-HT2C receptor (5-HT2CR) (Pentkowski et al, 2010). The ultimate level of functionality of the 5-HT2CR is determined by a culmination of factors, including availability of active pools of receptors at the membrane and effective coupling to and activation of G-protein-dependent and -independent downstream signaling (Millan et al, 2008). The 5-HT2CR functional status and subcellular localization can also be modulated by RNA editing, a mechanism that post-transcriptionally alters the coding properties of the RNA (Burns et al, 1997; Hoyer et al, 2002), and may impact alternative splicing mechanisms of the 5-HT2CR (Martin et al, 2013). RNA editing of 5-HT2CR reduces the efficiency of the interaction between the receptor and its signal transducers, which results in lower constitutive activity and signaling capacity (Burns et al, 1997). Thus, 5-HT2CR neurotransmission is malleable at transcriptional (eg, editing and alternative splicing), translational (eg, synthesis and post-translational modifications), and topological (eg, trafficking and protein complex composition) levels, which are elegantly controlled and responsive to internal and external stimuli.
The importance of 5-HT2CR function in the generation of impulsive action is supported by observations that the systemic administration of selective 5-HT2CR agonists or 5-HT2CR antagonists decrease and increase impulsive action, respectively (Anastasio et al, 2013a; Cunningham et al, 2012; Fletcher et al, 2011; Winstanley et al, 2004b). These effects reliably match the efficacy and directionality of the effects of these ligands in tasks that measure cocaine-seeking (Cunningham et al, 2011; Cunningham et al, 2012; Fletcher et al, 2008; Grottick et al, 2001). Despite the strong pharmacological data to support the involvement of the 5-HT2CR in these behaviors (Cunningham and Anastasio, 2013), there is currently little to no information as to whether the functional status of the 5-HT2CR in core brain circuits contributes to these vulnerability phenotypes. Here, we tested the hypothesis that dysregulation of 5-HT2CR function in mPFC may represent a mechanism that confers high inherent impulsive action (but see, Robinson et al, 2008) and cocaine cue reactivity.
The present study investigated the mPFC 5-HT2CR system as a neuromolecular driver of high inherent impulsive action and cocaine cue reactivity. The one-choice serial reaction time (1-CSRT) task, a simplified variant of the 5-CSRT task, is rapidly entrained and assesses deficits in behavioral control to appropriately withhold a prepotent response (action restraint) independent from complex visuospatial attentional processes (as in the 5-CSRT task) (Anastasio et al, 2011; Anastasio et al, 2013a; Cunningham et al, 2012; Dalley et al, 2002; Robbins, 2002). We tested the hypothesis that impulsive action and cocaine cue reactivity are interlocked constructs; the 1-CSRT task was used to identify impulsive action phenotypes in an outbred rat population before cocaine self-administration and assessment of cue reactivity in the form of lever presses reinforced by a cocaine-associated discrete cue complex during forced abstinence (FA) (Field and Cox, 2008). We then tested the hypothesis that phenotypic differences in inherent impulsive action associate with 5-HT2CR protein expression and specific patterns of 5-HT2CR mRNA variants in mPFC. Because the distribution pattern and functional status of the 5-HT2CR is also modulated by association with a repertoire of co-expressed PDZ proteins including postsynaptic density protein 95 (PSD95) (Becamel et al, 2002; Gavarini et al, 2006), we also tested the hypothesis that the protein complex formed between 5-HT2CR and PSD95 is differentially expressed in high and low impulsive (LI) action phenotypes. Finally, we tested the hypothesis that the genetic loss of 5-HT2CR in the mPFC evokes high impulsive action concomitant with high cocaine cue reactivity. Taken together, we propose that dysregulation of cortical 5-HT2CR may be a neurobiological mechanism underlying the intersection of impulsive action and cocaine cue reactivity.
MATERIALS AND METHODS
Animals
Male, Sprague–Dawley rats (n=176; Harlan, Houston, TX) weighing 250–275 g at arrival were housed two/cage under a 12-h light–dark cycle at constant temperature (21–23 °C) and humidity (40–50%). For the duration of 1-CSRT task acquisition and maintenance, rats were food restricted to 90% free-feeding weight; water was available ad libitum except during daily operant sessions. Rats were weighed daily to ensure that their body weights were maintained at 90% of free-feeding levels. During the self-administration assay, food and water were available ad libitum. All experiments were carried out in accordance with the NIH Guide for the Care and Use of Laboratory Animals (2011) and with the University of Texas Medical Branch Institutional Animal Care and Use Committee approval.
General Methods
1-Choice serial reaction time task
Procedures occurred in standard five-hole nosepoke operant chambers equipped with a houselight, food tray, and an external pellet dispenser capable of delivering 45 mg pellets (Bio-Serv, Frenchtown, NJ) housed within a ventilated and sound-attenuated chamber (MedAssociates, St Albans, VT). The 1-CSRT task methodology has been described previously (Anastasio et al, 2011; Anastasio et al, 2013a). In the pre-training stage, rats (n=176) were habituated to the test chamber and introduced to a nosepoke response for food pellets. During this stage, all responses made in the correctly lit (center) hole resulted in the illumination of the magazine light and presentation of a single food pellet. The training stages, thereafter, were each comprised of daily sessions of 100 trials to be completed in a maximum of 30 min; each training stage involved incrementally lowering the stimulus duration with a 5-s limited hold and an intertrial interval (ITI) of 5 s. Thus, a maximum of 100 correct responses in a session resulted in a maximum of 100 reinforcers delivered; incorrect or premature responses or omissions resulted in a 5-s time-out period and a reduction in potential reinforcers obtained. Rats were required to meet an acquisition criteria of a minimum of 50 correct responses, >80% accuracy (correct responses/(correct+incorrect) × 100) and <20% omissions (omitted responses/trials completed × 100) to move from one training stage to the next (see Anastasio et al, 2011 for details).
The total number of responses (premature, correct, incorrect, and omissions) were recorded (Anastasio et al, 2011; Anastasio et al, 2013a; Cunningham et al, 2012). Premature responses were used to assess impulsive action. The number of reinforcers earned provides a measure of task competency and a secondary assessment of impulsive action, while percent accuracy is a general indication of attentional capacity. Percent omissions indicate failures of detection of the visual stimuli in the center hole as well as the motivation level to perform the task.
Cocaine self-administration assay
Rats (n=64) were anesthetized (i.m.) with a cocktail containing xylazine (8.6 mg/kg), acepromazine (1.5 mg/kg), and ketamine (43 mg/kg) in bacteriostatic saline. The catheter was inserted into the right jugular vein and exited dorsally (Cunningham et al, 2011; Cunningham et al, 2012). Daily flushes with a solution of heparinized saline (10 U/ml) with streptokinase (0.67 mg/ml) and ticarcillin disodium (66.67 mg/ml) were performed. Rats were allowed 7 recovery days after surgery before initiation of cocaine self-administration.
Self-administration studies took place in standard operant chambers equipped with two retractable levers, a stimulus light above each lever, and a houselight opposite the levers housed within a ventilated and sound-attenuated chamber (MedAssociates). Cocaine self-administration sessions were initiated 7 days post catheterization and consisted of daily 180-min sessions during which rats were trained to lever press for a cocaine infusion (0.75 mg/kg/inf) on a fixed-ratio (FR) FR1 schedule of reinforcement before progressing to an FR5. Schedule completions on the active lever resulted in simultaneous illumination of the house and stimulus lights, and activation of the infusion pump (discrete cue complex paired with delivery of cocaine); responses on the inactive lever were recorded but had no scheduled consequences. Cocaine infusions were delivered by a syringe attached to an infusion pump located outside the chamber. (−)-Cocaine (NIDA) was dissolved in 0.9% saline. After stable self-administration was established, rats were returned to their home cages, weighed and handled daily for 14 days of FA. On FA day 14, rats were reintroduced to the chambers for a single cue reactivity test session, and responses on the previously active lever were reinforced by presentation of the discrete cue complex on an FR1 (cue-reinforced lever presses). The responses recorded are therefore under the control of a stimulus complex that includes the contextual cues plus the discrete cue complex (light and sound of infusion pump), previously associated with cocaine delivery; inactive lever presses were recorded but produced no scheduled consequences.
5-HT2CR protein analysis: immunoblotting and immunoprecipitation
The mPFC was homogenized in 10X w/v extraction buffer (10 mM HEPES, 1 mM EDTA, 2 mM EGTA, 1 mM DTT, 10 μl/ml protease inhibitor cocktail and 10 μl/ml phosphatase inhibitor cocktails 2 and 3 (Sigma-Aldrich, St Louis, MO). The homogenate was centrifuged at 1000 g for 10 min at 4 °C to pellet the nuclear fraction. The supernatant was removed and centrifuged at 20 000 g at 4 °C for 30 min to pellet the membrane-bound protein fraction. The membrane-enriched pellet was washed once and then resuspended in 200 μl resuspension buffer (20 mM HEPES, 400 mM NaCl, 1 mM EDTA, 1 mM EGTA, 1 mM DTT, protease and phosphatase inhibitors, and 0.5% dodecyl maltoside) (Anastasio et al, 2010; Anastasio et al, 2013a).
Equal amounts of mPFC membrane protein were separated by SDS-PAGE and transferred to a PVDF membrane for immunoblotting with the following antibodies: 5-HT2CR (sc-17797, 1 : 100; Santa Cruz Biotechnology, Dallas, TX), β-actin (MAB1501, 1 : 10000; Millipore, Billerica, MA), PSD95 (MAB1598, 1 : 1000; Millipore), or pan-cadherin (AB6528, 1 : 10000; Abcam, Cambridge, MA) (Anastasio et al, 2010; Anastasio et al, 2013a). Membranes were incubated with mouse IgG IRDye800 (1 : 10000) for detection by Odyssey Imaging System (LI-COR, Lincoln, NE). The integrated intensity of each band was analyzed with the Odyssey Software and normalized to pan-cadherin or β-actin immunoreactivity.
Co-immunoprecipitation methodology was used to assess the 5-HT2CR protein complex with PSD95 in the mPFC of HI and LI rats; a synaptosomal preparation enriched for pre- and postsynaptic proteins (ie, presynaptic terminals, postsynaptic membranes, postsynaptic density, and synaptic protein complexes) was used (Anastasio et al, 2010). Briefly, the mPFC was homogenized (10X w/v) in ice-cold Krebs buffer containing 0.32 M sucrose plus protease inhibitor cocktail and phosphatase inhibitor 2 and 3 cocktails (10 μl/ml, Sigma-Aldrich). The homogenate was then centrifuged at 1000 g at 4 °C for 10 min. The supernatant was collected and centrifuged at 20 000 g at 4 °C for 30 min to pellet the crude synaptosomes; the resultant pellet was resuspended in Krebs buffer plus 1% dodecyl maltoside. The PSD95 antibody was covalently crosslinked onto protein A/G resin as previously described with minor modifications (Anastasio et al, 2010; Anastasio et al, 2013a). Synaptosomal protein was incubated with the antibody-crosslinked resin for 48 h at 4 °C with constant shaking. The eluted protein was resuspended in Laemmli buffer and subjected to SDS-PAGE. Immunoblotting for 5-HT2CR and PSD95 was performed as described above.
5-HT2CR mRNA analyses: isolation and isoform profile analyses
The mPFC was homogenized in TRI Reagent and RNA isolated using the RiboPure kit (Life Technologies, Grand Island, NY) (Lanfranco et al, 2009). Reverse transcription was performed on 250 ng RNA using SuperScript III First Strand Synthesis System (Life Technologies) with random hexamer primers. RT-PCR reactions were assayed in triplicate on a 7500 Fast RT PCR System using TaqMan Fast Advanced Master Mix and TaqMan gene-specific primer/probes (Htr2c: Rn00562748_m1; Cyclophilin A (Ppia): Rn00690933_m1; Life Technologies). Data are presented in terms of crossing threshold (Ct), which was calculated as ΔCt=Ct(Htr2c)–Ct(Cyclophilin).
Quantification of 5-HT2CR mRNA isoform profiles was determined using a high-throughput sequencing strategy (Morabito et al, 2010). First-strand cDNA was synthesized with an 5-HT2CR-specific antisense primer containing the adapter sequence required for the Illumina sequencing platform (5′-CAAGCAGAAGACGGCATACGAGCTCTTCCGATCTATCAAAGCTTGACGGCGTAGGACGTAG-3′ lllumina adapter sequence is underlined) using avian myoblastosis virus reverse transcriptase (Promega, Madison, WI). The total reverse-transcription reaction was amplified by PCR with Phusion DNA polymerase (Finnzymes, Woburn, MA) using the same antisense primer and one of the twenty-four 5-HT2CR-specific sense primers containing a six nucleotide bar-code (NNNNNN) that allowed for multiplex sample analysis and an adapter sequence (single underline) with a region complementary to the standard Illumina sequencing primer (double underline) (5′-AATGATACGGCGACCACCGAGATCTACACTCTTTCCCTACACGACGCTCTTCCGATCT NNNNNNGCTGGACCGGTATGTAGCA-3′). Amplified products were separated on a 2% agarose gel and purified from excised gel slices using the Wizard SV Gel and PCR Purification Kit (Promega); PCR amplicons corresponding to alternatively spliced 5-HT2CR RNA isoform 2 (286 bp) were isolated for all experiments. The concentration of gel-purified fragments was measured by spectrophotometry (A260) and ∼20 ng of each individual sample was pooled with other products containing unique bar codes and subjected to single-end sequencing with the Illumina Genome Analyzer (Illumina, San Diego, CA) (Bentley et al, 2008). Data were filtered (Morabito et al, 2010) using a 72 nucleotide mouse 5-HT2CR reference sequence (5′-NNNNNNATTAACCCTCACTAAAGGGAGCTGGACCGGTATGTAGCARTRCGTRRTCCTRTTGAGCATAGCCGG-3′ bar code sequence position indicated in bold N) that allowed for either an adenosine/guanosine at the five editing sites (R, purine).
shRNA design and production
A 24-nucleotide sequence within the coding region of the Htr2c was identified using methods we have previously reported (Hommel et al, 2003). Two sets of oligonucleotides (Integrated DNA Technology, Coralville, IA) for cloning were synthesized (Htr2c shRNA (top, 5′-TTGAATCCAGACGGGGCACAAATATCCTTCCTGTCAGATATTTGTGCCCCGTCTGGATTATTTTT-3′ bottom, 5′-CTAGAAAAATAATCCAGACGGGGCACAAATATCTGACAGGAAGGATATTTGTGCCCCGTCTGGATTC-3′); non-silencing control (NSC) shRNA (top, 5′-TTTGTGGAGCCGAGTTTCTAAATTCCGCTTCCTGTCACGGAATTTAGAAACCCGGCTCCAATTTTT-3′ bottom, 5′-CTAGAAAAATTGGAGCCGGGTTTCTAAATTCCGTGACAGGAAGCGGAATTTAGAAACTCGGCTCCAC-3′)). Oligonucleotides were designed with Sap1 and Xbal overhangs to allow ligation downstream of the mU6pro region of a modified pAAV-MCS vector, pAAV-shRNA, which was designed to coexpress hairpin RNAs, under the control of a mU6pro and an SV40 polyadenylation site, as well as eGFP controlled by an independent CMV promoter and hGH polyadenylation sequence (Hommel et al, 2003). To assess the extent of 5-HT2CR knockdown in vitro, HEK293 cells were transiently co-transfected with the 5-HT2CR and the 5-HT2CR shRNA plasmid DNA or empty vector (1 : 10 μg); at 72 h post transfection, cells were harvested, RNA was extracted and RT-PCR was performed to quantify 5-HT2CR mRNA expression as described above. Adeno-associated viral (AAV) serotype type 2 vectors were packaged using a helper-free packaging system (Life Technologies) and purified viral stocks were assayed in camptothecin-treated HT1080 cells to confirm titers of 1-2 × 1011 transducing units/ml.
Viral-mediated gene transfer
Rats (n=48) were anesthetized (i.m.) with a cocktail containing xylazine (8.6 mg/kg), acepromazine (1.5 mg/kg), and ketamine (43 mg/kg) in bacteriostatic saline and placed in a stereotaxic apparatus with the upper incisor bar at −3.8 mm below the interaural line. Two microsyringes (28 gauge, Hamilton Company, Reno, NV) were lowered bilaterally at 15° from the midsaggital plane relative to bregma (Paxinos and Watson, 1998) to target the mPFC encompassing the ventral prelimbic and dorsal infralimbic subnuclei (Supplementary Figure 2); the coordinates were anteroposterior +3 mm, mediolateral +1.8 mm, and dorsoventral −5.1 mm from the skull. The NSC shRNA-eGFP AAV (‘control' 1.5 μl) or 5-HT2CR shRNA-eGFP (‘5-HT2CR knockdown' 1.5 μl) AAV vectors were infused bilaterally at 0.1 μl/min over 15 min. Rats were allowed 3 weeks to recover and to allow for stable transgene expression. AAV infection has been well-characterized with stabilization of gene expression in rodent brain at 3 weeks and with stability for at least 12–18 months post infection (Daly, 2004; Leff et al, 1999).
Research Design
Identification of impulsive action phenotypes
(Cohorts 1–4; see experimental timelines, Figures 1, 2, 3). Rats (n=128) were trained on the 1-CSRT task and were required to meet the acquisition criteria of a minimum of 50 correct responses, >80% accuracy and <20% omissions on the final training stage (0.5 s stimulus duration, 5 s limited hold, and 5 s ITI, ITI5) before being tested on an ITI8 challenge session (0.5 s stimulus duration, 5 s limited hold, and 8 s ITI) to more easily detect phenotypic differences in inherent impulsive action (Besson et al, 2013; Dalley et al, 2002). HI and LI rats were stratified as the upper and lower quartile based on the number of premature responses made during the ITI8 challenge session.
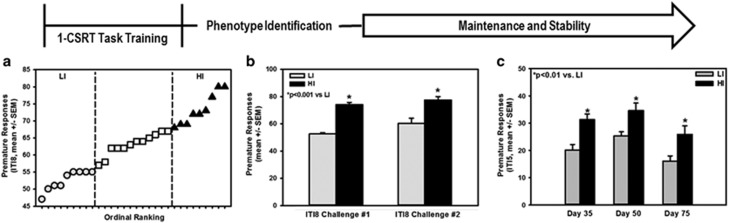
Figure 1.
Impulsive action phenotypes are identifiable in an outbred rat population. Rats were required to meet an acquisition criteria of a minimum of 50 correct responses, >80% accuracy and <20% omissions on the final one-choice serial reaction time (1-CSRT) task training stage (0.5 s stimulus duration, 5 s limited hold and 5 s intertrial interval, ITI5) before being tested on an ITI8 challenge session to more easily detect phenotypic differences in inherent impulsive action. (a) The number of premature responses made during the ITI8 challenge session was used to stratify rats as high impulsive (HI) or low impulsive (LI) relative to rats in the interquartile range. (b) HI rats exhibited higher numbers of premature responses vs LI rats on two separate ITI8 challenge sessions conducted when the cohort initially met criteria (ITI8 challenge #1; day 25–30 on average) and then upon retest (ITI8 challenge #2; day 92 of 1-CSRT task maintenance), respectively (*p<0.001 vs LI). (c) The impulsive phenotype was identifiable and stable throughout >75 days of 1-CSRT sessions (ITI5) (HI vs LI at day 35 (*p<0.05 vs LI), day 50 (*p<0.05 vs LI) and day 75 (*p<0.05 vs LI)).
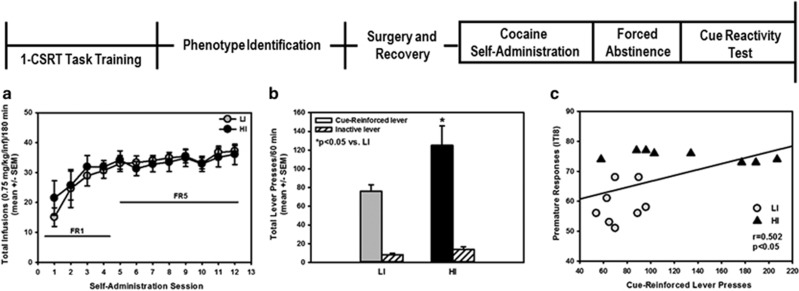
Figure 2.
Inherent impulsive action predicts cocaine cue reactivity. Following phenotype identification, the one-choice serial reaction time (1-CSRT) task sessions were terminated, and HI rats and LI rats were retained in their home cages and allowed to free-feed for 7 days before surgical implantation of a jugular catheter. (a) In daily 180 min sessions, HI and LI rats readily acquired cocaine self-administration (0.75 mg/kg/0.1 ml infusion) to stability. (b) On forced abstinence (FA) day 14, HI rats exhibited a higher number of previously active (*p<0.05 vs LI), but not inactive lever presses (n.s.), during the cue reactivity test session vs LI rats. (c) There was a positive correlation between premature responses on the 1-CSRT task and cue-reinforced lever presses during the cue reactivity test session for individual subjects (r=0.502; p<0.05).
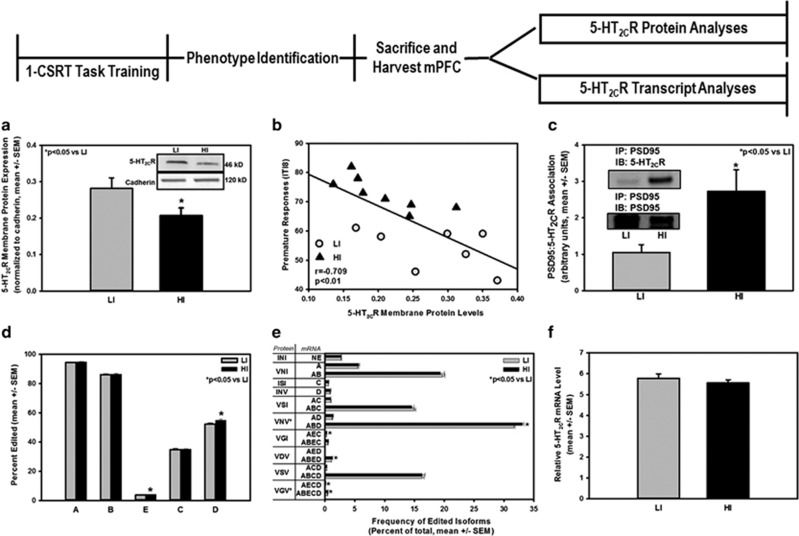
Figure 3.
Functional status of the 5-HT2CR is a neuromolecular substrate for inherent impulsive action. Following phenotype identification in two separate cohorts of rats, the one-choice serial reaction time (1-CSRT) task sessions were terminated, and HI and LI rats were retained in their home cages (food restriction was maintained) and killed within 2–3 days for ex vivo neurochemical analyses of 5-HT2CR protein and mRNA. (a) Qualitative (inset) and quantitative demonstration that HI rats expressed lower levels of membrane medial prefrontal cortex (mPFC) 5-HT2CR vs LI rats (*p<0.05 vs LI). (b) There was an inverse correlation between mPFC 5-HT2CR membrane protein levels and premature responding on the 1-CSRT task for individual subjects (r=−0.709; p<0.01). (c) Immunoprecipitation (IP) for postsynaptic density 95 (PSD95) followed by immunoblot (IB) for 5-HT2CR yielded 5-HT2CR immunoreactivity in both HI and LI rats (inset). Synaptosomal 5-HT2CR associated with PSD95 in the mPFC to a greater extent in HI vs LI rats (*p<0.05 vs LI); comparable levels of PSD95 were immunoprecipitated between HI and LI rats (inset). (d) The 5-HT2CR mRNA editing efficiency was significantly different in HI vs LI rats; editing at the E and D sites was higher in HI vs LI rats (*p<0.05). (e) The frequencies of ABD, AEC, ABED, AECD, and ABECD 5-HT2CR mRNA variants were elevated in HI rats compared with LI rats. HI rats expressed higher levels of predicted protein isoforms VNV and VGV vs LI rats (*p<0.05 vs LI). (f) Total mPFC 5-HT2CR mRNA levels as assessed by RT-PCR did not differ between HI and LI rats (n.s.).
Inherent impulsive action predicts cocaine cue reactivity
(Cohort 2; see experimental timeline, Figure 2). Following phenotype identification, the 1-CSRT task sessions were terminated and HI rats (n=8) and LI rats (n=8) were retained in their home cages and allowed to free-feed for 7 days before surgical implantation of a jugular catheter. Cocaine self-administration commenced 7 days post catheterization; cue reactivity was assessed on FA day 14.
Functional 5-HT2CR status in impulsive action phenotypes
(Cohorts 3 and 4; see experimental timeline, Figure 3). Following phenotype identification in two separate cohorts of rats (n=64), the 1-CSRT task sessions were terminated and HI (n=16) and LI rats (n=16) were retained in their home cages (food restriction was maintained). Within 2–3 days, rats were anesthetized (chloral hydrate, 400 mg/kg) and decapitated, and the brain was microdissected immediately, flash frozen in liquid nitrogen, and stored at −80 °C for subsequent protein (n=16) (Anastasio et al, 2010; Anastasio et al, 2013a; Liu et al, 2007) or RNA analysis (n=16) (Lanfranco et al, 2009).
Genetic loss of mPFC 5-HT2CR confers aggregate impulsive action/cocaine cue reactivity
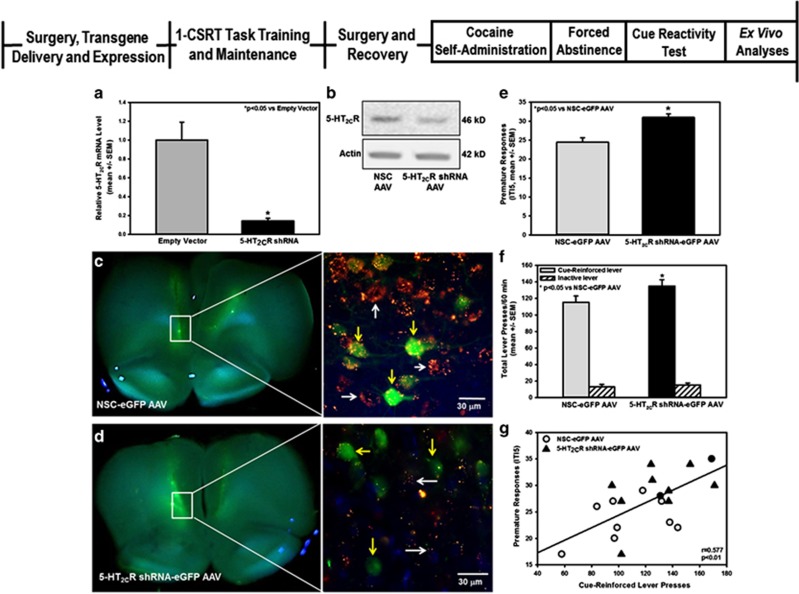
(Cohort 5; see experimental timeline, Figure 4). Following intra-mPFC transgene delivery and stable viral vector expression, control and 5-HT2CR knockdown rats (n=48) were trained to criteria on the 1-CSRT task. Upon completion of assessments on the final 1-CSRT task training stage (0.5 s stimulus duration, 5-s limited hold, ITI5), control and 5-HT2CR knockdown rats were retained in their home cages and allowed to free-feed for 7 days before surgical implantation of a jugular catheter. Cocaine self-administration commenced 7 days post catheterization; cue reactivity was assessed on FA day 14. At the termination of the cue reactivity test, rats were removed from the operant chambers and returned to their home cages. Seven days later, rats were anesthetized (chloral hydrate; 400 mg/kg, i.p.) and killed, and tissue samples were taken for visualization, immunoblot analyses, or immunohistochemical verification of 5-HT2CR protein knockdown. A 1-mm coronal section containing the mPFC was placed on a cold glass slide and rapid visualization of eGFP ex vivo was accomplished with a DFP-1 Dual Fluorescent Protein Flashlight by the investigator wearing a pair of VG2 barrier filter glasses (Nightsea, Bedford, MA) (Li and Wolf, 2011). Photomicrographs of coronal sections were taken with a DSLR camera equipped with a macro lens and yellow filter (Li and Wolf, 2011). Fluorescent regions from the mPFC were then microdissected and assayed for immunoblotting to assess knockdown ex vivo. A subset of rats (n=6) were anesthetized (sodium pentobarbital; 100 mg/kg, i.p.) and perfused transcardially with 3% paraformaldehyde for immunohistochemical analyses (Bubar et al, 2011). Brains were removed, post fixed (2 h), and cryoprotected in 30% sucrose solution. Free-floating coronal sections at the level of the mPFC (30 μm) were incubated in 0.5% sodium borohydride to reduce autofluorescence. Sections were blocked (1.5% normal goat serum in 0.4% triton-PBS) before incubation with 5-HT2CR antibody (1 : 100; 2 h 25 °C, 18 h 4 °C) followed by AlexaFluor 555 to mouse IgG (A21424, 1 : 2000; Life Technologies). Slides were coverslipped with Vectashield fluorescent mounting medium with DAPI (Vector Laboratories, Burlingame, CA).
Figure 4.
Genetic loss of 5-HT2CR in medial prefrontal cortex (mPFC) confers aggregate impulsive action/cue reactivity. (a) The shRNA expression plasmid targeting the 5-HT2CR efficiently silenced 5-HT2CR mRNA in a cell line transiently expressing 5-HT2CR (*p<0.05 vs LI). (b) The 5-HT2CR shRNA-eGFP AAV knocked down 5-HT2CR protein levels in the mPFC (∼50% decrease) relative to the NSC-eGFP AAV. (c) Stereotaxic placement and NSC-eGFP AAV infection (green) in mPFC (left). The NSC-eGFP AAV (green) did not alter 5-HT2CR protein expression (red) in infected neurons (yellow arrows) relative to non-AAV infected neurons (white arrows) (right). (d) Stereotaxic placement and 5-HT2CR shRNA-eGFP AAV infection (green) in mPFC (left). The 5-HT2CR shRNA-eGFP AAV (green) induced a significant knockdown of 5-HT2CR protein (red) in infected neurons (yellow arrows) relative to non-infected neurons (white arrows) (right). (e) Following intra-mPFC transgene delivery and stable viral vector expression, control and 5-HT2CR knockdown rats were subjected to the one-choice serial reaction time (1-CSRT) task. The 5-HT2CR knockdown rats expressed significantly higher premature responses vs control rats on the final 1-CSRT task training stage (0.5 s stimulus duration, 5 s limited hold and 5 s intertrial interval (ITI5)) (*p<0.05 vs NSC-eGFP AAV). Upon completion of 1-CSRT task assessments, control and 5-HT2CR knockdown rats were retained in their home cages and allowed to free-feed for 7 days before surgical implantation of a jugular catheter. Cocaine self-administration commenced 7 days post catheterization. (f) On forced abstinence (FA) day 14, 5-HT2CR knockdown rats exhibited higher cue-reinforced lever presses (*p<0.05 vs NSC-eGFP AAV), but not inactive, lever presses (n.s.), during a cue reactivity test session vs control rats. (g) There was a positive correlation between premature responses and cue-reinforced lever presses during the cue reactivity test session for individual subjects (r=0.577; p<0.01).
Statistical Analyses
Outcome measures from the 1-CSRT task, cocaine self-administration and cue reactivity assays, as well as 5-HT2CR protein and mRNA analyses, were assessed by Student's t-test or ANOVA as appropriate to research design (Anastasio et al, 2011; Anastasio et al, 2013a; Cunningham et al, 2012; Keppel, 1973). Based upon preliminary data and published literature, a priori group comparisons were specifically defined before the start of the experiment and were assessed with the Tukey test (for multiple pairwise comparisons of means) (Keppel, 1973). To analyze the relationship between premature responses and cue-reinforced lever presses, or premature responses and protein expression, we used Pearson's partial correlation (Keppel, 1973). These analyses were performed in SAS (version 9.3) with an experimentwise error rate of α=0.05.
RESULTS
Impulsive Action Phenotypes are Stable in the 1-CSRT Task
Four separate cohorts of outbred rats were trained on the 1-CSRT task and, within each cohort (n=32), HI (n=7-8/cohort) and LI rats (n=7-9/cohort) were stratified as the top and bottom 25% of rats based upon premature responses on the ITI8 challenge session. Supplementary Table 1 illustrates the consistency of premature responses, reinforcers earned, accuracy and percent omissions on the ITI8 challenge observed across the four cohorts of HI and LI rats used for these analyses. There was no main effect of cohort on premature responses for LI rats (F(3,27)=2.47, n.s.) or HI rats (F(3,27)=2.47, n.s.; Supplementary Table 1). Accuracy averaged 95–99% for all cohorts and did not differ between HI and LI rats; levels of premature responses, reinforcers earned, and percent omissions were consistently different in HI vs LI rats in all four cohorts (statistical analyses presented in Supplementary Table 1). The results across the four cohorts support the utility, reliability, and consistency of the 1-CSRT task to identify phenotypic differences in impulsive action.
Cohort 1 was used to test the hypothesis that individual differences in levels of impulsive action were observable and stable on the 1-CSRT task (Figure 1a–c; experimental timeline). The ordinal distribution of individual rats in Cohort 1 are plotted by premature responses to illustrate the upper (HI) and lower (LI) quartile of rats relative to those in the interquartile range (Figure 1a). HI rats exhibited higher numbers of premature responses vs LI rats on two separate ITI8 challenge sessions conducted when each rat initially met criteria (25–30 sessions) and then upon retest on session 92, respectively (Figure 1b; p<0.05). For rats phenotypically identified on the ITI8 challenge session, impulsive action was significantly higher in HI vs LI rats on their ITI5 maintenance sessions on day 35 (p<0.05), day 50 (p<0.05), and day 75 (p<0.05) (Figure 1c). Both HI and LI rats exhibited characteristically high accuracy and low omissions (Supplementary Table 1), indicating that all rats detected the task stimuli and performed effectively. HI rats earned fewer reinforcers and displayed lower percent omissions relative to LI rats (Supplementary Table 1), suggesting that HI rats may have greater motivational drive to perform the task vs LI rats (Frijda, 2010). Taken together, these data support the identification of a stable, trait-like impulsive action phenotype in an outbred rat population.
Impulsive Action and Cocaine Cue Reactivity are Interlocked Phenotypes
Cohort 2 was used to test the hypothesis that inherent impulsive action predicted levels of cue reactivity as measured by cue-reinforced lever presses (Figure 2, experimental timeline). After screening in the 1-CSRT task, HI and LI rats were trained to self-administer cocaine and then subjected to a 14-day FA. HI and LI rats readily acquired cocaine self-administration to stability (Figure 2a; Supplementary Figure 1); across the last three sessions, there was no main effect of phenotype (F(1,47)=0.56, n.s.), session (F(2,47)=0.99, n.s.), or a phenotype × session interaction (F(2,47)=1.00, n.s.) for the total number of infusions (Figure 2a). Average total daily cocaine intake over the last three self-administration sessions did not differ between phenotypes (HI=10.3±1.1 mg/kg/day; LI=9.4±1.7 mg/kg/day; n.s.). On FA day 14, HI rats exhibited higher cue-reinforced (Figure 2b; p<0.05), but not inactive, lever presses (Figure 2b; n.s.) vs LI rats; a positive correlation was observed between premature responses and cue-reinforced lever presses in individual rats (Figure 2c; r=0.502; p<0.05). Notably, LI rats (gray circles) expressed the lowest levels of cue reactivity and did not overlap with HI rats (black triangles) (Figure 2c). In contrast, HI rats consistently exhibited high levels of cue reactivity with exceedingly high levels (⩾180–210 lever presses/60 min) expressed in some HI rats (Figure 2c). Thus, inherent impulsive action predicted the level of cocaine cue reactivity in an outbred strain of rats.
Impaired 5-HT2CR Functional Status may Underlie Impulsive Action
The ultimate level of functionality of the 5-HT2CR is determined by a culmination of factors, including the availability of active pools of receptors in the synaptic compartment and effective coupling to, and activation of, downstream signaling and/or scaffolding components. In Cohorts 3 and 4 of HI and LI rats, we evaluated 5-HT2CR protein and transcript expression in the mPFC to gain insight into the functionality of this receptor as a neuromolecular substrate for impulsive action.
In Cohort 3, we tested the hypothesis that HI and LI rats would exhibit differential expression of the 5-HT2CR protein in a crude membrane preparation of the mPFC. We observed that 5-HT2CR membrane protein expression in the mPFC was lower in HI vs LI rats (Figure 3a; p<0.05) and that 5-HT2CR membrane protein expression in individual rats was inversely correlated to premature responses (Figure 3b; r=−0.709; p<0.01). Notably, levels of 5-HT2CR protein expression in HI rats did not overlap with those in LI rats (Figure 3b).
The 5-HT2CR in the membrane exists in a macromolecular complex (Becamel et al, 2002) and one key regulatory partner is PSD95, which, through its association with the 5-HT2CR, promotes internalization and desensitization of the receptor, thereby attenuating the responsivity of 5-HT2CR signaling (Abbas et al, 2009; Becamel et al, 2002; Gavarini et al, 2006). Thus, we tested the hypothesis that, in addition to lower membrane 5-HT2CR in HI vs LI rats, HI rats may present with a greater association of PSD95 with 5-HT2CR, which would be expected to further reduce the responsivity of 5-HT2CR signaling. We used co-immunoprecipitation techniques to assess this physical association of PSD95 with the 5-HT2CR in the synaptosomal fraction of the mPFC of HI and LI rats. The synaptosome contains the presynaptic molecular machinery for the uptake, storage, and release of neurotransmitters, as well as the postsynaptic milieu that is composed of receptors and downstream effectors of neuronal transmission without contamination from membranous organelles found in crude membrane protein fractions (Breukel et al, 1997). Immunoprecipitation (IP) for PSD95 followed by immunoblot (IB) for 5-HT2CR yielded 5-HT2CR immunoreactivity in both HI and LI rats (Figure 3c), indicating that a 5-HT2CR:PSD95 protein complex is assembled in native mPFC tissue and can be examined ex vivo (Anastasio et al, 2010). In addition, HI rats exhibited higher levels of the 5-HT2CR:PSD95 complex in the synaptosomal fraction of the mPFC vs LI rats (Figure 3c; p<0.05). These data suggest that the functionality of available receptors may be reduced by higher coupling of PSD95 to the 5-HT2CR in the mPFC of HI vs LI rats (Abbas et al, 2009; Becamel et al, 2002; Gavarini et al, 2006). Immunoblot for PSD95 after IP for PSD95 indicated that comparable levels of PSD95 protein were immunoprecipitated between HI and LI rats (Figure 3c). No difference in synaptosomal PSD95 protein levels in the mPFC were detected between HI (9.9±0.4 arbitrary units) and LI rats (11.6±1.4 arbitrary units; n.s.). Taken together, the lower 5-HT2CR membrane protein expression and the higher 5-HT2CR:PSD95 complex in mPFC of HI rats suggest that reduced responsivity of the 5-HT2CR in mPFC may confer in part high inherent impulsive action.
The functional capacity of the 5-HT2CR protein and its signaling network is regulated by editing of the 5-HT2CR pre-mRNA, which generates up to 32 mRNA isoforms that encode up to 24 predicted receptor protein isoforms (Burns et al, 1997; Herrick-Davis et al, 1999; Marion et al, 2004). Editing drives localization of the receptor (eg, cellular membrane vs endocytic vesicles), constitutive activity and trafficking, and responsiveness to ligands with the degree of editing generally associated with the degree of receptor stimulation (Marion et al, 2004); editing can also influence alternative splicing of the 5-HT2CR (Martin et al, 2013). The nonedited isoform (5-HT2C-INIR) exhibits the highest constitutive activity, whereas the fully edited isoform (5-HT2C-VGVR) is incapable of coupling to its G protein and exhibits no constitutive activity (Burns et al, 1997; Hoyer et al, 2002; Marion et al, 2004). Partially edited isoforms exhibit profiles between these two extremes. Although these multiple isoforms are predicted as key in 5-HT2CR function, there are no published methods to separately analyze expression of isoform-specific proteins; however, high-throughput sequencing provides the opportunity to evaluate expression of mRNA for all 5-HT2CR isoforms across phenotypes (Morabito et al, 2010; Zhu et al, 2012).
In Cohort 4, we tested the hypothesis that the editing efficiency and 5-HT2CR mRNA profiles would distinguish inherent impulsive action phenotypes, providing a window on the predicted protein isoforms that may contribute to differential 5-HT2CR protein expression seen in HI vs LI rats. RNA extracted from the mPFC collected from HI and LI rats was analyzed for efficiency of editing at each of the five editing sites (A, B, E, C, D) as well as the frequency of mRNA variants using high-throughput multiplexed transcript analysis (Morabito et al, 2010; Zhu et al, 2012); all 32 possible mRNA variants were detected in the rat mPFC (Supplementary Table 2). A main effect of phenotype (F(1,56)=9.86, p<0.01), site (F(4,56)=22744.6, p<0.001), and a phenotype × site interaction (F(4,56)=3.22, p<0.05) on editing efficiency at the five editing sites was observed; HI rats expressed higher levels of editing at the E and D sites vs LI rats (Figure 3d; p<0.05). We observed a trend toward a main effect of phenotype (F(1,399)=4.00, p=0.06), a main effect of mRNA variant (F(31,399)=4372.9, p<0.001), and a phenotype × mRNA variant interaction (F(31,399)=1.58, p<0.05) on the frequency of expressed mRNA variants; HI rats expressed higher levels of mRNA variants ABD, ABECD, ABED, AEC, and AECD vs LI rats (Figure 3e; p<0.05), all of which include editing at the E and/or D sites.
The 32 possible 5-HT2CR mRNA variants can lead to a predicted 24 resultant 5-HT2CR protein isoforms (Supplementary Table 2). A main effect of phenotype (F(1,313)=5.3, p<0.05), a main effect of predicted protein isoform (F(23,313)=4452.58, p<0.001) and a trend toward a phenotype × predicted protein isoform interaction (F(23,313)=1.43, p=0.09) was observed on the frequency of predicted protein isoforms; the frequency of the predicted isoforms for the most abundant, partially edited 5-HT2C-VNVR (encoded by AD and ABD), and the fully edited 5-HT2C-VGVR (encoded by AECD and ABECD) were higher in HI vs LI rats (Figure 3e; p<0.05). Although statistical differences in the AEC and ABED mRNA variants between HI and LI rats were detected, there was no difference in the predicted protein isoforms (5-HT2C-VGIR and 5-HT2C-VDVR, respectively) in HI vs LI rats. A probe spanning the boundary of exons 4 and 5 of translated mRNA was used to detect total 5-HT2CR (full-length and all splice variants) mRNA levels in the mPFC of HI and LI rats; total 5-HT2CR mRNA levels did not differ between HI and LI rats (Figure 3f; n.s.), suggesting that differential protein expression profiles in HI vs LI rats (above) is under the regulatory control of post-transcriptional, rather than transcriptional, mechanisms. Thus, the frequency of edited 5-HT2CR mRNA variants was elevated with the predicted protein population in HI rats, favoring the isoforms linked to reduced signaling capacity and constitutive activity.
Loss of 5-HT2CR in mPFC Evokes Aggregate Impulsive Action/Cocaine Cue Reactivity
Cohort 5 was used to test the hypothesis that genetic knockdown of the 5-HT2CR in the mPFC in behavioral- and drug-naive rats would recapitulate high inherent impulsive action and cocaine cue reactivity (Figure 4, experimental timeline). To study the functional consequences of disrupted 5-HT2CR function directly in the mPFC in vivo, we used an RNA interference strategy (Hommel et al, 2003) to engineer AAV vectors to suppress all endogenous 5-HT2CR RNA variants. In vitro analyses indicated that the selected 5-HT2CR shRNA plasmid effectively silenced 5-HT2CR RNA expression vs the empty vector in HEK293 cells expressing the 5-HT2CR (Figure 4a; p<0.05). Ex vivo analyses indicated that after microinfusion into the mPFC, the 5-HT2CR shRNA-eGFP AAV curtailed 5-HT2CR protein production (∼50% decrease) relative to the NSC-eGFP AAV (Figure 4b). Cross-sections through the mPFC of a rat infused with NSC-eGFP AAV (Figure 4c) or 5-HT2CR shRNA-eGFP AAV (Figure 4d) demonstrated reduced 5-HT2CR immunoreactivity in infected neurons (compare yellow arrows in Figure 4c and d); analysis of microinfusion placements in mPFC of individual rats indicated that viral infection was localized within the mPFC along the border of the infralimbic/prelimbic subnuclei (Supplementary Figure 2).
Knockdown of the 5-HT2CR in the mPFC enhanced premature responses in the 1-CSRT task vs control (Figure 4e; p<0.05). Descriptive statistics for the effects of 5-HT2CR knockdown on additional 1-CSRT task measures are presented in Supplementary Figure 3. Of note, the number of reinforcers earned, accuracy, percent omissions, and latency to start the task did not differ between knockdown and control rats (Supplementary Figure 3), suggesting that the 5-HT2CR in the mPFC selectively regulates prepotent responding in the 1-CSRT task, a key facet of impulsive action. Both 5-HT2CR knockdown and control rats readily acquired cocaine self-administration to stability; across the last three sessions of stable self-administration, no main effect of phenotype (F(1,81)=0.63, n.s.), session (F(2,81)=0.21, n.s.) and no phenotype × session interaction (F(2,81)=1.24, n.s.) was observed for total number of cocaine infusions (Supplementary Figure 4). Total daily cocaine intake did not differ between treatment groups (knockdown=10.5±0.5 mg/kg/day; control=11.3±0.5 mg/kg/day; n.s.). On FA day 14, knockdown rats displayed higher cue-reinforced lever presses (Figure 4f; p<0.05) vs controls; inactive lever presses during the cue reactivity test did not differ between 5-HT2CR knockdown and control rats. There was a positive correlation between premature responses and cue-reinforced lever presses in individual rats (Figure 4g; r=0.577; p<0.01). Thus, genetic loss of the 5-HT2CR in mPFC induces aggregate impulsive action/cocaine cue reactivity, suggesting that depressed 5-HT2CR tone in this region confers vulnerability to these interlocked behaviors.
DISCUSSION
We provide the first direct evidence that 5-HT2CR neurotransmission in the mPFC is an important neuromolecular driver of inherent impulsive action and cocaine cue reactivity in an outbred population of rats. Our results demonstrate that high inherent impulsive action is associated with lower cortical 5-HT2CR membrane protein and higher 5-HT2CR:PSD95 complex expression, and a shift toward edited and less functional 5-HT2CR isoforms. We are the first to engineer and use virally mediated 5-HT2CR genetic deletion methods to discover that the loss of 5-HT2CR in the mPFC results in aggregate impulsive action/cocaine cue reactivity. Thus, we demonstrate that phenotypes in rats that correlate in humans and drive relapse-like behaviors appear to neuromechanistically overlap, with the 5-HT2CR functional status acting as a neural rheostat to regulate inherent impulsive action, as well as the intersection between impulsive action and cocaine cue reactivity.
Control of impulsive behavior is a critical factor in the propensity to relapse (Moeller et al, 2001b). Tasks of impulsive action can be reasonably matched to provide valid translational research approaches (Robbins, 2002; Voon et al, 2013), although few studies have used such tasks to address questions concerning the relationship between impulsive action and cue-reinforced cocaine-seeking. Here, we evaluated the relationship between impulsive action and cocaine-seeking, following a period of FA that is more similar to the situation in cocaine addicts that do not experience extinction training or seeking-taking chained paradigms (Belin et al, 2008; Economidou et al, 2009). Trait impulsive action does not consistently predict higher levels of cocaine intake (eg, current study; Besson et al, 2013), although HI rats acquire cocaine self-administration at a faster rate with higher intake levels than LI rats when trained on submaximal doses of cocaine (Anastasio and Cunningham, unpublished observations; Belin et al, 2008; Dalley et al, 2007) (but, see Besson et al, 2013). Trait impulsive action has also been linked to the persistence of cocaine-seeking behavior in the absence of cocaine (Belin et al, 2008) or following punishment (Economidou et al, 2009). To our knowledge, our findings comprise the first report that HI animals exhibit elevated cocaine-seeking under conditions in which each lever press is reinforced by discrete cocaine-paired cues; no association was observed under conditions in which lever presses were not reinforced by discrete cocaine-paired cues. These data support a direct relationship between inherent impulsive action and the motivational attributes of discrete cocaine-associated cues. Thus, impulsive action and cue reactivity are related processes that may be mediated by a common underlying neurobiology and mutual ‘top-down' cortical circuitry (Chen et al, 2013; Dalley et al, 2011).
The mPFC is a major neural director of reward-driven behavior, impulse control, and integration of internal states with environmental cues (Kalivas and Volkow, 2005; Koob and Volkow, 2010). Disruptions in brain glutamate and dopamine homeostasis within the corticolimbic circuit are involved in impulsive action and cue reactivity (Dalley et al, 2007; Koob and Volkow, 2010; Volkow et al, 2009). Both of these core neurotransmitter systems and the response of the neurocircuitry to acute and chronic exposure to cocaine are under the regulatory control of the 5-HT system (for review, see Cunningham and Anastasio, 2013). Integration of the few studies that have directly addressed the involvement of 5-HT in impulsive action (Dalley et al, 2002; Winstanley et al, 2004a) and cocaine-seeking (Bradberry and Rubino, 2004; Parsons et al, 1995) is hampered not only by the complexity of the tasks used and the state in which these behaviors are assessed, but also by the tonic and phasic mechanisms by which the 5-HT system modulates the mPFC microcircuitry (Komlosi et al, 2012) and output to subcortical regions (Warden et al, 2012). Nonetheless, previous findings coupled with the present results suggest that heterogeneity in 5-HT neurotransmission and perturbations in its balance may contribute to impulsive action and cocaine-seeking, driven in part by altered forebrain 5-HT2CR function.
The sites of action and regulatory processes underlying 5-HT2CR functional status have not yet been fully characterized in the impulsive action phenotype, and few have evaluated the integrity and fidelity of the 5-HT2CR system in impulsive action ex vivo. As shown here, differences in total 5-HT2CR mRNA levels in the mPFC are not evident between HI and LI rats (Besson et al, 2013). Multiple factors impact functionality of 5-HT2CR, including (but not limited to) divergent and convergent signaling through G-protein-dependent and -independent mechanisms, constitutive activity, availability of active pools of receptors at the membrane, and the composition of the associated macromolecular protein complex. An important regulator of 5-HT2CR constitutive activity is RNA editing. There are no reports of how editing alters the coupling efficiency between the receptor and its G-protein in vivo; however, editing of the 5-HT2CR restricts its ability to activate intracellular cascades, and also affects receptor desensitization and internalization in cellular models (Werry et al, 2008). Further, RNA editing may interact with the alternative splicing machinery of the 5-HT2CR, thereby shifting the expression ratio of the full-length protein variant relative to the truncated variant, a mechanistic process that has been proposed to influence the density of 5-HT2CR in the plasma membrane in vitro (Martin et al, 2013). We suggest that differential expression of greater edited 5-HT2CR variants and lower 5-HT2CR membrane protein may translate into depressed basal levels of 5-HT2CR intracellular signaling and subsequent dampened activation of cortical neurons, which could account for the HI phenotype. In keeping with this concept, the functional status of the 5-HT2CR is also modulated by association with a repertoire of co-expressed PDZ proteins, such as PSD95 (Becamel et al, 2002; Gavarini et al, 2006). Given that the association of 5-HT2CR with PSD95 promotes desensitization and internalization of the 5-HT2CR (Becamel et al, 2002; Gavarini et al, 2006), our discovery of an elevated cortical 5-HT2CR:PSD95 complex in HI rats may reflect phenotypic distinctions in neurobiological trait (inherent) or state (ligand-mediated) markers of 5-HT2R coupling to its G-proteins or downstream signaling capacity. Taken together, these multi-dimensional neurochemical facets that dictate 5-HT2CR functional status would be expected to contribute to generation of impulsive action through control of neuronal firing and mPFC output.
Activation of the mPFC is required for suppression of impulsive action (Dalley et al, 2011; Murphy et al, 2012) and cocaine-seeking behavior (Koya et al, 2009; LaLumiere et al, 2012). Our observation that 5-HT2CR knockdown in the mPFC resulted in elevated impulsive action and cocaine cue reactivity suggests that loss of the 5-HT2CR inactivates the mPFC suppression circuit to drive aggregate expression of these behaviors. Of note intra-mPFC administration of the selective 5-HT2CR antagonist SB242084 did not significantly alter impulsive action under training conditions in the 5-CSRT task (Robinson et al, 2008). Differences in the sensitivity of the task parameters as well as the tools used to manipulate 5-HT2CR function (genetic vs pharmacological) most likely underlie the discrepancies between the present study and previous work (Robinson et al, 2008). Nonetheless, it is tempting to speculate that 5-HT2CR signaling skewed toward a lower basal tone, and/or less constitutively active 5-HT2CR may account in part for a shift in the 5-HT2CR homeostatic framework that governs output from the mPFC to generate relapse-predictive behaviors in cocaine-dependent individuals. This is an entirely innovative concept, and taken together, our data support the idea that the functional status of the 5-HT2CR is differentially regulated in impulsive action phenotypes, and may contribute to a mechanistic imbalance within the mPFC that confers interlocked impulsive action and cue reactivity.
This contribution is significant because it is the first step in a continuum of research that will lead to the targeted development of pharmacological and molecular strategies to restore serotonergic function and minimize deleterious behaviors that promote relapse. Furthermore, this study provides not only insight into neural dysfunction in cocaine dependence but also into the molecular basis of normal brain function, as well as insight into the therapy of other related diseases associated with 5-HT2CR dysfunction (eg, obesity and eating disorders) and impulse control deficits.
FUNDING AND DISCLOSURE
This work was supported by the Jeane B Kempner Scholarship (NCA), NIDA grants K99 DA033374 (NCA), P20 DA024157 (KAC), K05 DA020087 (KAC), K02 DA000403 (FGM.), and the Center for Addiction Research at the University of Texas Medical Branch. Dr Cunningham is an editor of Neuropsychopharmacology Reviews for which she receives compensation from the American College of Neuropsychopharmacology and a consultant for Arena Pharmaceuticals. Dr Moeller is a consultant for Boehringer-Ingelheim. All other authors declare no conflicts of interest.
Acknowledgments
We thank Dr Joel Steinberg for statistical guidance, Mr Philip C Anastasio for assistance with the macrophotography and Dr Marcy Bubar Jordan for helpful discussions and comments on the manuscript.
Footnotes
Supplementary Information accompanies the paper on the Neuropsychopharmacology website (http://www.nature.com/npp)
Supplementary Material
References
- Abbas AI, Yadav PN, Yao WD, Arbuckle MI, Grant SG, Caron MG, et al. PSD-95 is essential for hallucinogen and atypical antipsychotic drug actions at serotonin receptors. J Neurosci. 2009;29:7124–7136. doi: 10.1523/JNEUROSCI.1090-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Gilbertson SR, Bubar MJ, Agarkov A, Stutz SJ, Jeng YJ, et al. Peptide inhibitors disrupt the serotonin 5-HT2C receptor interaction with phosphatase and tensin homolog to allosterically modulate cellular signaling and behavior. J Neurosci. 2013a;33:1615–1630. doi: 10.1523/JNEUROSCI.2656-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Lanfranco MF, Bubar MJ, Seitz PK, Stutz SJ, McGinnis AG, et al. Serotonin 5-HT(2C) receptor protein expression is enriched in synaptosomal and post-synaptic compartments of rat cortex. J Neurochem. 2010;113:1504–1515. doi: 10.1111/j.1471-4159.2010.06694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anastasio NC, Stoffel EC, Fox RG, Bubar MJ, Rice KC, Moeller FG, et al. Serotonin (5-hydroxytryptamine) 5-HT2A receptor: Association with inherent and cocaine-evoked behavioral disinhibition in rats. Behav Pharmacol. 2011;22:248–261. doi: 10.1097/FBP.0b013e328345f90d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becamel C, Alonso G, Galeotti N, Demey E, Jouin P, Ullmer C, et al. Synaptic multiprotein complexes associated with 5-HT(2C) receptors: a proteomic approach. EMBO J. 2002;21:2332–2342. doi: 10.1093/emboj/21.10.2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley DR, Balasubramanian S, Swerdlow HP, Smith GP, Milton J, Brown CG, et al. Accurate whole human genome sequencing using reversible terminator chemistry. Nature. 2008;456:53–59. doi: 10.1038/nature07517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, Pelloux Y, Dilleen R, Theobald DE, Lyon A, Belin-Rauscent A, et al. Cocaine modulation of frontostriatal expression of Zif268, D2, and 5-HT2c receptors in high and low impulsive rats. Neuropsychopharmacology. 2013;38:1963–1973. doi: 10.1038/npp.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradberry CW, Rubino SR. Phasic alterations in dopamine and serotonin release in striatum and prefrontal cortex in response to cocaine predictive cues in behaving rhesus macaques. Neuropsychopharmacology. 2004;29:676–685. doi: 10.1038/sj.npp.1300386. [DOI] [PubMed] [Google Scholar]
- Breukel AI, Besselsen E, Ghijsen WE. Synaptosomes. A model system to study release of multiple classes of neurotransmitters. Methods Mol Biol. 1997;72:33–47. doi: 10.1385/0-89603-394-5:33. [DOI] [PubMed] [Google Scholar]
- Bubar MJ, Stutz SJ, Cunningham KA. 5-HT(2C) receptors localize to dopamine and GABA neurons in the rat mesoaccumbens pathway. PLoS ONE. 2011;6:e20508. doi: 10.1371/journal.pone.0020508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns CM, Chu H, Rueter SM, Hutchinson LK, Canton H, Sanders-Bush E, et al. Regulation of serotonin-2C receptor G-protein coupling by RNA editing. Nature. 1997;387:303–308. doi: 10.1038/387303a0. [DOI] [PubMed] [Google Scholar]
- Carpenter KM, Schreiber E, Church S, McDowell D. Drug Stroop performance: relationships with primary substance of use and treatment outcome in a drug-dependent outpatient sample. Addict Behav. 2006;31:174–181. doi: 10.1016/j.addbeh.2005.04.012. [DOI] [PubMed] [Google Scholar]
- Carter BL, Tiffany ST. Cue-reactivity and the future of addiction research. Addiction. 1999;94:349–351. [PubMed] [Google Scholar]
- Chen BT, Yau HJ, Hatch C, Kusumoto-Yoshida I, Cho SL, Hopf FW, et al. Rescuing cocaine-induced prefrontal cortex hypoactivity prevents compulsive cocaine seeking. Nature. 2013;496:359–362. doi: 10.1038/nature12024. [DOI] [PubMed] [Google Scholar]
- Cunningham KA, Anastasio NC.2013Serotonin at the nexus of impulsivity and cue ceactivity in cocaine addiction Neuropharmacology(In press). [DOI] [PMC free article] [PubMed]
- Cunningham KA, Anastasio NC, Fox RG, Stutz SJ, Bubar MJ, Swinford SE, et al. Synergism between a serotonin 5-HT2A receptor (5-HT2AR) antagonist and 5-HT2CR agonist suggests new pharmacotherapeutics for cocaine addiction. ACS Chemical Neuroscience. 2012;4:110–121. doi: 10.1021/cn300072u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunningham KA, Fox RG, Anastasio NC, Bubar MJ, Stutz SJ, Moeller FG, et al. Selective serotonin 5-HT2C receptor activation suppresses the reinforcing efficacy of cocaine and sucrose but differentially affects the incentive-salience value of cocaine- vs. sucrose-associated cues. Neuropharmacology. 2011;61:513–523. doi: 10.1016/j.neuropharm.2011.04.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, et al. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Theobald DE, Eagle DM, Passetti F, Robbins TW. Deficits in impulse control associated with tonically-elevated serotonergic function in rat prefrontal cortex. Neuropsychopharmacology. 2002;26:716–728. doi: 10.1016/S0893-133X(01)00412-2. [DOI] [PubMed] [Google Scholar]
- Daly TM. Overview of adeno-associated viral vectors. Methods Mol Biol. 2004;246:157–165. doi: 10.1385/1-59259-650-9:157. [DOI] [PubMed] [Google Scholar]
- Economidou D, Pelloux Y, Robbins TW, Dalley JW, Everitt BJ. High impulsivity predicts relapse to cocaine-seeking after punishment-induced abstinence. Biol Psychiatry. 2009;65:851–856. doi: 10.1016/j.biopsych.2008.12.008. [DOI] [PubMed] [Google Scholar]
- Field M, Cox WM. Attentional bias in addictive behaviors: a review of its development, causes, and consequences. Drug Alcohol Depend. 2008;97:1–20. doi: 10.1016/j.drugalcdep.2008.03.030. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Noble K, Higgins GA. Impulsive action induced by amphetamine, cocaine and MK801 is reduced by 5-HT(2C) receptor stimulation and 5-HT(2A) receptor blockade. Neuropharmacology. 2011;61:468–477. doi: 10.1016/j.neuropharm.2011.02.025. [DOI] [PubMed] [Google Scholar]
- Fletcher PJ, Rizos Z, Sinyard J, Tampakeras M, Higgins GA. The 5-HT(2C) receptor agonist RO 60-0175 reduces cocaine self-administration and reinstatement induced by the stressor yohimbine and contextual cues. Neuropsychopharmacology. 2008;33:1402–1412. doi: 10.1038/sj.npp.1301509. [DOI] [PubMed] [Google Scholar]
- Frijda NH. Impulsive action and motivation. Biol Psychol. 2010;84:570–579. doi: 10.1016/j.biopsycho.2010.01.005. [DOI] [PubMed] [Google Scholar]
- Gavarini S, Becamel C, Altier C, Lory P, Poncet J, Wijnholds J, et al. Opposite effects of PSD-95 and MPP3 PDZ proteins on serotonin 5-hydroxytryptamine2C receptor desensitization and membrane stability. Mol Biol Cell. 2006;17:4619–4631. doi: 10.1091/mbc.E06-03-0218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green CE, Moeller FG, Schmitz JM, Lucke JF, Lane SD, Swann AC, et al. Evaluation of heterogeneity in pharmacotherapy trials for drug dependence: a Bayesian approach. Am J Drug Alcohol Abuse. 2009;35:95–102. doi: 10.1080/00952990802647503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grottick AJ, Corrigall WA, Higgins GA. Activation of 5-HT2C receptors reduces the locomotor and rewarding effects of nicotine. Psychopharmacology. 2001;157:292–298. doi: 10.1007/s002130100801. [DOI] [PubMed] [Google Scholar]
- Herrick-Davis K, Grinde E, Niswender CM. Serotonin 5-HT2C receptor RNA editing alters receptor basal activity: implications for serotonergic signal transduction. J Neurochem. 1999;73:1711–1717. doi: 10.1046/j.1471-4159.1999.731711.x. [DOI] [PubMed] [Google Scholar]
- Hommel JD, Sears RM, Georgescu D, Simmons DL, DiLeone RJ. Local gene knockdown in the brain using viral-mediated RNA interference. Nat Med. 2003;9:1539–1544. doi: 10.1038/nm964. [DOI] [PubMed] [Google Scholar]
- Hoyer D, Hannon JP, Martin GR. Molecular, pharmacological and functional diversity of 5-HT receptors. Pharmacol Biochem Behav. 2002;71:533–554. doi: 10.1016/s0091-3057(01)00746-8. [DOI] [PubMed] [Google Scholar]
- Kalivas PW, Volkow ND. The neural basis of addiction: A pathology of motivation and choice. Am J Psychiatry. 2005;162:1403–1413. doi: 10.1176/appi.ajp.162.8.1403. [DOI] [PubMed] [Google Scholar]
- Keppel G. Design and Analysis: A Researcher's Handbook. Prentice-Hall, Inc.: Englewood Cliffs, NJ, USA; 1973. [Google Scholar]
- Komlosi G, Molnar G, Rozsa M, Olah S, Barzo P, Tamas G. Fluoxetine (prozac) and serotonin act on excitatory synaptic transmission to suppress single layer 2/3 pyramidal neuron-triggered cell assemblies in the human prefrontal cortex. J Neurosci. 2012;32:16369–16378. doi: 10.1523/JNEUROSCI.2618-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–238. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koya E, Uejima JL, Wihbey KA, Bossert JM, Hope BT, Shaham Y. Role of ventral medial prefrontal cortex in incubation of cocaine craving. Neuropharmacology. 2009;56 (Suppl 1:177–185. doi: 10.1016/j.neuropharm.2008.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaLumiere RT, Smith KC, Kalivas PW. Neural circuit competition in cocaine-seeking: roles of the infralimbic cortex and nucleus accumbens shell. Eur J Neurosci. 2012;35:614–622. doi: 10.1111/j.1460-9568.2012.07991.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanfranco MF, Seitz PK, Morabito MV, Emeson RB, Sanders-Bush E, Cunningham KA. An innovative real-time PCR method to measure changes in RNA editing of the serotonin 2C receptor (5-HT(2C)R) in brain. J Neurosci Methods. 2009;179:247–257. doi: 10.1016/j.jneumeth.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leff SE, Spratt SK, Snyder RO, Mandel RJ. Long-term restoration of striatal L-aromatic amino acid decarboxylase activity using recombinant adeno-associated viral vector gene transfer in a rodent model of Parkinson's disease. Neuroscience. 1999;92:185–196. doi: 10.1016/s0306-4522(98)00741-6. [DOI] [PubMed] [Google Scholar]
- Li X, Wolf ME. Visualization of virus-infected brain regions using a GFP-illuminating flashlight enables accurate and rapid dissection for biochemical analysis. J Neurosci Methods. 2011;201:177–179. doi: 10.1016/j.jneumeth.2011.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linley SB, Hoover WB, Vertes RP. Pattern of distribution of serotonergic fibers to the orbitomedial and insular cortex in the rat. J Chem Neuroanat. 2013;48-49:29–45. doi: 10.1016/j.jchemneu.2012.12.006. [DOI] [PubMed] [Google Scholar]
- Liu S, Bubar MJ, Lanfranco MF, Hillman GR, Cunningham KA. Serotonin(2C) receptor localization in GABA neurons of the rat medial prefrontal cortex: Implications for understanding the neurobiology of addiction. Neuroscience. 2007;146:1667–1688. doi: 10.1016/j.neuroscience.2007.02.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu S, Lane SD, Schmitz JM, Waters AJ, Cunningham KA, Moeller FG. Relationship between attentional bias to cocaine-related stimuli and impulsivity in cocaine-dependent subjects. Am J Drug Alcohol Abuse. 2011;37:117–122. doi: 10.3109/00952990.2010.543204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marion S, Weiner DM, Caron MG. RNA editing induces variation in desensitization and trafficking of 5-hydroxytryptamine 2c receptor isoforms. J Biol Chem. 2004;279:2945–2954. doi: 10.1074/jbc.M308742200. [DOI] [PubMed] [Google Scholar]
- Martin CB, Ramond F, Farrington DT, Aguiar AS, Jr., Chevarin C, Berthiau AS, et al. RNA splicing and editing modulation of 5-HT(2C) receptor function: relevance to anxiety and aggression in VGV mice. Mol Psychiatry. 2013;18:656–665. doi: 10.1038/mp.2012.171. [DOI] [PubMed] [Google Scholar]
- Millan MJ, Marin P, Bockaert J, la Cour CM. Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions. Trends Pharmacol Sci. 2008;29:454–464. doi: 10.1016/j.tips.2008.06.007. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Barratt ES, Dougherty DM, Schmitz JM, Swann AC. Psychiatric aspects of impulsivity. Am J Psychiatry. 2001a;158:1783–1793. doi: 10.1176/appi.ajp.158.11.1783. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Dougherty DM, Barratt ES, Schmitz JM, Swann AC, Grabowski J. The impact of impulsivity on cocaine use and retention in treatment. J Subst Abuse Treat. 2001b;21:193–198. doi: 10.1016/s0740-5472(01)00202-1. [DOI] [PubMed] [Google Scholar]
- Moeller FG, Schmitz JM, Steinberg JL, Green CM, Reist C, Lai LY, et al. Citalopram combined with behavioral therapy reduces cocaine use: a double-blind, placebo-controlled trial. Am J Drug Alcohol Abuse. 2007;33:367–378. doi: 10.1080/00952990701313686. [DOI] [PubMed] [Google Scholar]
- Morabito MV, Ulbricht RJ, O'Neil RT, Airey DC, Lu P, Zhang B, et al. High-throughput multiplexed transcript analysis yields enhanced resolution of 5-hydroxytryptamine 2C receptor mRNA editing profiles. Mol Pharmacol. 2010;77:895–902. doi: 10.1124/mol.109.061903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy ER, Fernando AB, Urcelay GP, Robinson ES, Mar AC, Theobald DE, et al. Impulsive behaviour induced by both NMDA receptor antagonism and GABAA receptor activation in rat ventromedial prefrontal cortex. Psychopharmacology. 2012;219:401–410. doi: 10.1007/s00213-011-2572-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons LH, Koob GF, Weiss F. Extracellular serotonin is decreased in the nucleus accumbens during withdrawal from cocaine self-administration. Behav Brain Res. 1995;73:225–228. doi: 10.1016/0166-4328(96)00101-5. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. Academic Press: Sydney, Australia; 1998. [Google Scholar]
- Pentkowski NS, Duke FD, Weber SM, Pockros LA, Teer AP, Hamilton EC, et al. Stimulation of medial prefrontal cortex serotonin 2C 5-HT2C receptors attenuates cocaine-seeking behavior. Neuropsychopharmacology. 2010;35:2037–2048. doi: 10.1038/npp.2010.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW. The 5-choice serial reaction time task: behavioural pharmacology and functional neurochemistry. Psychopharmacology. 2002;163:362–380. doi: 10.1007/s00213-002-1154-7. [DOI] [PubMed] [Google Scholar]
- Robinson ES, Dalley JW, Theobald DE, Glennon JC, Pezze MA, Murphy ER, et al. Opposing roles for 5-HT2A and 5-HT2C receptors in the nucleus accumbens on inhibitory response control in the 5-choice serial reaction time task. Neuropsychopharmacology. 2008;33:2398–2406. doi: 10.1038/sj.npp.1301636. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Baler R, Telang F. Imaging dopamine's role in drug abuse and addiction. Neuropharmacology. 2009;56 (Suppl 1:3–8. doi: 10.1016/j.neuropharm.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voon V, Irvine MA, Derbyshire K, Worbe Y, Lange I, Abbott S, et al. Measuring "waiting" impulsivity in substance addictions and binge eating disorder in a novel analogue of rodent serial reaction time task. Biol Psychiatry. 2013;S0006-3223:00456–3. doi: 10.1016/j.biopsych.2013.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warden MR, Selimbeyoglu A, Mirzabekov JJ, Lo M, Thompson KR, Kim SY, et al. A prefrontal cortex-brainstem neuronal projection that controls response to behavioural challenge. Nature. 2012;492:428–432. doi: 10.1038/nature11617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werry TD, Stewart GD, Crouch MF, Watts A, Sexton PM, Christopoulos A. Pharmacology of 5HT(2C) receptor-mediated ERK1/2 phosphorylation: agonist-specific activation pathways and the impact of RNA editing. Biochem Pharmacol. 2008;76:1276–1287. doi: 10.1016/j.bcp.2008.08.024. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Dalley JW, Theobald DE, Robbins TW. Fractionating impulsivity: contrasting effects of central 5-HT depletion on different measures of impulsive behavior. Neuropsychopharmacology. 2004a;29:1331–1343. doi: 10.1038/sj.npp.1300434. [DOI] [PubMed] [Google Scholar]
- Winstanley CA, Theobald DE, Dalley JW, Glennon JC, Robbins TW. 5-HT2A and 5-HT2C receptor antagonists have opposing effects on a measure of impulsivity: interactions with global 5-HT depletion. Psychopharmacology. 2004b;176:376–385. doi: 10.1007/s00213-004-1884-9. [DOI] [PubMed] [Google Scholar]
- Zhu H, Urban DJ, Blashka J, McPheeters MT, Kroeze WK, Mieczkowski P, et al. Quantitative analysis of focused a-to-I RNA editing sites by ultra-high-throughput sequencing in psychiatric disorders. PLoS ONE. 2012;7:e43227. doi: 10.1371/journal.pone.0043227. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.