Abstract
Ubiquitination is a reversible post-translational modification that plays a dynamic role in regulating most eukaryotic processes. Deubiquitinating enzymes (DUBs), which hydrolyze the isopeptide or peptide linkages joining ubiquitin to substrate lysines or N-termini, therefore play a key role in ubiquitin signaling. Cells employ multiple mechanisms to regulate DUB activity and thus ensure the appropriate biological response. Recent structural studies have shed light on several different mechanisms by which DUB activity and specificity is regulated.
Keywords: ubiquitin, deubiquitinating enzymes, UBL, DUB
Introduction
The attachment of the small protein, ubiquitin, to protein substrate lysines plays a pivotal role in regulating a vast range of physiological processes in eukaryotes.1 While the first function of ubiquitin to be characterized was its essential role in targeting proteins for proteasomal degradation, ubiquitination is now understood to play many nondegradative roles in processes including transcription,2 the DNA damage response,3 endosomal sorting,4 and the inflammatory response.5 The ability of ubiquitin to play diverse signaling roles is the result of the different types of ubiquitin modifications that can be assembled and their reversibility. The C-terminus of ubiquitin is joined by an isopeptide linkage to lysine side chains in a three-enzyme cascade (reviewed in6,7). The E1 ubiquitin activating enzyme charges an E2 ubiquitin conjugating enzyme with ubiquitin, yielding an E2 with a thioester linkage between its active site cysteine and the ubiquitin C-terminus (E2∼Ub). An E3 ubiquitin ligase then binds to both substrate and charged E2∼Ub and facilitates the attack of the substrate lysine on the E2-Ub thioester, yielding the ubiquitinated lysine and the free E2. Ubiquitin itself can be ubiquitinated at any of its seven lysines, giving rise to a polyubiquitin chain. Ubiquitin can also be joined by a peptide bond to the N-terminus of another ubiquitin, yielding a linear ubiquitin chain,8 or to the N-terminus of the ubiquitin-like protein, SUMO.9 Polyubiquitin chains with different linkage types signal distinct outcomes for the modified substrates1: K48-linked chains target substrates for proteasomal degradation,10 whereas K63-linked chains are non-degradative signals that play a role in the DNA damage response.11 Monoubiquitination plays a variety of non-degradative roles including regulation of transcription.2 The reader is referred to a number of recent reviews covering the physiological roles of ubiquitination12–15 as well as the enzymatic machinery that targets specific substrates for ubiquitination and governs the nature and multiplicity of the ubiquitin modification.1,16–18
Deubiquitinating enzymes (DUBs) play a key role in ubiquitin homeostasis by regulating the nature and extent of substrate ubiquitination as well as the available pool of free ubiquitin. The human genome encodes over 90 DUBs that fall into five structural classes (Fig. 1): the ubiquitin-specific protease (USP), ovarian tumor (OTU), ubiquitin C-terminal hydrolase UCH and Josephin (MJD) cysteine proteases and the Jab1/Mov34/Mpr1 (JAMM) metalloproteases (reviewed in12,19–21). Outside of the conserved catalytic domain that defines each of these families, DUBs vary greatly in the number and type of additional domains12 they contain as well as in the higher-order complexes they form with other proteins.22 While only a fraction of these enzymes have been well-characterized, it is clear that DUBs vary greatly in their specificity for specific substrates and selectivity for cleaving particular types of polyubiquitin chains.19 Studies to date have revealed a variety of mechanisms by which DUB activity and specificity is regulated, including post-translational modification,23 oxidation,24,25 and through interactions with partner proteins.26–28
Figure 1.
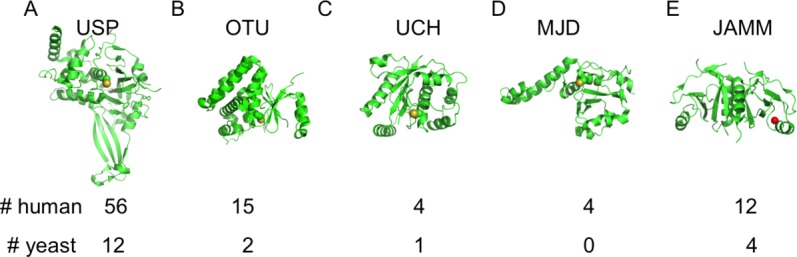
11.The five classes of DUBs. Active site cysteine (yellow) or zinc (red) shown as spheres. The number of each class in humans and in yeast is indicated below (from Ref.12). A: USP–USP7/HAUSP (1NB8)31. B: OTU–OTUB2 (1ZFF).70 C: UCH–UCH-L3 (1UCH).71 D: MJD–Ataxin-3 (3O65).72 E: JAMM–AfJAMM (1R5X).73
A defining characteristic of DUBs is that many are found in complex with other proteins.22,26 While an understanding of how these associations govern DUB activity is in its infancy, several principles have emerged. One important role of the association of DUBs with other proteins is to activate the intrinsic enzymatic activity of the DUB. Many DUBs have weak isopeptidase activity that is enhanced upon binding to one or more subunits.20 Conversely, binding to partner proteins can also downregulate DUB activity. Finally, DUBs can be targeted to specific substrates by binding partners. One intriguing observation to emerge from mass spectrometry studies is that many DUBs form complexes with E3 ligases, while a number also bind to E2 enzymes.22,28 This points to coregulation of ubiquitin conjugation and removal, although the way in which the balance of ubiquitination is controlled through these associations remains to be determined.
Understanding how DUBs are regulated in cells is key to elucidating the regulatory networks that control ubiquitin signaling as well as for providing a basis for designing chemotherapeutic agents that target DUBs.29 This brief review covers recent advances in understanding the molecular mechanisms by which DUB activity is modulated.
Active Site Rearrangements in USP7 and OTULIN
Although the cysteine protease DUBs differ widely in folds, they share a common papain-like catalytic triad (Cys-His-Asn/Asp) and mechanism of isopeptide or peptide bond cleavage.30 These residues must be properly aligned so that the histidine can activate the cysteine for nucleophilic attack on the peptide linkage. Some DUBs, however, have misaligned or occluded active sites that must undergo rearrangements to accommodate their substrates (reviewed in Ref.20). Structural studies of DUBs from the USP31 and OTU32,33 families have revealed that these active site residues are in some cases misaligned, and must undergo substrate-induced rearrangements to adopt a catalytically competent configuration. The requirement for substrate-induced alignment of active site residues was first shown for USP7 (HAUSP)31 [Fig. 1(A)]. USP7 plays multiple roles in responses to stress, DNA damage and viral infection,34 and its substrates include the p53 tumor suppressor.35 The catalytic cysteine and histidine are in a nonproductive conformation in the apoenzyme and must undergo a significant conformational rearrangement that is triggered by ubiquitin binding31 [Fig. 2(A)].
Figure 2.
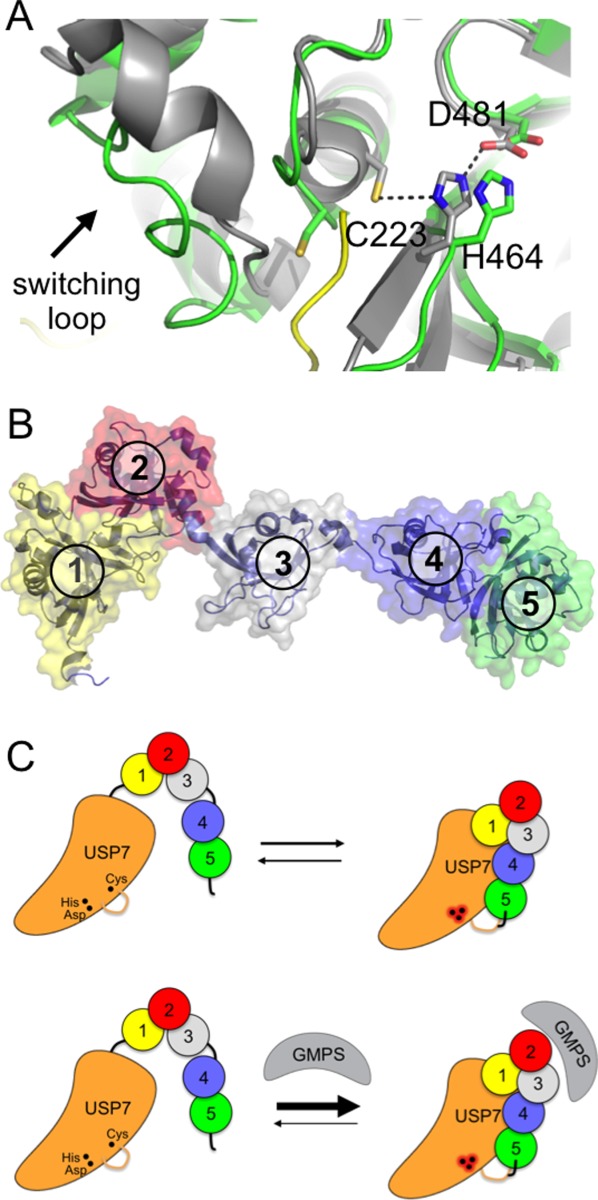
Activation of USP7 by the HUBL domain. A: Active site of USP7 in the presence (gray) and absence (green) of bound ubiquitin aldehyde (yellow; 1NB8 and 1NBF). Arrow indicates switching loop. B: HUBL domain of USP7, consisting of five UBL domains (numbered). C: Model for activation of USP7 by the HUBL domain, which is promoted by GMPS binding. The HUBL 4 and 5 domains bind to USP7 and trigger a conformational change in the switching loop, which in turn results in a reconfiguration of the catalytic triad to the active conformation. GMPS binding stabilizes the association of the HUBL-4, 5 domains and thereby maintains the active state. Adapted from Ref.36.
Recent studies have shed light on how this feature of USP7 is exploited to regulate its activity. In addition to its conserved USP catalytic domain, USP7 contains five C-terminal ubiquitin-like (UBL) domains collectively known as the HAUSP UBL (HUBL) domain [Fig. 2(B)], which is required for full enzymatic activity.36 UBL domains are found in many USP-class DUBs, where they play diverse roles in regulating enzymatic activity.37 The HUBL domain of USP7 contacts the catalytic domain and increases its affinity for ubiquitin.36 The HUBL domain also stimulates catalysis, an effect that is mediated by a set of residues called the “switching loop” that are located adjacent to the active site and that govern the configuration of the active site residues [Fig. 2(A)]. This stimulatory effect of the HUBL domain is promoted by GMP synthase, which was shown by mutagenesis studies and small-angle x-ray scattering to promote the activated conformation of USP7 in which the HUBL domain associates with the USP domain.36 The HUBL domain governs the conformation of the switching loop and thus the catalytically competent configuration of active site residues [Fig. 2(C)]. Future high-resolution structural studies will be needed to shed light on the precise mechanism by which the HUBL domain communicates changes to the enzyme active site.
Active site rearrangements can also be triggered by the substrate itself, and thereby play a role in determining the specificity of a DUB for particular substrates. Members of the OTU family of DUBs exhibit especially high selectivity for cleaving polyubiquitin chains with particular linkage types38: OTUB1, for example, cleaves only K48-linked chains32,39 whereas OTUD2 preferentially cleaves K11-linked polyubiquitin.38 OTULIN/Fam105b is a newly identified OTU class DUB that specifically cleaves linear ubiquitin and plays a role in cytokine signaling33,40 and angiogenesis.41 In contrast with polyubiquitin chains in which each ubiquitin C-terminus is linked by an isopeptide bond to a lysine in another ubiquitin, the individual ubiquitins in a linear ubiquitin chain (also known as M1 polyubiquitin) are joined by peptide bonds between the C-terminus of one ubiquitin and the N-terminus of the next.8 While peptide and isopeptide bonds are chemically identical and are cleaved by the same catalytic mechanism, OTULIN discriminates between these two types of linkages by relying on structural differences between peptide and isopeptide linkages. The active site histidine of OTULIN [Fig. 3(A)] is not aligned for catalysis in the apoenzyme [Fig. 3(B)], but is coaxed into position by the carbonyl oxygen of the N-terminal methionine of the proximal ubiquitin [Fig. 3(C)]. The absence of an analogous group in isopeptide-linked polyubiquitin chains, with the aliphatic carbons of lysine in place of the peptide backbone, favors linear ubiquitin substrates. In addition, a residue (Glu16) in the proximal ubiquitin further promotes the active conformation of the histidine in two ways. First, Glu16 displaces another acidic side chain that interacts with the catalytic histidine in the apo enzyme and promotes a catalytically incompetent conformation. Glu16 also hydrogen bonds with the catalytic asparagine and orients it to hydrogen bond with the catalytic histidine [Fig. 3(B)]. Since the precise positioning of Glu16 relative to the active site is unique to linear polyubiquitin chains, its requirement for efficient chain hydrolysis33 helps to ensure OTULIN specificity for linear chains.
Figure 3.
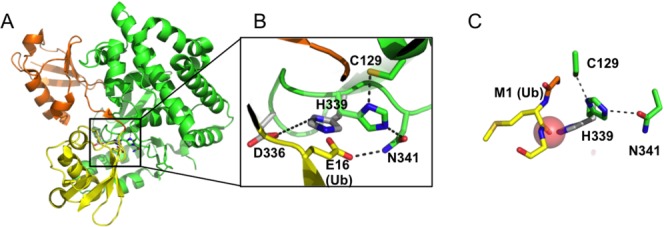
3Active site rearrangements in OTULIN. A: OTULIN (green) bound to linear di-ubiquitin (orange and yellow; 3ZNZ). B: Inset shows rearrangement of active site residues in the absence (gray) and presence (green) of bound linear di-ubiquitin. In the absence of ubiquitin, D336 favors a position of H339 that is far from the active site cysteine (3ZNZ and 3ZNV). C: Role of the carbonyl oxygen of residue M1 in the proximal ubiquitin (yellow) in favoring the active (green) over inactive (gray) conformation of H339. Transparent red sphere indicates van der Waals radius of oxygen.
Whereas misalignment of catalytic residues is exploited to regulate DUB activity in the case of USP7 and OTULIN, it is not clear how universal this is. An intriguing proposal42 is that misalignment of the catalytic triad, in particular the displacement of the histidine that is needed to activate the cysteine sulfur for nucleophilic attack, may guard against oxidative damage. Most DUBs are cysteine proteases and are thus potentially susceptible to oxidation of the active site cysteine by reactive oxygen species, which inactivates the enzyme. Indeed, several DUBs, including the OTU protein, A20,42 and several USP class DUBs,24,25 including USP1, can undergo reversible oxidation of the cysteine sulfhydryl to sulphenic acid. By reducing the reactivity of the cysteine in the absence of substrate, misalignment of the catalytic center may reduce the chance of cysteine oxidation and resulting inactivation of the DUB.
The Role of Partner Protein Interactions in Regulating Ubp8
Some DUBs must be incorporated into larger macromolecular complexes in order to be fully active. The yeast USP class enzyme, Ubp8, is an example of a DUB that is inactive unless complexed with several partner proteins that function, at least in part, as scaffolds.43,44 Ubp8 is a subunit of the ∼19 protein SAGA (Spt-Ada-Gcn5-Acetyltransferase) transcriptional coactivator complex, which deubiquitinates histone H2B and acetylates histone H3 during transcription activation and elongation.45 SAGA's deubiquitinating activity resides in a four-protein complex known as the DUB module, which contains Ubp8 bound to Sgf11, Sus1, and Sgf73.46,47 The ∼100 N-terminal residues of Sgf73 are integral to the DUB module and are sufficient for full activity, while the remainder of the protein tethers the DUB module to the rest of SAGA.46,47 Structural studies48,49 revealed that the four DUB module subunits are highly intertwined and are organized around the two globular domains of Ubp8: the C-terminal catalytic USP domain and an N-terminal zinc finger-ubiquitin binding protein (ZnF-UBP) domain [Fig. 4(A)]. A notable feature of the complex is that Sgf11, Sus1, and Sgf73 are largely non-globular, but instead depend upon interactions with other subunits for their observed structure. Whereas some ZnF-UBP domains, such as the ZnF-UBP domain of IsoT (USP5), bind the ubiquitin C-terminus,50 the Ubp8 ZnF-UBP domain lacks the corresponding residues that would bind ubiquitin. Instead, this domain in Ubp8 plays a role in DUB module assembly by associating with the long N-terminal helix of Sgf11 and with Sus1.48,51 The C-terminal zinc finger domain of Sgf11 binds near the Ubp8 active site and is connected to the N-terminal helix by a long linker that spans the two Ubp8 globular domains [Fig. 4(A)]. Sgf73 is like the “glue” that cements the two lobes of the complex together, filling in the gaps that would otherwise be found between the two lobes while possibly aligning the two domains.
Figure 4.
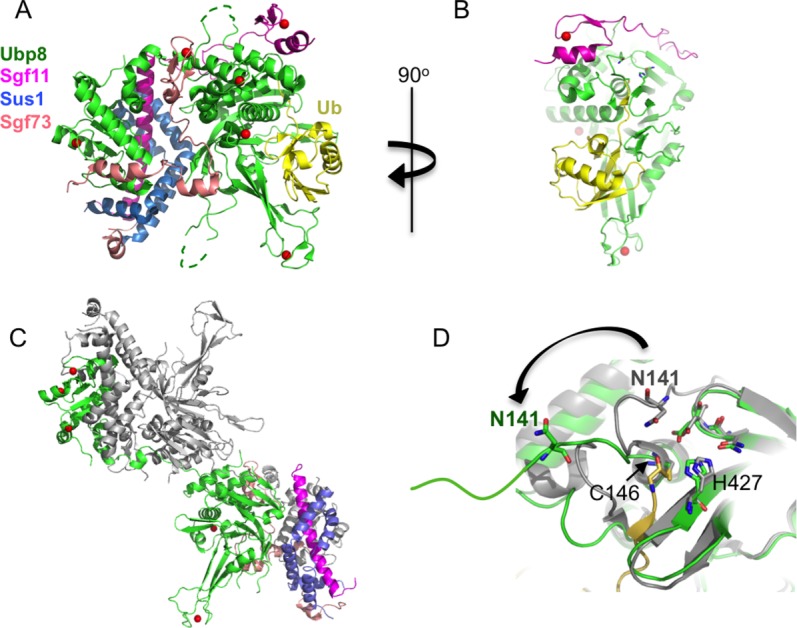
Activation of the Ubp8 enzyme in the SAGA deubiquitinating module. A: The SAGA DUB module bound to ubiquitin (yellow). The color code for the four DUB module subunits is indicated at left. Zinc atoms are depicted as red spheres (3MHS). B: View of the DUB module rotated 90o showing USP domain of Ubp8 (green) and the Sgf11 zinc finger (magenta) bound near the Ubp8 active site. Ubiquitin is yellow. C: “Domain-swapped dimer” of DUB modules that occurs when the Sgf11 zinc finger is deleted. The USP domain of one Ubp8 protein is in the lower complex and the ZnF-UBP domain is in the upper complex. There are two copies each of Ubp8, Sgf11, Sus1, and Sgf73. One set of proteins is colored as in (A); those in the second set are colored gray (4FJC). D: Conformation of Ubp8 in the “domain-swapped” dimer (green) displaces the oxyanion-stabilizing residue, N141, from the active site as compared with its position in the wild-type complex (gray).
Structures of the DUB module in the presence49 and absence48,49 of ubiquitin suggested that Ubp8's partner proteins may contribute to enzymatic activity by stabilizing an active conformation of the catalytic USP domain that is both capable of binding to substrate and has its active site residues prealigned. In contrast with USP family members such as USP1452 and USP8,53 which have partially occluded substrate-binding sites, the ubiquitin-binding site of Ubp8 is little changed in the presence and absence of bound ubiquitin aldehyde (a covalent inhibitor whose C-terminus forms a thiohemiacetal bond with the active site cysteine54). While the structure of Ubp8 in the absence of its binding partners is not known, it has been proposed that Sgf73, Sgf11, and Sus1 may help to stabilize a conformation of Ubp8 that can readily accommodate the substrate ubiquitin without the need for energetically costly conformational rearrangements.49 Interestingly, the Ubp8 residues (230-235) corresponding to the USP7 switching loop36 are contacted by Sgf11 but are only ordered when ubiquitin is bound,48,49,55 leaving open the question of the involvement of this loop in regulating Ubp8 activity.
The Sgf11 zinc finger is critical to enzymatic activity, as deleting this domain dramatically reduces DUB module enzymatic activity.48 Recent structural studies addressing the contribution of Sgf11 to Ubp8 activity uncovered a role for Sgf11 in stabilizing an active conformation of the Ubp8 catalytic center.55 Sgf11 contains a C-terminal zinc finger domain that binds adjacent to the Ubp8 active site [Fig. 4(B)]. Structural and solution studies55 unexpectedly revealed that deletion of Sgf11 zinc finger gives rise to domain swapping between two DUB modules. As shown in Figure 4(C), the domain-swapping arises because each Ubp8 protein has its ZnF-UBP domain in one DUB module assembly and its USP domain in another. This exchange of Ubp8 domains between two DUB module assemblies preserves the overall relation of protein domains to one another, with the exception of the polypeptide strand that connects the Ubp8 globular domains [Fig. 4(C,D)]. Importantly, the dramatic reconfiguration of this strand has the critical consequence of displacing a key active site residue, N141 [Fig. 4(D)], which is proposed to stabilize the oxyanion intermediate in the isopeptidase reaction. Gain of function mutations that stabilize the “monomeric” form of the DUB module even in the absence of the Sgf11 zinc finger domain increase enzymatic activity,55 consistent with a role for Sgf11 in positioning the oxyanion hole residue near the active site cysteine by preventing domain swapping and favoring the “monomeric” form of the four-protein DUB module. The “monomeric” DUB module lacking the Sgf11 zinc finger is, however, still 5-fold less active (as measured by kcat/KM ratio) than an intact DUB module, pointing to additional roles for Sgf11 that remain to be elucidated.
Whether the dependence of Ubp8 activity on its incorporation into the DUB module is exploited to regulate the activity of Ubp8 remains an open question; in other words, is Ubp8 ever found in the cell in the absence of its four partners? The activity of the human homologue of Ubp8, USP22, also depends upon its association with the other three human DUB module subunits.56 Since the DUB module does not appear to be highly specific for particular substrates, at least in vitro,55 it is possible that its dependence upon partner proteins that anchor it to the SAGA complex and direct its activity towards chromatin may also prevent off-target deubiquitination in the cell.
Cross-Regulation by OTUB1 and E2 Enzyme Complexes
The OTU class enzyme, OTUB1, forms complexes with E2 ubiquitin conjugating enzymes that impact the activity of both the E2 and OTUB1. As mentioned above, OTU DUBs specifically degrade polyubiquitin chains and exhibit preferences for particular chain linkage types.32,33,38,39,57 OTUB1 is highly specific for cleaving K48-linked polyubiquitin chains and contains two distinct binding sites for the proximal and distal ubiquitins that flank the K48 linkage.32,39 A proteomics study22 first revealed that OTUB1 can be found in complex with the E2 ubiquitin conjugating enzymes, UBE2N (UBC13) and members of the UBE2D (UBCH5) and UBE2E family, which has been confirmed by subsequent studies.28,58 These complexes turn out to have dual functions: OTUB1 suppresses the ubiquitin conjugating activity of the E2,28 while the E2 stimulates OTUB1 DUB activity.59 Recent structural studies have elucidated the mechanism of this reciprocal regulation by enzymes that conjugate and remove ubiquitin.59–62
The first evidence that OTUB1-E2 interactions serve a regulatory role came from studies of the cellular response to DNA double strand breaks.28 These lesions trigger a cascade of ubiquitination events at the break sites, including the accumulation of K63-linked polyubiquitin chains, which recruit the BRCA1 complex (for a recent review, see Ref.3). An RNAi screen for DUBs that prevent K63 chains from persisting at damage sites paradoxically identified OTUB1,28 which does not cleave K63-linked polyubiquitin.32,39 Moreover, the catalytic activity of OTUB1 was not needed for the observed suppression of K63 polyubiquitin accumulation. Instead, OTUB1 binds preferentially to the charged E2∼Ub reaction intermediate and prevents ubiquitin transfer.28 This preferential binding, as well as the ability of OTUB1 to inhibit E2 activity, was dependent on a C-terminal extension of the OTU domain [Fig. 1(B)] that is disordered in the absence of substrate.32,60 Although the initial study focused on inhibition of UBC13, the E2 that synthesizes K63-linked chains,11 OTUB1 also inhibits other E2s including UBCH5 isoforms.28
Structural and solution studies have revealed that binding of OTUB1 to the E2∼Ub conjugate is regulated by binding of a free ubiquitin monomer to the distal site of OTUB1, which allosterically regulates the binding of OTUB1 to charged E2 enzymes.60,61 In the absence of free ubiquitin, OTUB1 binds with equal affinity to both charged and uncharged E2.60,61 However, the addition of either free ubiquitin or the covalent inhibitor, ubiquitin aldehyde, preferentially increases the affinity of OTUB1 for the charged E2∼Ub,60,61 and increases the ability of OTUB1 to inhibit K63-linked polyubiquitin synthesis.60 The structural basis for these observations was revealed by studies of OTUB1 with the E2, UBC13,60 and with UBCH5.59,61 The structure of OTUB1 bound to ubiquitin aldehyde and a UBC13∼Ub conjugate60 is shown in Figure 5(A). Ubiquitin aldehyde (mimicking free ubiquitin) binds in the distal site of OTUB1 while the ubiquitin conjugated to UBC13 binds in the OTUB1 proximal site [Fig. 5(A)]. A comparison with the structure of OTUB1 bound to UBC13 in the absence of ubiquitin shows that ubiquitin binding triggers conformational changes in OTUB1 [Fig. 5(B)]. The structural changes include rearrangements within the OTU globular domain that maximize contacts with the ubiquitin bound in the OTUB1 proximal site60 as well as formation of a ∼20-residue ubiquitin-binding helix in the OTUB1 N-terminus,60,61 which is disordered in the apoenzyme.32,60 The spatial relationship between the ubiquitins bound to OTUB1 mimics that of K48 diubiquitin [Fig. 5(C)], suggesting that the ability of OTUB1 to bind to K48-linked diubiquitin substrates was adapted to convert OTUB1 into an inhibitor. The resulting inhibitory complex blocks binding of E3 ligases as well as MMS2 (or UEV1A), a subunit that must bind to UBC13 in order to direct synthesis of K63-linked chains.11
Figure 5.
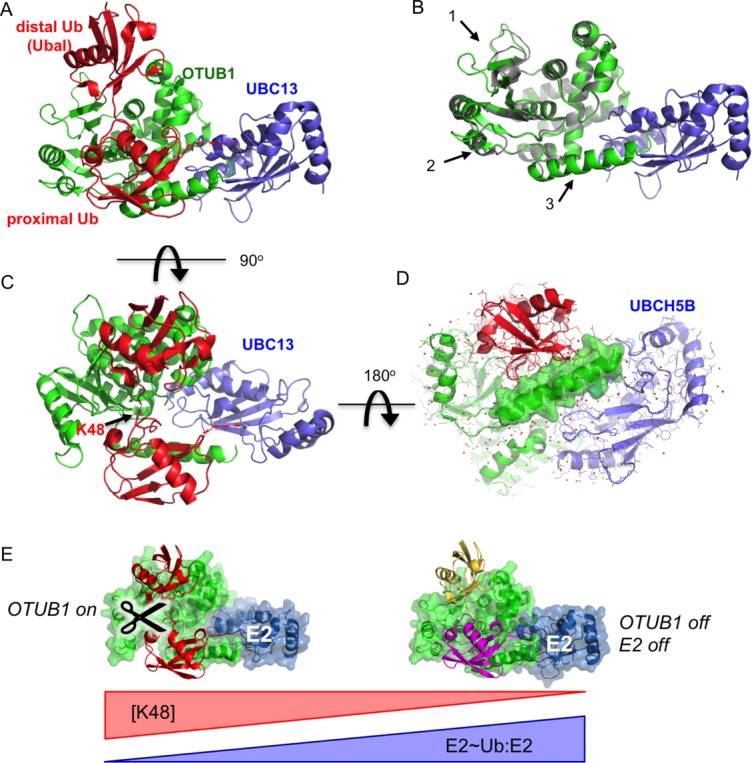
Cross-regulation by OTUB1 and E2 enzymes. A: Structure of OTUB1 bound to ubiquitin aldehyde (Ubal) and a UBC13∼Ub conjugate. OTUB1 in this figure is a hybrid containing the catalytic domain of C. elegans OTUB1 and the N-terminal helix of human OTUB160 (4DHZ). B: Superposition of OTUB1 in the presence (green) and absence (gray) of bound ubiquitin. UBC13 shown in blue. Arrows indicate conformational changes in the globular domain (1 and 2) and formation of an N-terminal ubiquitin-binding helix (3) (4DHZ and 4DHI). C: Binding of ubiquitin to distal (top) and proximal (bottom) sites in OTUB1 mimics K48-linked diubiquitin substrate. View of complex in (A) rotated by 90° shows juxtaposition of K48 of proximal ubiquitin and the C-terminus of the distal ubiquitin. D: Structure of OTUB1-Ubal bound to UBCH5B∼Ub59 (4LDT). View of complex is rotated 180° relative to view of OTUB1-Ubal-UBC13∼Ub complex shown in (C). Surface rendering of OTUB1 C-terminal ubiquitin-binding helix illustrates how this helix, which forms only in OTUB1-E2 complexes containing two bound ubiquitins, forms contacts with both UBCH5B and the proximal ubiquitin. E: Model for regulation of OTUB1 activity in response to changes in ratio of uncharged to charged E2 (E2:E2∼Ub) as well as the concentration of K48-linked polyubiquitin. At low levels of E2 charging (left), E2 binding to OTUB1 stimulates cleavage of K48-linked polyubiquitin. A high proportion of charged E2∼Ub (right), the repressed complex will form between the E2∼Ub, OTUB1 and a free ubiquitin monomer (yellow), which blocks both OTUB1 DUB activity and the ability of the E2 to conjugate ubiquitin to substrate.
The ability of OTUB1 to form complexes with E2 enzymes impacts OTUB1 enzymatic activity, as well. Although the studies described above focused on the ability of OTUB1 to suppress E2 activity, the inhibitory complex consisting of OTUB1 bound to an E2∼Ub thioester plus an additional free ubiquitin [Fig. 5(A)] also blocks OTUB1 deubiquitinating activity by preventing substrate binding. What then is the impact on OTUB1 deubiquitinating activity when an uncharged E2 enzyme binds? This question was explored in a recent study that screened human E2 enzymes for their effect on the ability of OTUB1 cleavage of K48-linked polyubiquitin.59 Remarkably, six E2s – the three UBCH5/UBE2D isoforms, UBE2E1, UBE2N, and UBE2W– stimulated OTUB1 cleavage of K48-linked substrates by lowering the KM by as much as ∼35 fold.59 This stimulation of substrate binding to OTUB1 depended on the same interactions between OTUB1 and E2 that are required to stabilize the inhibitory complex. A key contribution of the E2 to OTUB1 activity stems from direct contacts between the E2 and the N-terminal substrate-binding helix of OTUB1 [Fig. 5(C)], which is also essential to the ability of OTUB1 to repress E2 enzymes.28 Although a structure of OTUB1 bound to a true K48-linked ubiquitin substrate has yet to be reported, a complex of OTUB1 bound to both a K48 diubiquitin substrate and UBCH5B could be modeled based on a high-resolution structure of OTUB1 bound to ubiquitin aldehyde and a UBCH5B∼Ub conjugate59 [Fig. 5(D)]. The structure shows that UBCH5B contacts the proximal ubiquitin-binding helix of OTUB1, which is disordered in the apoenzyme.32,60 By promoting folding of the OTUB1 N-terminus, which contacts the proximal ubiquitin in the K48-linked substrate, the E2 helps to prepay the entropic penalty and thereby increase the affinity of OTUB1 for substrate.
Whether or not E2 enzymes stimulate OTUB1 DUB activity or form a complex that represses both the E2 and the DUB depends upon E2 charging, since charged E2s form the repressive OTUB1/E2∼Ub/Ub complex while uncharged E2s stimulate OTUB1 cleavage of K48 polyubiquitin chains. For at least two of the OTUB1 partners that have been examined, UBC13 and UBCH5B, a significant proportion of the E2s are uncharged in cultured cells,59 indicating that both types of OTUB1-E2 complexes may coexist. The balance between the activated and repressed OTUB1 may thus be governed by E2 charging ratios as well as by the concentration of K48-linked polyubiquitin [Fig. 5(E)], although the degree to which these may vary under different conditions or in response to cellular stress remains to be investigated. The importance of E2 interactions for OTUB1 deubiquitinating activity in vivo remains to be explored using validated substrates such as c-IAP163 and the E3 ligase, GRAIL,64 whose activity and levels have previously been shown to be regulated through the deubiquitinating activity of OTUB1.
Outlook
We have only just begun to scratch the surface in terms of a molecular understanding of how DUB activity is regulated. Cell-based and solution studies have revealed numerous well-documented examples of DUB regulation21 that await structural and mechanistic studies. In particular, there are now many examples of DUBs that are regulated through interactions with partner proteins in ways that are poorly understood. A notable example is UAF1 (USP1-Associated Factor 1), which regulates USP165 as well as several other DUBs from the USP family.27,66 UAF1, a member of the WD40 family of seven β-propeller proteins,67,68 forms a complex with USP proteins and increases activity. Nearly 20 USP class DUBs are found in complex with other WD40 proteins,66 although the significance and regulatory consequences of these complexes remains to be explored. Similarly, more than two dozen DUBs have been found in complex with E3 ligases,22 although our understanding of the regulatory implications of these complexes are similarly at very early stages. The central role DUBs play in so many pathways and the keen interest in DUBs as drug targets69 virtually guarantee that we can look forward to many more studies that shed light on the diverse mechanisms by which these enzymes are regulated.
Acknowledgments
The author is indebted to the members of her laboratory for their many contributions to studies of ubiquitin signaling, including recent work covered in this review.
References
- 1.Komander D, Rape M. The ubiquitin code. Annu Rev Biochem. 2012;81:203–229. doi: 10.1146/annurev-biochem-060310-170328. [DOI] [PubMed] [Google Scholar]
- 2.Wright DE, Wang CY, Kao CF. Histone ubiquitylation and chromatin dynamics. Front Biosci. 2012;17:1051–1078. doi: 10.2741/3973. [DOI] [PubMed] [Google Scholar]
- 3.Jackson SP, Durocher D. Regulation of DNA damage responses by ubiquitin and SUMO. Mol Cell. 2013;49:795–807. doi: 10.1016/j.molcel.2013.01.017. [DOI] [PubMed] [Google Scholar]
- 4.Piper RC, Lehner PJ. Endosomal transport via ubiquitination. Trends Cell Biol. 2011;21:647–655. doi: 10.1016/j.tcb.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen J, Chen ZJ. Regulation of NF-kappaB by ubiquitination. Curr Opin Immunol. 2013;25:4–12. doi: 10.1016/j.coi.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kerscher O, Felberbaum R, Hochstrasser M. Modification of proteins by ubiquitin and ubiquitin-like proteins. Annu Rev Cell Dev Biol. 2006;22:159–180. doi: 10.1146/annurev.cellbio.22.010605.093503. [DOI] [PubMed] [Google Scholar]
- 7.Pickart CM, Eddins MJ. Ubiquitin: structures, functions, mechanisms. Biochim Biophys Acta. 2004;1695:55–72. doi: 10.1016/j.bbamcr.2004.09.019. [DOI] [PubMed] [Google Scholar]
- 8.Kirisako T, Kamei K, Murata S, Kato M, Fukumoto H, Kanie M, Sano S, Tokunaga F, Tanaka K, Iwai K. A ubiquitin ligase complex assembles linear polyubiquitin chains. EMBO J. 2006;25:4877–4887. doi: 10.1038/sj.emboj.7601360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tatham MH, Plechanovova A, Jaffray EG, Salmen H, Hay RT. Ube2W conjugates ubiquitin to alpha-amino groups of protein N-termini. Biochem J. 2013;453:137–145. doi: 10.1042/BJ20130244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pickart CM, Cohen RE. Proteasomes and their kin: proteases in the machine age. Nat Rev Mol Cell Biol. 2004;5:177–187. doi: 10.1038/nrm1336. [DOI] [PubMed] [Google Scholar]
- 11.Hofmann RM, Pickart CM. Noncanonical MMS2-encoded ubiquitin-conjugating enzyme functions in assembly of novel polyubiquitin chains for DNA repair. Cell. 1999;96:645–653. doi: 10.1016/s0092-8674(00)80575-9. [DOI] [PubMed] [Google Scholar]
- 12.Clague MJ, Barsukov I, Coulson JM, Liu H, Rigden DJ, Urbe S. Deubiquitylases from genes to organism. Physiol Rev. 2013;93:1289–1315. doi: 10.1152/physrev.00002.2013. [DOI] [PubMed] [Google Scholar]
- 13.Jacq X, Kemp M, Martin NM, Jackson SP. Deubiquitylating enzymes and DNA damage response pathways. Cell Biochem Biophys. 2013;67:25–43. doi: 10.1007/s12013-013-9635-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Geng F, Tansey WP. Polyubiquitylation of histone H2B. Mol Biol Cell. 2008;19:3616–3624. doi: 10.1091/mbc.E08-01-0050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hurley JH, Stenmark H. Molecular mechanisms of ubiquitin-dependent membrane traffic. Annu Rev Biophys. 2011;40:119–142. doi: 10.1146/annurev-biophys-042910-155404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wenzel DM, Stoll KE, Klevit RE. E2s: structurally economical and functionally replete. Biochem J. 2011;433:31–42. doi: 10.1042/BJ20100985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lorenz S, Cantor AJ, Rape M, Kuriyan J. Macromolecular juggling by ubiquitylation enzymes. BMC Biol. 2013;11:65. doi: 10.1186/1741-7007-11-65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Deshaies RJ, Joazeiro CA. RING domain E3 ubiquitin ligases. Annu Rev Biochem. 2009;78:399–434. doi: 10.1146/annurev.biochem.78.101807.093809. [DOI] [PubMed] [Google Scholar]
- 19.Komander D, Clague MJ, Urbe S. Breaking the chains: structure and function of the deubiquitinases. Nat Rev Mol Cell Biol. 2009;10:550–563. doi: 10.1038/nrm2731. [DOI] [PubMed] [Google Scholar]
- 20.Reyes-Turcu FE, Ventii KH, Wilkinson KD. Regulation and cellular roles of ubiquitin-specific deubiquitinating enzymes. Annu Rev Biochem. 2009;78:363–397. doi: 10.1146/annurev.biochem.78.082307.091526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Eletr ZM, Wilkinson KD. Regulation of proteolysis by human deubiquitinating enzymes. Biochim Biophys Acta. 2014;1843:114–128. doi: 10.1016/j.bbamcr.2013.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sowa ME, Bennett EJ, Gygi SP, Harper JW. Defining the human deubiquitinating enzyme interaction landscape. Cell. 2009;138:389–403. doi: 10.1016/j.cell.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kessler BM, Edelmann MJ. PTMs in conversation: activity and function of deubiquitinating enzymes regulated via post-translational modifications. Cell Biochem Biophys. 2011;60:21–38. doi: 10.1007/s12013-011-9176-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee JG, Baek K, Soetandyo N, Ye Y. Reversible inactivation of deubiquitinases by reactive oxygen species in vitro and in cells. Nat Commun. 2013;4:1568. doi: 10.1038/ncomms2532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Cotto-Rios XM, Bekes M, Chapman J, Ueberheide B, Huang TT. Deubiquitinases as a signaling target of oxidative stress. Cell Rep. 2012;2:1475–1484. doi: 10.1016/j.celrep.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ventii KH, Wilkinson KD. Protein partners of deubiquitinating enzymes. Biochem J. 2008;414:161–175. doi: 10.1042/BJ20080798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cohn MA, Kee Y, Haas W, Gygi SP, D'Andrea AD. UAF1 is a subunit of multiple deubiquitinating enzyme complexes. J Biol Chem. 2009;284:5343–5351. doi: 10.1074/jbc.M808430200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nakada S, Tai I, Panier S, Al-Hakim A, Iemura S, Juang YC, O'Donnell L, Kumakubo A, Munro M, Sicheri F, Gingras AC, Natsume T, Suda T, Durocher D. Non-canonical inhibition of DNA damage-dependent ubiquitination by OTUB1. Nature. 2010;466:941–946. doi: 10.1038/nature09297. [DOI] [PubMed] [Google Scholar]
- 29.Sippl W, Collura V, Colland F. Ubiquitin-specific proteases as cancer drug targets. Future Oncol. 2011;7:619–632. doi: 10.2217/fon.11.39. [DOI] [PubMed] [Google Scholar]
- 30.Storer AC, Menard R. Catalytic mechanism in papain family of cysteine peptidases. Methods Enzymol. 1994;244:486–500. doi: 10.1016/0076-6879(94)44035-2. [DOI] [PubMed] [Google Scholar]
- 31.Hu M, Li P, Li M, Li W, Yao T, Wu JW, Gu W, Cohen RE, Shi Y. Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell. 2002;111:1041–1054. doi: 10.1016/s0092-8674(02)01199-6. [DOI] [PubMed] [Google Scholar]
- 32.Edelmann MJ, Iphofer A, Akutsu M, Altun M, di Gleria K, Kramer HB, Fiebiger E, Dhe-Paganon S, Kessler BM. Structural basis and specificity of human otubain 1-mediated deubiquitination. Biochem J. 2009;418:379–390. doi: 10.1042/BJ20081318. [DOI] [PubMed] [Google Scholar]
- 33.Keusekotten K, Elliott PR, Glockner L, Fiil BK, Damgaard RB, Kulathu Y, Wauer T, Hospenthal MK, Gyrd-Hansen M, Krappmann D, Hofmann K, Komander D. OTULIN antagonizes LUBAC signaling by specifically hydrolyzing Met1-linked polyubiquitin. Cell. 2013;153:1312–1326. doi: 10.1016/j.cell.2013.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nicholson B, Suresh Kumar KG. The multifaceted roles of USP7: new therapeutic opportunities. Cell Biochem Biophys. 2011;60:61–68. doi: 10.1007/s12013-011-9185-5. [DOI] [PubMed] [Google Scholar]
- 35.Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J, Gu W. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416:648–653. doi: 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- 36.Faesen AC, Dirac AM, Shanmugham A, Ovaa H, Perrakis A, Sixma TK. Mechanism of USP7/HAUSP activation by its C-terminal ubiquitin-like domain and allosteric regulation by GMP-synthetase. Mol Cell. 2011;44:147–159. doi: 10.1016/j.molcel.2011.06.034. [DOI] [PubMed] [Google Scholar]
- 37.Faesen AC, Luna-Vargas MP, Sixma TK. The role of UBL domains in ubiquitin-specific proteases. Biochem Soc Trans. 2012;40:539–545. doi: 10.1042/BST20120004. [DOI] [PubMed] [Google Scholar]
- 38.Mevissen TE, Hospenthal MK, Geurink PP, Elliott PR, Akutsu M, Arnaudo N, Ekkebus R, Kulathu Y, Wauer T, El Oualid F, Freund SM, Ovaa H, Komander D. OTU deubiquitinases reveal mechanisms of linkage specificity and enable ubiquitin chain restriction analysis. Cell. 2013;154:169–184. doi: 10.1016/j.cell.2013.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang T, Yin L, Cooper EM, Lai MY, Dickey S, Pickart CM, Fushman D, Wilkinson KD, Cohen RE, Wolberger C. Evidence for bidentate substrate binding as the basis for the K48 linkage specificity of otubain 1. J Mol Biol. 2009;386:1011–1023. doi: 10.1016/j.jmb.2008.12.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fiil BK, Damgaard RB, Wagner SA, Keusekotten K, Fritsch M, Bekker-Jensen S, Mailand N, Choudhary C, Komander D, Gyrd-Hansen M. OTULIN restricts Met1-linked ubiquitination to control innate immune signaling. Mol Cell. 2013;50:818–830. doi: 10.1016/j.molcel.2013.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rivkin E, Almeida SM, Ceccarelli DF, Juang YC, MacLean TA, Srikumar T, Huang H, Dunham WH, Fukumura R, Xie G, Gondo Y, Raught B, Gingras AC, Sicheri F, Cordes SP. The linear ubiquitin-specific deubiquitinase gumby regulates angiogenesis. Nature. 2013;498:318–324. doi: 10.1038/nature12296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kulathu Y, Garcia FJ, Mevissen TE, Busch M, Arnaudo N, Carroll KS, Barford D, Komander D. Regulation of A20 and other OTU deubiquitinases by reversible oxidation. Nat Commun. 2013;4:1569. doi: 10.1038/ncomms2567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kohler A, Pascual-Garcia P, Llopis A, Zapater M, Posas F, Hurt E, Rodriguez-Navarro S. The mRNA export factor Sus1 is involved in Spt/Ada/Gcn5 acetyltransferase-mediated H2B deubiquitinylation through its interaction with Ubp8 and Sgf11. Mol Biol Cell. 2006;17:4228–4236. doi: 10.1091/mbc.E06-02-0098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Samara NL, Wolberger C. A new chapter in the transcription SAGA. Curr Opin Struct Biol. 2011;21:767–774. doi: 10.1016/j.sbi.2011.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Koutelou E, Hirsch CL, Dent SY. Multiple faces of the SAGA complex. Curr Opin Cell Biol. 2010;22:374–382. doi: 10.1016/j.ceb.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kohler A, Schneider M, Cabal GG, Nehrbass U, Hurt E. Yeast Ataxin-7 links histone deubiquitination with gene gating and mRNA export. Nat Cell Biol. 2008;10:707–715. doi: 10.1038/ncb1733. [DOI] [PubMed] [Google Scholar]
- 47.Lee KK, Swanson SK, Florens L, Washburn MP, Workman JL. Yeast Sgf73/Ataxin-7 serves to anchor the deubiquitination module into both SAGA and Slik(SALSA) HAT complexes. Epigenetics Chromatin. 2009;2:2. doi: 10.1186/1756-8935-2-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kohler A, Zimmerman E, Schneider M, Hurt E, Zheng N. Structural basis for assembly and activation of the heterotetrameric SAGA histone H2B deubiquitinase module. Cell. 2010;141:606–617. doi: 10.1016/j.cell.2010.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Samara NL, Datta AB, Berndsen CE, Zhang X, Yao T, Cohen RE, Wolberger C. Structural insights into the assembly and function of the SAGA deubiquitinating module. Science. 2010;328:1025–1029. doi: 10.1126/science.1190049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Reyes-Turcu FE, Horton JR, Mullally JE, Heroux A, Cheng X, Wilkinson KD. The ubiquitin binding domain ZnF UBP recognizes the C-terminal diglycine motif of unanchored ubiquitin. Cell. 2006;124:1197–1208. doi: 10.1016/j.cell.2006.02.038. [DOI] [PubMed] [Google Scholar]
- 51.Ellisdon AM, Jani D, Koehler A, Hurt E, Stewart M. Structural basis for the interaction between yeast saga complex components SGF11 and SUS1. J Biol Chem. 2009;285:3850–3856. doi: 10.1074/jbc.M109.070839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu M, Li P, Song L, Jeffrey PD, Chenova TA, Wilkinson KD, Cohen RE, Shi Y. Structure and mechanisms of the proteasome-associated deubiquitinating enzyme USP14. EMBO J. 2005;24:3747–3756. doi: 10.1038/sj.emboj.7600832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Avvakumov GV, Walker JR, Xue S, Finerty PJ, Jr, Mackenzie F, Newman EM, Dhe-Paganon S. Amino-terminal dimerization, NRDP1-rhodanese interaction, and inhibited catalytic domain conformation of the ubiquitin-specific protease 8 (USP8) J Biol Chem. 2006;281:38061–38070. doi: 10.1074/jbc.M606704200. [DOI] [PubMed] [Google Scholar]
- 54.Pickart CM, Rose IA. Mechanism of ubiquitin carboxyl-terminal hydrolase. Borohydride and hydroxylamine inactivate in the presence of ubiquitin. J Biol Chem. 1986;261:10210–10217. [PubMed] [Google Scholar]
- 55.Samara NL, Ringel AE, Wolberger C. A role for intersubunit interactions in maintaining SAGA deubiquitinating module structure and activity. Structure. 2012;20:1414–1424. doi: 10.1016/j.str.2012.05.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lang G, Bonnet J, Umlauf D, Karmodiya K, Koffler J, Stierle M, Devys D, Tora L. The tightly controlled deubiquitination activity of the human SAGA complex differentially modifies distinct gene regulatory elements. Mol Cell Biol. 2011;31:3734–3744. doi: 10.1128/MCB.05231-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bergeron E, Albarino CG, Khristova ML, Nichol ST. Crimean-Congo hemorrhagic fever virus-encoded ovarian tumor protease activity is dispensable for virus RNA polymerase function. J Virol. 2010;84:216–226. doi: 10.1128/JVI.01859-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Havugimana PC, Hart GT, Nepusz T, Yang H, Turinsky AL, Li Z, Wang PI, Boutz DR, Fong V, Phanse S, Babu M, Craig SA, Hu P, Wan C, Vlasblom J, Dar VU, Bezginov A, Clark GW, Wu GC, Wodak SJ, Tillier ER, Paccanaro A, Marcotte EM, Emili A. A census of human soluble protein complexes. Cell. 2012;150:1068–1081. doi: 10.1016/j.cell.2012.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wiener R, DiBello AT, Lombardi PM, Guzzo CM, Zhang X, Matunis MJ, Wolberger C. E2 ubiquitin-conjugating enzymes regulate the deubiquitinating activity of OTUB1. Nat Struct Mol Biol. 2013;20:1033–1039. doi: 10.1038/nsmb.2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wiener R, Zhang X, Wang T, Wolberger C. The mechanism of OTUB1-mediated inhibition of ubiquitination. Nature. 2012;483:618–622. doi: 10.1038/nature10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Juang YC, Landry MC, Sanches M, Vittal V, Leung CC, Ceccarelli DF, Mateo AR, Pruneda JN, Mao DY, Szilard RK, Orlicky S, Munro M, Brzovic PS, Klevit RE, Sicheri F, Durocher D. OTUB1 co-opts Lys48-linked ubiquitin recognition to suppress E2 enzyme function. Mol Cell. 2012;45:384–397. doi: 10.1016/j.molcel.2012.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Sato Y, Yamagata A, Goto-Ito S, Kubota K, Miyamoto R, Nakada S, Fukai S. Molecular basis of Lys-63-linked polyubiquitination inhibition by the interaction between human deubiquitinating enzyme OTUB1 and ubiquitin-conjugating enzyme UBC13. J Biol Chem. 2012;287:25860–25868. doi: 10.1074/jbc.M112.364752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Goncharov T, Niessen K, Almagro de MC, Izrael-Tomasevic A, Fedorova AV, Varfolomeev E, Arnott D, Deshayes K, Kirkpatrick DS, Vucic D. OTUB1 modulates c-IAP1 stability to regulate signalling pathways. EMBO J. 2013;32:1103–1114. doi: 10.1038/emboj.2013.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Soares L, Seroogy C, Skrenta H, Anandasabapathy N, Lovelace P, Chung CD, Engleman E, Fathman CG. Two isoforms of otubain 1 regulate T cell anergy via GRAIL. Nat Immunol. 2004;5:45–54. doi: 10.1038/ni1017. [DOI] [PubMed] [Google Scholar]
- 65.Cohn MA, Kowal P, Yang K, Haas W, Huang TT, Gygi SP, D'Andrea AD. A UAF1-containing multisubunit protein complex regulates the Fanconi anemia pathway. Mol Cell. 2007;28:786–797. doi: 10.1016/j.molcel.2007.09.031. [DOI] [PubMed] [Google Scholar]
- 66.Villamil MA, Liang Q, Zhuang Z. The WD40-repeat protein-containing deubiquitinase complex: catalysis, regulation, and potential for therapeutic intervention. Cell Biochem Biophys. 2013;67:111–126. doi: 10.1007/s12013-013-9637-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gaudet R, Bohm A, Sigler PB. Crystal structure at 2.4 angstroms resolution of the complex of transducin betagamma and its regulator, phosducin. Cell. 1996;87:577–588. doi: 10.1016/s0092-8674(00)81376-8. [DOI] [PubMed] [Google Scholar]
- 68.Sprague ER, Redd MJ, Johnson AD, Wolberger C. Structure of the C-terminal domain of Tup1, a corepressor of transcription in yeast. EMBO J. 2000;19:3016–3027. doi: 10.1093/emboj/19.12.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Fraile JM, Quesada V, Rodriguez D, Freije JM, Lopez-Otin C. Deubiquitinases in cancer: new functions and therapeutic options. Oncogene. 2012;31:2373–2388. doi: 10.1038/onc.2011.443. [DOI] [PubMed] [Google Scholar]
- 70.Balakirev MY, Tcherniuk SO, Jaquinod M, Chroboczek J. Otubains: a new family of cysteine proteases in the ubiquitin pathway. EMBO Rep. 2003;4:517–522. doi: 10.1038/sj.embor.embor824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Johnston SC, Larsen CN, Cook WJ, Wilkinson KD, Hill CP. Crystal structure of a deubiquitinating enzyme (human UCH-L3) at 1.8 A resolution. EMBO J. 1997;16:3787–3796. doi: 10.1093/emboj/16.13.3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Weeks SD, Grasty KC, Hernandez-Cuebas L, Loll PJ. Crystal structure of a Josephin-ubiquitin complex: evolutionary restraints on ataxin-3 deubiquitinating activity. J Biol Chem. 2011;286:4555–4565. doi: 10.1074/jbc.M110.177360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ambroggio XI, Rees DC, Deshaies RJ. JAMM: a metalloprotease-like zinc site in the proteasome and signalosome. PLoS Biol. 2004;2:E2. doi: 10.1371/journal.pbio.0020002. [DOI] [PMC free article] [PubMed] [Google Scholar]


