Abstract
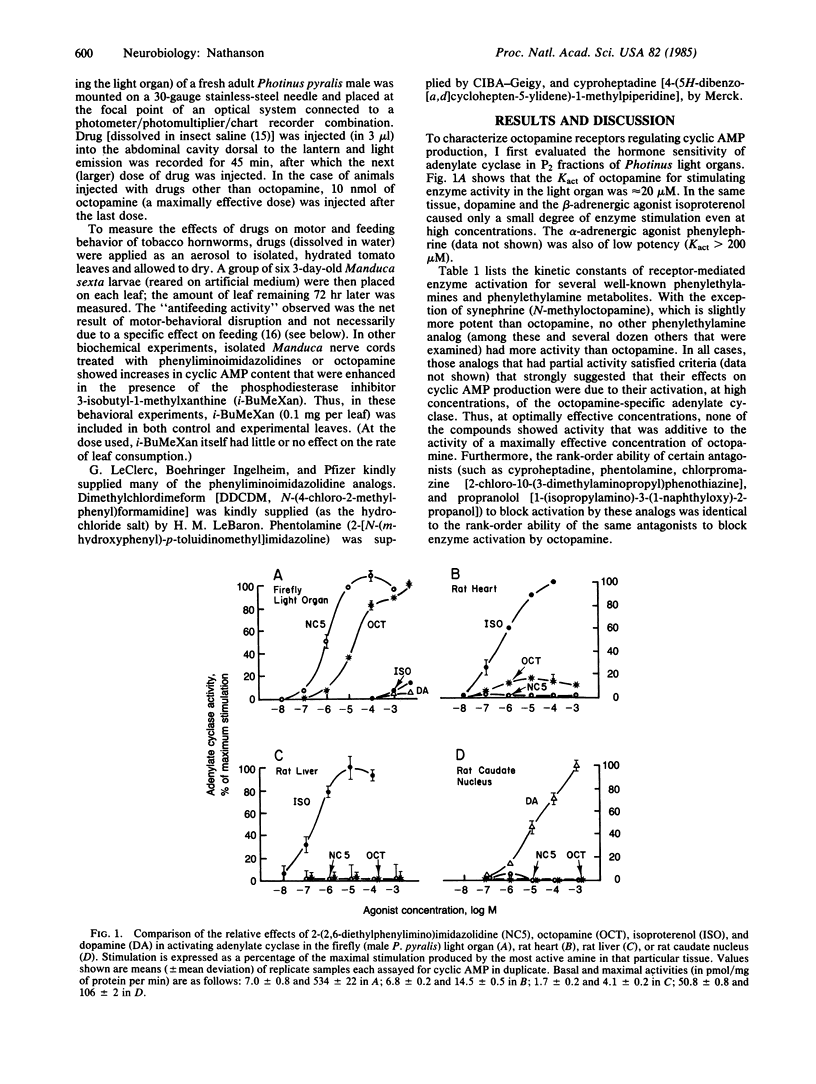
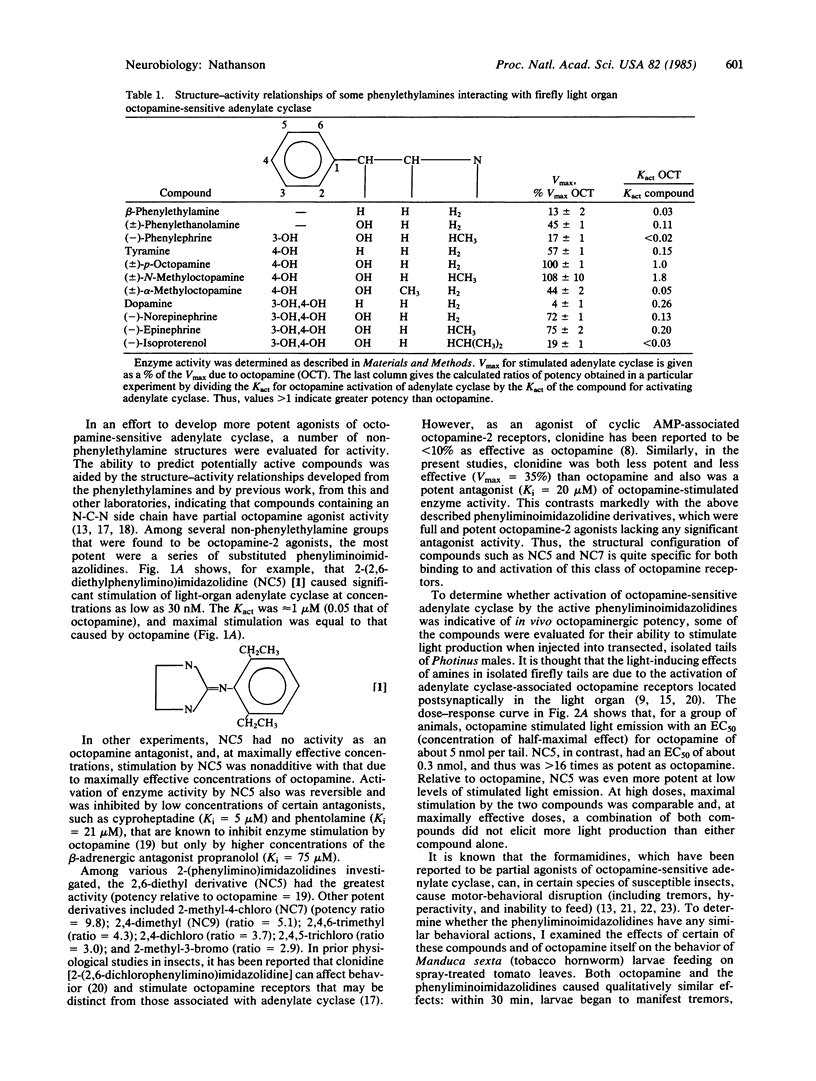
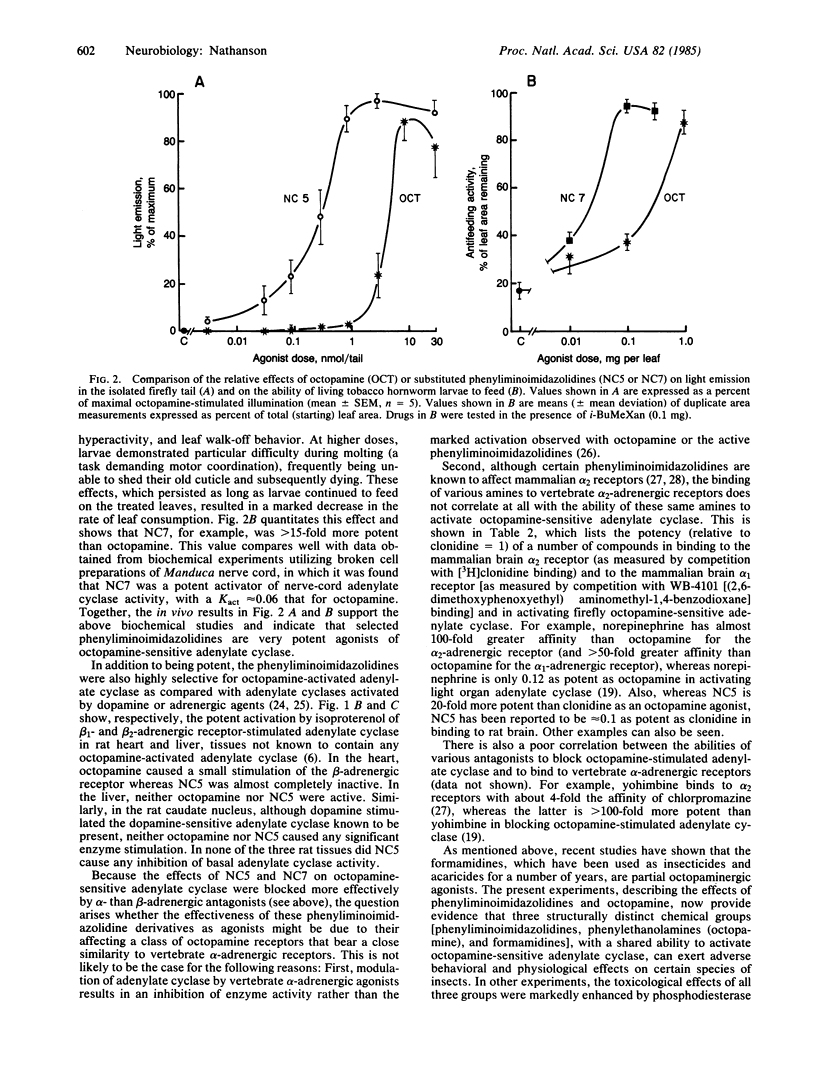
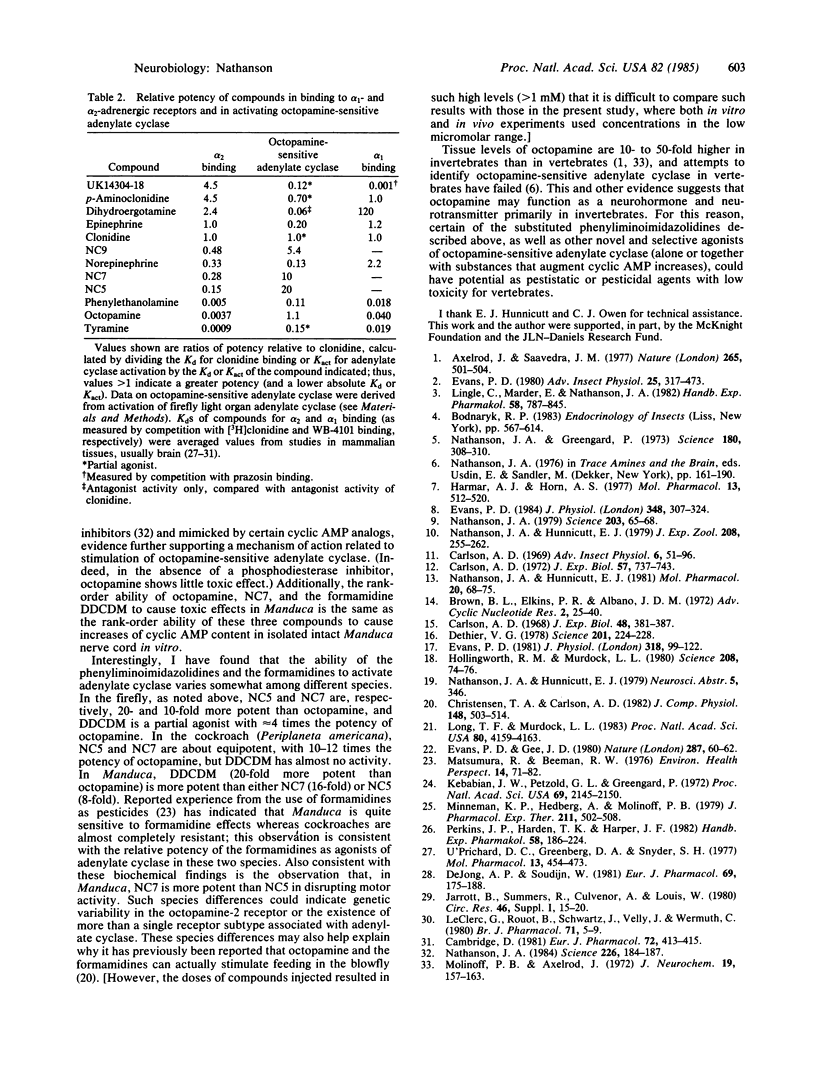
Octopamine-2 receptors, associated with activation of adenylate cyclase, mediate a number of the important hormonal and neurotransmitter functions of octopamine in invertebrates. By utilizing the highly enriched octopamine-sensitive adenylate cyclase present in the firefly light organ, it has been possible to pharmacologically characterize octopamine-2 receptors and to define a new class of highly potent and selective octopamine-2 agonists. At low concentrations, these substituted phenyliminoimidazolidines stimulate light emission when injected into fireflies. At somewhat higher concentrations, these compounds, when ingested by tobacco hornworms, cause disruption of motor and feeding behavior, leading to insect death. The effects of these compounds are markedly potentiated by phosphodiesterase inhibitors and mimicked by other activators of octopamine-sensitive adenylate cyclase, including octopamine itself. Because octopamine-2 receptors appear to be present primarily in invertebrates, these findings, together with other data, raise the possibility that potent and selective octopamine agonists could be useful as insect toxins with low toxicity in vertebrates.
Full text
PDF
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Axelrod J., Saavedra J. M. Octopamine. Nature. 1977 Feb 10;265(5594):501–504. doi: 10.1038/265501a0. [DOI] [PubMed] [Google Scholar]
- Brown B. L., Ekins R. P., Albano J. D. Saturation assay for cyclic AMP using endogenous binding protein. Adv Cyclic Nucleotide Res. 1972;2:25–40. [PubMed] [Google Scholar]
- Cambridge D. UK-14,304, a potent and selective alpha2-agonist for the characterisation of alpha-adrenoceptor subtypes. Eur J Pharmacol. 1981 Jul 10;72(4):413–415. doi: 10.1016/0014-2999(81)90588-4. [DOI] [PubMed] [Google Scholar]
- Carlson A. D. Effect of adrenergic drugs on the lantern of the larval Photuris firefly. J Exp Biol. 1968 Apr;48(2):381–387. doi: 10.1242/jeb.48.2.381. [DOI] [PubMed] [Google Scholar]
- De Jong A. P., Soudijn W. Relationships between structure and alpha-adrenergic receptor affinity of clonidine and some related cyclic amidines. Eur J Pharmacol. 1981 Jan 16;69(2):175–188. doi: 10.1016/0014-2999(81)90412-x. [DOI] [PubMed] [Google Scholar]
- Dethier V. G. Other tastes, other worlds. Science. 1978 Jul 21;201(4352):224–228. doi: 10.1126/science.663651. [DOI] [PubMed] [Google Scholar]
- Evans P. D. A modulatory octopaminergic neurone increases cyclic nucleotide levels in locust skeletal muscle. J Physiol. 1984 Mar;348:307–324. doi: 10.1113/jphysiol.1984.sp015112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans P. D., Gee J. D. Action of formamidine pesticides on octopamine receptors. Nature. 1980 Sep 4;287(5777):60–62. doi: 10.1038/287060a0. [DOI] [PubMed] [Google Scholar]
- Evans P. D. Multiple receptor types for octopamine in the locust. J Physiol. 1981 Sep;318:99–122. doi: 10.1113/jphysiol.1981.sp013853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmar A. J., Horn A. S. Octopamine-sensitive adenylate cyclase in cockroach brain: effects of agonists, antagonists, and guanylyl nucleotides. Mol Pharmacol. 1977 May;13(3):512–520. [PubMed] [Google Scholar]
- Hollingworth R. M., Murdock L. L. Formamidine pesticides: octopamine-like actions in a firefly. Science. 1980 Apr 4;208(4439):74–76. doi: 10.1126/science.208.4439.74. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Petzold G. L., Greengard P. Dopamine-sensitive adenylate cyclase in caudate nucleus of rat brain, and its similarity to the "dopamine receptor". Proc Natl Acad Sci U S A. 1972 Aug;69(8):2145–2149. doi: 10.1073/pnas.69.8.2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc G., Rouot B., Schwartz J., Velly J., Wermuth C. G. Studies on some para-substituted clonidine derivatives that exhibit an alpha-adrenoceptor stimulant activity. Br J Pharmacol. 1980;71(1):5–9. doi: 10.1111/j.1476-5381.1980.tb10902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long T. F., Murdock L. L. Stimulation of blowfly feeding behavior by octopaminergic drugs. Proc Natl Acad Sci U S A. 1983 Jul;80(13):4159–4163. doi: 10.1073/pnas.80.13.4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumura F., Beeman R. W. Biochemical and physiological effects of chlordimeform. Environ Health Perspect. 1976 Apr;14:71–82. doi: 10.1289/ehp.761471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minneman K. P., Hedberg A., Molinoff P. B. Comparison of beta adrenergic receptor subtypes in mammalian tissues. J Pharmacol Exp Ther. 1979 Dec;211(3):502–508. [PubMed] [Google Scholar]
- Molinoff P. B., Axelrod J. Distribution and turnover of octopamine in tissues. J Neurochem. 1972 Jan;19(1):157–163. doi: 10.1111/j.1471-4159.1972.tb01265.x. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A. Caffeine and related methylxanthines: possible naturally occurring pesticides. Science. 1984 Oct 12;226(4671):184–187. doi: 10.1126/science.6207592. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A., Greengard P. Octopamine-sensitive adenylate cyclse: evidence for a biological role of octopamine in nervous tissue. Science. 1973 Apr 20;180(4083):308–310. doi: 10.1126/science.180.4083.308. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A., Hunnicutt E. J. N-demethylchlordimeform: a potent partial agonist of octopamine-sensitive adenylate cyclase. Mol Pharmacol. 1981 Jul;20(1):68–75. [PubMed] [Google Scholar]
- Nathanson J. A., Hunnicutt E. J. Neural control of light emission in Photuris larvae: identification of octopamine-sensitive adenylate cyclase (1). J Exp Zool. 1979 May;208(2):255–262. doi: 10.1002/jez.1402080213. [DOI] [PubMed] [Google Scholar]
- Nathanson J. A. Octopamine receptors, adenosine 3',5'-monophosphate, and neural control of firefly flashing. Science. 1979 Jan 5;203(4375):65–68. doi: 10.1126/science.214856. [DOI] [PubMed] [Google Scholar]
- U'Prichard D. C., Greenberg D. A., Snyder S. H. Binding characteristics of a radiolabeled agonist and antagonist at central nervous system alpha noradrenergic receptors. Mol Pharmacol. 1977 May;13(3):454–473. [PubMed] [Google Scholar]


