Abstract
The number of cervical vertebrae in mammals is highly conserved at seven. We have shown that changes of this number are selected against due to a coupling with major congenital abnormalities (pleiotropic effects). Here we show that the incidence of abnormal cervical vertebral numbers in Late Pleistocene mammoths from the North Sea is high (33.3%) and approximately 10 times higher than that of extant elephants (3.6%). Abnormal numbers were due to the presence of large cervical ribs on the seventh vertebra, which we deduced from the presence of rib articulation facets on sixth (posterior side) and seventh (anterior side) cervical vertebrae. The incidence of abnormal cervical vertebral numbers in mammoths appears to be much higher than in other mammalian species, apart from exceptional sloths, manatees and dugongs and indicates a vulnerable condition. We argue that the increased incidence of cervical ribs in mammoths is probably caused by inbreeding and adverse conditions that impact early pregnancies in declining populations close to extinction in the Late Pleistocene.
Keywords: Mammoths, Extinction, Loxodonta, Elephas, Vertebral column, Body plan, Inbreeding
Introduction
The number of cervical vertebrae in mammals is remarkably constant at seven. In other tetrapods, the number of cervical vertebrae varies considerably, and in mammals the number of vertebrae in more caudal vertebral regions is variable as well (Leboucq, 1898; Schultz, 1961; Starck, 1979; Narita & Kuratani, 2005). Only manatees (Trichechus, Sirenia) and sloths (Bradypus and Choloepus, Xenarthra) have an exceptional number of cervical vertebrae (Bateson, 1894; Starck, 1979; Varela-Lasheras et al., 2011). Despite the extreme evolutionary conservation of the number of cervical vertebrae, intraspecific variation is not uncommon in mammals. The most common variation is represented by ribs on the seventh vertebra, so-called cervical ribs, which can be considered a partial or complete homeotic transformation of a cervical into a thoracic vertebra (involving a change in the activity of Hox genes (Galis, 1999; Li & Shiota, 2000; Varela-Lasheras et al., 2011; Wéry et al., 2003)). The strong conservation of the number of cervical vertebrae implies that there must be selection against intraspecific variation of this number. Indeed, very strong selection against cervical ribs was shown to exist in humans (Galis, 1999; Galis et al., 2006; Furtado et al., 2011; ten Broek et al., 2012). Approximately 90 percent of individuals possessing a cervical rib die before reaching reproductive age (Galis et al., 2006). The severe selection is due to the strong association of cervical ribs with multiple and major congenital abnormalities. In other mammalian species, we have also found an association with abnormalities (Varela-Lasheras et al., 2011). A cervical rib itself is relatively harmless, but its development is induced by a (genetic or environmental) disturbance of early embryogenesis (Li & Shiota, 2000; Wéry et al., 2003; Chernoff & Rogers, 2004; Galis et al., 2006). Such a disturbance usually has multiple effects, due to the highly interactive nature of early embryogenesis. Hence, the strong selection against cervical ribs is indirect and due to the severity of the associated medical problems (Galis et al., 2006; ten Broek et al., 2012).
Of three caudal cervical vertebrae from Mammuthus primigenius, a sixth (C6) and two seventh (C7), that were recently found in the North Sea, during infrastructural works for an extension of the Rotterdam Harbour (Maasvlakte 2) and donated to the Natural History Museum in Rotterdam, two possessed articulation facets for cervical ribs (the C6 and one of the C7). This surprising finding aroused our interest, and we searched the extensive collection of Late Pleistocene M. primigenius material in the Naturalis Biodiversity Centre (Leiden) to make an estimate of the incidence of this developmental abnormality. Additionally, we determined for comparison the incidence of cervical ribs in skeletons of the most closely related extant species, the Asian and African elephants (Elephas maximus and Loxodonta africana).
Methods
Specimens
We analyzed 6 sixth cervical vertebrae (C6) and 10 seventh cervical vertebrae (C7) of Late Pleistocene mammoths (M. primigenius), from two collections: the Natural History Museum Rotterdam (NMR, Table 1) and Naturalis Biodiversity Center (Naturalis, Table 1). The cervical vertebrae were identified as C6 and C7 based on the relative size of the spinous processes and anterior tubercles. We analysed 28 specimens of extant elephants, 21 E. maximus and 7 L. africana, from 5 collections: the Natural History Museum of Denmark, Copenhagen (ZMUC), Naturhistorisches Museum Wien, Vienna (NHMW), The University of Vienna, the Swedish Museum of Natural History, Stockholm (NRM), the Royal Museum for Central Africa Tervuren (RMCA) and Naturalis Biodiversity Center (Naturalis). All mammoth specimens (see Table 1 for collection numbers) are of Late Pleistocene age and originate from the North Sea. Two specimens (C6, inv.nr. NMR999100006627 and C7, inv. nr. NMR999100007602) were recently found during infrastructural works in the Rotterdam harbor area (“Maasvlakte 2”) on the North Sea seabed (Maasvlakte Zandwingebied, i.e., the source area for sand extraction, c. 51°59′N/3°53′E) and allocated to the NMR by the Rotterdam Port Authorities.
Table 1. List of investigated specimens and scores of articulation facets of cervical ribs.
The presence of articulation facets of ribs was indicated posteriorly on the sixth cervical vertebra (C6) and/or anteriorly on the seventh cervical vertebra (C7).
| Species | Institute | Collection no. | Sex | Vertebra | Rib facets (left/right) |
|---|---|---|---|---|---|
| Mammuthus primigenius | Naturalis | RGM592809 | n.a. | C7 | 0 |
| RGM103337 | n.a. | C7 | n.a. | ||
| RGM132902 | n.a. | C7 | 0 | ||
| RGM139079 | n.a. | C7 | 0 | ||
| RGM172327 | n.a. | C7 | n.a. | ||
| RGM20026 | n.a. | C7 | n.a. | ||
| RGM20313 | n.a. | C6 | n.a. | ||
| RGM369465 | n.a. | C6 | n.a. | ||
| RGM40098 | n.a. | C6 | 0 | ||
| RGM40120 | n.a. | C7 | 0 | ||
| RGM4445989 | n.a. | C7 | n.a. | ||
| RGM79245 | n.a. | C6 | n.a. | ||
| RGM146248 | n.a. | C6 | 1 (left) | ||
| NMR | NMR999100007602 | n.a. | C7 | 1 (left) | |
| NMR999100006627 | n.a. | C6 | 1 (right) | ||
| NMR999100007479 | n.a. | C7 | 0 | ||
| Elephas maximus | Naturalis | RMNH.MAM.46016 | n.a. | C6, C7 | 0 |
| RMNH.MAM.46024 | M | C6, C7 | 0 | ||
| RMNH.MAM.39235 | F | C6, C7 | 0 | ||
| RMNH.MAM.39234 | n.a. | C6, C7 | 0 | ||
| ZMA 13483 | n.a. | C6, C7 | 0 | ||
| RMNH.MAM.46018 | n.a. | C6, C7 | 0 | ||
| ZMA.MAM.30069 | M | C6, C7 | 0 | ||
| NRM | A609596* | F | C6, C7 | 0 | |
| A591540 | n.a. | C6, C7 | 0 | ||
| A600572 | n.a. | C6, C7 | 0 | ||
| A589489* | F | C6, C7 | 0 | ||
| NMW | 16545 | n.a. | C6, C7 | 0 | |
| 5505* | M | C6, C7 | 0 | ||
| UAV | n.a. | n.a. | C6, C7 | 0 | |
| ZMUC | ZMUC CN2 | F | C6, C7 | 0 | |
| ZMUC CN4196* | n.a. | C6, C7 | 0 | ||
| ZMUC CN1399* | F | C6, C7 | 0 | ||
| ZMUC CN1 | M | C6, C7 | 0 | ||
| ZMUC CN2293* | M | C6, C7 | 0 | ||
| ZMUC CN639* | F | C6, C7 | 1 (right, C7) | ||
| ZMUC CN 558* | M | C6, C7 | 0 | ||
| Loxodonta africana | Naturalis | RMNH.MAM.45488 | M | C6, C7 | 0 |
| NRM | A601286 | M | C6, C7 | 0 | |
| A600551 | M | C6, C7 | 0 | ||
| NMW | 287* (exhibition) | n.a. | C6, C7 | 0 | |
| RMCA | RMCA 4559 | n.a. | C6, C7 | 0 | |
| ZMUC | ZMUC CN708* | M | C6, C7 | 0 | |
| ZMUC CN3684* | M | C6, C7 | 0 |
Notes.
Died in captivity (wild born).
- n.a.
- not available
- Naturalis
- Naturalis Biodiversity Center Leiden
- NRM
- Naturhistoriska Riksmuseet Stockholm
- NMW
- Naturhistorisches Museum Wien
- UAV
- University Anatomy Vienna
- ZMUC
- Zoologisk Museum University Copenhagen
- RMCA
- Royal Museum Central Africa Tervuren
Cervical ribs
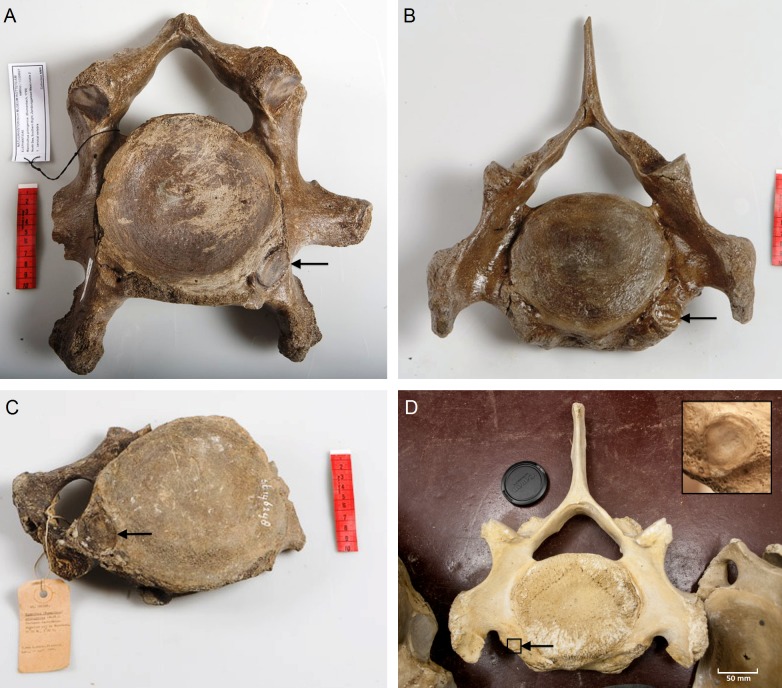
We analyzed the C6 and C7 vertebrae for the presence or absence of articulation facets of cervical ribs. The presence of cervical ribs can be deduced from articulation facets on the anterior side of C7 (Figs. 1A and 1D) and, if the cervical ribs are large enough, on the posterior side of C6, as well (Figs. 1B and 1C).
Figure 1. Presence of rib articulation facets on cervical vertebrae of woolly mammoths (A–C) and Asian elephant (D).
(A) Posterior view of a C6 of a Mammuthus primigenius from the North Sea (NMR999100006627), showing an articulation facet of a cervical rib on the right side. (B) Anterior view of a C7 of a Mammuthus primigenius from the North Sea (NMR999100007602), showing a sinistral articulation facet (lower right in the picture). (C) Posterior view of a C6 of a Mammuthus primigenius from the North Sea (Naturalis St 146248), showing an articulation facet of a cervical rib on the left side. (D) Anterior view of a C7 of an Elephas maximus (ZMUC CN639), showing a minute articulation facet of a cervical rib on the right side (see inset for articulation facet). The size of cervical ribs is presumably associated with the strength of associated abnormalities. Arrows indicate articulation facets.
Statistical tests
To compare the prevalence of cervical rib facets between mammoths and elephants we used a G-test of independence, which is particularly appropriate for variable samples sizes as is often the case with paleopathological data (Farke, 2007). Furthermore we also used Barnard’s test for 2 × 2 tables, which is appropriate for small sample sizes and yields greater power than Fisher’s exact test (Barnard, 1945). P-values <0.05 were considered as significant. All analyses were carried out in R.
Results
Articulation facets for cervical ribs on cervical vertebrae are characterized by the following combination of characteristics: (i) they have a smooth, polished-looking surface, visibly smoother than the (surrounding) cortical surface of the vertebrae; (ii) the surfaces have no vascular or nervous foramina; and (iii) the facets are bordered by a clear edge, distinguishing them from the surrounding cortex.
We found one C7 with a unilateral sinistral anterior rib facet indicating a left cervical rib (Fig. 1A). Five C7 did not have rib facets anteriorly and four could not be judged due to the absence of the relevant part of the vertebra. We found two C6 with rib facets on the posterior side indicating cervical ribs: one on the right side and one on the left side (Figs. 1B and 1C respectively). We found one C6 without rib facets posteriorly and three that could not be judged.
Thus, out of the nine C6 and C7 that could be evaluated, three indicate the presence of a cervical rib, i.e., an incidence of cervical rib facets of 33.3%. We found in one of the 21 E. maximus a minute cervical rib facet on C7 (Fig. 1D, 4.8%) and no articulation facet visible posteriorly on C6 of the same individual. None of the seven L. africana individuals had cervical rib facets, nor were rudimentary cervical ribs found. The overall incidence of cervical ribs in the two species is, thus, 3.6%. This is significantly lower than the 33.3% in mammoths, if we only consider vertebrae that can be evaluated for cervical rib articulation facets (G-test for independence, p = 0.035, Barnard’s exact test, p = 0.031).
Discussion
The incidence of cervical rib facets in our set of Late Pleistocene M. primigenius recovered from the North Sea is extremely high (3 out of 9, 33.3%), almost ten times higher than that of extant elephants (1 out of 29, 3.6%). In humans, an incidence higher than 1% has only been found in hospitals or isolated populations (Galis et al., 2006). An incidence higher than 25% has only been found in children with leukemia, brain tumours and neuroblastoma (Schumacher, Mai & Gutjahr, 1992; Galis & Metz, 2003; Merks et al., 2005) and in deceased fetuses and infants (Galis et al., 2006; Furtado et al., 2011; ten Broek et al., 2012). Along with the high incidence of cervical ribs in mammoths, the size of the articulation facets is particularly large (Figs. 1A–1C), substantially larger than the articulation facet found in the one E. maximus (Fig. 1D) and, pointing to substantially larger cervical ribs than usually found in humans (see Bots et al., 2011; ten Broek et al., 2012 for examples). Size of cervical ribs was found to be negatively correlated with fitness in transgenic mice (Jeannotte et al., 1993; see also Bots et al., 2011).
The exceptionally high incidence of large cervical ribs in our set of Late Pleistocene mammoths can be due to two factors. Firstly, it can be due to a high rate of inbreeding in declining populations, before final extinction. A high incidence of cervical ribs (7.46%) has been observed in an isolated human population (Palma & Carini, 1990) in Sicily, in inbred pedigreed dogs (11.4% Breit & Kunzel, 1998) and inbred minipigs (11% at birth, Jørgensen, 1998). Generally, in inbred mammals there is an increased incidence of congenital anomalies (Cristescu et al., 2009; Räikkönen et al., 2013). Recent studies have shown that the genetic diversity was extremely low in Late Pleistocene mammoth populations in Siberia (Miller et al., 2008; Nyström et al., 2012). Additionally, the increased incidence of cervical ribs may be due to harsh conditions that impact early pregnancies, because diseases, famine, cold and other stressors can lead to disturbances of early organogenesis, that can result in the induction of cervical ribs (e.g., Sawin, 1937; Li & Shiota, 2000; Wéry et al., 2003; Chernoff & Rogers, 2004; Steigenga et al., 2006). Harsh conditions during the Late Pleistocene, a period of intense climatic fluctuations and ecosystem instability, are plausible (Brace et al., 2012). Furthermore, bone dystrophy in mammoth calves of Northern Eurasian Late Pleistocene populations is found regularly and assumed to be caused by mineral deficiencies in pregnant females (Leshchinskiy, 2012). Hence, a combination of inbreeding and harsh conditions may be the most likely explanation for the extremely high incidence of cervical ribs. Our results, thus, are in agreement with inbreeding in populations in North-Western Eurasia, just as has been found for Siberian populations (Miller et al., 2008; Nyström et al., 2012). Finally, the high incidence and large size of the cervical ribs indicates a strong vulnerability, given the association of cervical ribs with diseases and congenital abnormalities in mammals. The vulnerable condition may well have contributed to the eventual extinction of the woolly mammoths.
Acknowledgments
We thank the Rotterdam Port Authorities for the donation of all bones that are found during the extension of the Rotterdam harbor in the North Sea to the Natural History Museum in Rotterdam. We thank Mogens Andersen, Alex Bibl, Daniela Kalthoff, Steven van der Mije, Wim Wendelen and Reinier van Zelst for making specimens available and Alexandra van der Geer, Jacques van Alphen, Russell Lande, Natasja den Ouden and Rienk de Jong for comments. We thank Joris van Alphen for making the photographs of Figs. 1A–1C.
Funding Statement
FG acknowledges Synthesys travel grants to visit the Royal Museum for Central Africa Tervuren, the Zoological Museum Copenhagen, and the Natural History Museum of Stockholm (BE-TAF-1649, DK-TAF-2183, DE-TAF-2114, SE-TAF-3009). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare no competing interests.
Author Contributions
Jelle W.F. Reumer and Frietson Galis conceived and designed the experiments, performed the experiments, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Clara M.A. ten Broek performed the experiments, analyzed the data, prepared figures and/or tables, reviewed drafts of the paper.
References
- Barnard (1945).Barnard G. A new test for 2 × 2 tables. Nature. 1945;156:783–784. doi: 10.1038/156783b0. [DOI] [Google Scholar]
- Bateson (1894).Bateson W. Materials for the study of variation. London: MacMillan; 1894. [Google Scholar]
- Bots et al. (2011).Bots J, Wijnaendts LC, Delen S, Van Dongen S, Heikinheimo K, Galis F. Analysis of cervical ribs in a series of human fetuses. Journal of Anatomy. 2011;219:403–409. doi: 10.1111/j.1469-7580.2011.01400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brace et al. (2012).Brace S, Palkopoulou E, Dalén L, Lister AM, Miller R, Otte M, Germonpré M, Blockley SP, Stewart JR, Barnes I. Serial population extinctions in a small mammal indicate Late Pleistocene ecosystem instability. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:20532–20536. doi: 10.1073/pnas.1213322109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breit & Kunzel (1998).Breit S, Kunzel W. Osteologische Besonderheiten an Wirbelsaulen von Rassehunden: eine rontgenologische und morphologische Studie. Wiener Tierarztliche Monatsschrift. 1998;85:340–350. [Google Scholar]
- Chernoff & Rogers (2004).Chernoff N, Rogers JM. Supernumerary ribs in developmental toxicity bioassays and in human populations: incidence and biological significance. Journal of Toxicology and Environmental Health, Part B. 2004;7:437–449. doi: 10.1080/10937400490512447. [DOI] [PubMed] [Google Scholar]
- Cristescu et al. (2009).Cristescu R, Cahill V, Sherwin WB, Handasyde K, Carlyon K, Whisson D, Herbert CA, Carlsson BLJ, Wilton AN, Cooper DW. Inbreeding and testicular abnormalities in a bottlenecked population of koalas (Phascolarctos cinereus) Wildlife Research. 2009;36:299–308. doi: 10.1071/WR08010. [DOI] [Google Scholar]
- Farke (2007).Farke AA. Reexamination of paleopathology in plesiosaurs. Journal of Vertebrate Paleontology. 2007;27:724–726. doi: 10.1671/0272-4634(2007)27[724:ROPIPA]2.0.CO;2. [DOI] [Google Scholar]
- Furtado et al. (2011).Furtado LV, Thaker HM, Erickson LK, Shirts BH, Opitz JM. Cervical ribs are more prevalent in stillborn fetuses than in live-born infants and are strongly associated with fetal aneuploidy. Pediatric and Developmental Pathology. 2011;14:431–437. doi: 10.2350/11-01-0974-OA.1. [DOI] [PubMed] [Google Scholar]
- Galis (1999).Galis F. Why do almost all mammals have seven cervical vertebrae? Developmental constraints, Hox genes, and cancer. Journal of Experimental Zoology. 1999;285:19–26. doi: 10.1002/(SICI)1097-010X(19990415)285:1<19::AID-JEZ3>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- Galis & Metz (2003).Galis F, Metz JA. Anti-cancer selection as a source of developmental and evolutionary constraints. BioEssays. 2003;25:1035–1039. doi: 10.1002/bies.10366. [DOI] [PubMed] [Google Scholar]
- Galis et al. (2006).Galis F, Van Dooren TJ, Feuth JD, Metz JA, Witkam A, Ruinard S, Steigenga MJ, Wijnaendts LC. Extreme selection in humans against homeotic transformations of cervical vertebrae. Evolution. 2006;60:2643–2654. doi: 10.1111/j.0014-3820.2006.tb01896.x. [DOI] [PubMed] [Google Scholar]
- Jeannotte et al. (1993).Jeannotte L, Lemieux M, Charron J, Poirier F, Robertson E. Specification of axial identity in the mouse: role of the Hoxa-5 (Hox1. 3) gene. Genes & Development. 1993;7:2085–2096. doi: 10.1101/gad.7.11.2085. [DOI] [PubMed] [Google Scholar]
- Jørgensen (1998).Jørgensen KD. Minipig in reproduction toxicology. Scandinavian Journal of Laboratory Animal Science. 1998;25:63–75. [Google Scholar]
- Leboucq (1898).Leboucq HLF. Recherches sur les variations anatomiques de la première côte chez l’homme. Archives de Biologie. 1898;15:9–178. [Google Scholar]
- Leshchinskiy (2012).Leshchinskiy SV. Paleoecological investigation of mammoth remains from the Kraków Spadzista Street (B) site. Quaternary International. 2012;276–277:155–169. doi: 10.1016/j.quaint.2012.05.025. [DOI] [Google Scholar]
- Li & Shiota (2000).Li ZL, Shiota K. Stage-specific homeotic vertebral transformations in mouse fetuses induced by maternal hyperthermia during somitogenesis. Developmental Dynamics. 2000;216:336–348. doi: 10.1002/(SICI)1097-0177(199912)216:4/5<336::AID-DVDY3>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Merks et al. (2005).Merks JH, Smets AM, Van Rijn RR, Kobes J, Caron HN, Maas M, Hennekam RC. Prevalence of rib anomalies in normal Caucasian children and childhood cancer patients. European Journal of Medical Genetics. 2005;48:113–129. doi: 10.1016/j.ejmg.2005.01.029. [DOI] [PubMed] [Google Scholar]
- Miller et al. (2008).Miller W, Drautz DI, Ratan A, Pusey B, Qi J, Lesk AM, Tomsho LP, Packard MD, Zhao F, Sher A. Sequencing the nuclear genome of the extinct woolly mammoth. Nature. 2008;456:387–390. doi: 10.1038/nature07446. [DOI] [PubMed] [Google Scholar]
- Narita & Kuratani (2005).Narita Y, Kuratani S. Evolution of the vertebral formulae in mammals: a perspective on developmental constraints. Journal of Experimental Zoology Part B: Molecular and Developmental Evolution. 2005;304:91–106. doi: 10.1002/jez.b.21029. [DOI] [PubMed] [Google Scholar]
- Nyström et al. (2012).Nyström V, Humphrey J, Skoglund P, McKeown NJ, Vartanyan S, Shaw PW, Lidén K, Jakobsson M, Barnes I, Angerbjörn A. Microsatellite genotyping reveals end-Pleistocene decline in mammoth autosomal genetic variation. Molecular Ecology. 2012;21:3391–3402. doi: 10.1111/j.1365-294X.2012.05525.x. [DOI] [PubMed] [Google Scholar]
- Palma & Carini (1990).Palma A, Carini F. Variazioni dellápofisi trasversa della settima vertebra cervicale: studio anatomo-radiologico su una popolazione “segregate”. Archivio Italiano di Anatomia e di Embrioligia. 1990;95:11–16. [PubMed] [Google Scholar]
- Räikkönen et al. (2013).Räikkönen J, Vucetich JA, Vucetich LM, Peterson RO, Nelson MP. What the inbred scandinavian wolf population tells us about the nature of conservation. PLoS ONE. 2013;8:e67218. doi: 10.1371/journal.pone.0067218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawin (1937).Sawin PB. Preliminary studies of hereditary variation in the axial skeleton of the rabbit. The Anatomical Record. 1937;69:407–428. doi: 10.1002/ar.1090690403. [DOI] [Google Scholar]
- Schultz (1961).Schultz AH. Vertebral column and thorax. Basel: S Karger Ag; 1961. [Google Scholar]
- Schumacher, Mai & Gutjahr (1992).Schumacher R, Mai A, Gutjahr P. Association of rib anomalies and malignancy in childhood. European Journal of Pediatrics. 1992;151:432–434. doi: 10.1007/BF01959357. [DOI] [PubMed] [Google Scholar]
- Starck (1979).Starck D. Vergleichende anatomie der wirbeltiere. Berlin: Springer Verlag; 1979. [Google Scholar]
- Steigenga et al. (2006).Steigenga MJ, Ruinard S, de Koning J, Helmerhorst FM, Tijssen AMI, Galis F. Evolutionary conserved structures as indicators of medical risks: increased incidence of cervical ribs after ovarian hyperstimulation in mice. Animal Biology. 2006;56:63–68. doi: 10.1163/157075606775904696. [DOI] [Google Scholar]
- ten Broek et al. (2012).ten Broek CMA, Bakker AJ, Varela-Lasheras I, Bugiani M, Van Dongen S, Galis F. Evo-devo of the human vertebral column: on homeotic transformations, pathologies and prenatal selection. Evolutionary Biology. 2012;39:456–471. doi: 10.1007/s11692-012-9196-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varela-Lasheras et al. (2011).Varela-Lasheras I, Bakker AJ, van der Mije S, van Alphen J, Galis F. Breaking evolutionary and pleiotropic constraints in mammals: On sloths, manatees and homeotic mutations. EvoDevo. 2011;2:11. doi: 10.1186/2041-9139-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wéry et al. (2003).Wéry N, Narotsky MG, Pacico N, Kavlock RJ, Picard JJ, Gofflot F. Defects in cervical vertebrae in boric acid-exposed rat embryos are associated with anterior shifts of hox gene expression domains. Birth Defects Research Part A: Clinical and Molecular Teratology. 2003;67:59–67. doi: 10.1002/bdra.10031. [DOI] [PubMed] [Google Scholar]