Abstract
Rheumatoid arthritis (RA) is characterized by hyperplastic synovial pannus tissue, which mediates destruction of cartilage and bone. Fibroblast-like synoviocytes (FLS) are a key component of this invasive synovium and have a major role in the initiation and perpetuation of destructive joint inflammation. The pathogenic potential of FLS in RA stems from their ability to express immunomodulating cytokines and mediators as well as a wide array of adhesion molecule and matrix-modelling enzymes. FLS can be viewed as ‘passive responders’ to the immunoreactive process in RA, their activated phenotype reflecting the proinflammatory milieu. However, FLS from patients with RA also display unique aggressive features that are autonomous and vertically transmitted, and these cells can behave as primary promoters of inflammation. The molecular bases of this ‘imprinted aggressor’ phenotype are being clarified through genetic and epigenetic studies. The dual behaviour of FLS in RA suggests that FLS-directed therapies could become a complementary approach to immune-directed therapies in this disease. Pathophysiological characteristics of FLS in RA, as well as progress in targeting these cells, are reviewed in this manuscript.
Introduction
Fibroblast-like synoviocytes (FLS; also known as synovial fibroblasts or type B synoviocytes) are unique cells that populate the intimal lining of the synovium. FLS normally assure the structural and dynamic integrity of diarthrodial joints by controlling the composition of the synovial fluid and the extracellular matrix (ECM) of the joint lining. In rheumatoid arthritis (RA), however, FLS display surprisingly pathogenic behaviour; they increase in number and become a prominent component of the destructive pannus that characterizes synovia of patients with RA. Furthermore, FLS in RA acquire an aggressive phenotype and mediate inflammation and destruction of the joint.
What transforms FLS from friend to foe in RA? As these cells are exquisitely sensitive to the inflammatory milieu of the rheumatoid synovium, a conventional model suggests that FLS act as ‘passive responders’ in RA. In this scenario, FLS react appropriately to proinflammatory stimuli driven by innate and adaptive immunity, and would rapidly revert to a quiescent state if the inciting stimuli were to be withdrawn. The observation, however, that FLS obtained from synovia of patients with RA and cultured in vitro (RA FLS) retain their aggressive phenotype in the absence of exogenous stimulation is difficult to explain with the passive-responder model.1 This inconsistency, and additional observations of tumour-like behaviour of RA FLS (discussed later), led to the mooting of an alternative model, in which imprinted anomalies of FLS in patients with RA—for example, mutations or epigenetic changes—conspire to drive inflammation in the disease. In this model, previously called ‘transformed aggressor’,1 but which could perhaps more accurately be termed ‘imprinted aggressor’, FLS possess and/or acquire autonomous pathogenic features in RA that actively contribute to the mechanisms of disease. RA FLS can erode human cartilage when co-implanted with the tissue into mice with severe combined immunodeficiency (SCID) many months after removal from the RA synovial milieu, providing convincing in vivo evidence that FLS are imprinted—perhaps before and otherwise during the course of RA—with a phenotype that subsequently enhances tissue destruction.2 Implanted RA FLS can seed and damage joints distant from the site of implant, according to work in mice with SCID, suggesting that RA FLS might even have a primary ability to ‘metastasize’.3
The passive responder and imprinted aggressor models of FLS behaviour are not mutually exclusive. For instance, a persistent inflammatory environment is likely to contribute to the development of molecular anomalies, which can then lead passively responding effector FLS to become active aggressors. The recent emergence of epigenetic changes as possible drivers of the FLS phenotype in RA suggests that molecular anomalies in FLS could even predate the onset of disease and potentially explain a component of its heritability. In this Review, we describe pathophysiological features of FLS in RA at the functional and molecular levels, outline how these characteristics are acquired, and summarize therapeutic opportunities that will arise from understanding the roles of these cells in RA.
FLS physiology
FLS are a critical component of the intimal lining layer of the healthy synovium. The synovial intima is a delicate structure composed of 1–2 layers of FLS inter digitated with macrophage-like synoviocytes (also called type A synoviocytes). The intimal lining lacks tight junctions and a true basement membrane, enabling cells and proteins to move into the synovial fluid with ease. In common with other types of fibroblasts, FLS are mesenchymal-derived cells that express ECM proteins such as vimentin, type IV and type V collagens, adhesion molecules such as integrins of the α5β1 class, integrin receptors such as intracellular adhesion molecule 1, and surface markers such as Thy-1 membrane glycoprotein (also known as CD90) (Table 1).4 However, compared with fibroblasts in other anatomical locations (including those in the subintimal layer between the intimal layer and other joint tissues), FLS are characterized by the expression of UDP-glucose 6-dehydrogenase, an enzyme required for the synthesis of hyaluronic acid and of complement decay-accelerating factor (also known as CD55).5 FLS also express a higher level of vascular cell adhesion protein 1 than other fibroblast types.4 FLS also display high expression of a relatively selective adhesion molecule, cadherin-11, which is also expressed by osteoblasts.6 Cadherin-11 is essential for maintaining the integrity of the normal synovium. The synovial intimal lining of mice deficient in cadherin-11 contains markedly reduced numbers of FLS in diarthrodial joints, in comparison with wild-type mice.7 This adhesion molecule is responsible for homotypic aggregation of FLS in vitro, and its expression in mouse fibroblasts is sufficient to induce their organization into synovial-like intimal lining structures in vitro.8 Cadherin-11 also regulates the secretion of IL-6 and other proinflammatory properties of FLS.8,9
Table 1.
Key proteins expressed by FLS
| Protein | Specific marker of FLS? |
Possible clinical target? |
|---|---|---|
| Surface proteins | ||
| Integrins | No | Yes, such as α4-integrin (which is targeted by natalizumab) Blocking integrin signalling has a substantial effect on adaptive immunity |
| ICAM-1 | No | Yes, but limited efficacy observed with anti-ICAM-1 antibody85 |
| VCAM-1 | Relatively specific, compared with other fibroblast types, but expressed on many immune cell types |
Yes, but blocking VCAM-1 has possible effects on adaptive immunity by interfering with cell migration |
| Cadherin-11 | Relatively specific (also expressed by osteoblasts) |
Yes, can be blocked with anti-cadherin-11 antibody or cadherin-11–Fc construct |
| CD55* | No | Less likely than other surface proteins listed, because of its widespread expression, for example, by erythrocytes |
| CD90‡ | No | Yes, but blocking CD90 signalling has a substantial effect on adaptive immunity |
| Intracellular proteins | ||
| Vimentin | No | No |
| UDPGDH | Yes | Less likely than surface proteins listed |
| Collagen proteins | ||
| Type IV collagen | No | Less likely than surface proteins listed, but targeting of its integrin receptor is possible |
| Type V collagen | No | Less likely than surface proteins listed, but targeting of its integrin receptor is possible |
Also known as complement decay-accelerating factor.
Also known as Thy-1 membrane glycoprotein.
Abbreviations: FLS, fibroblast-like synoviocyte; ICAM-1, intracellular adhesion molecule 1; UDPGDH, UDP-glucose 6-dehydrogenase; VCAM-1, vascular cell adhesion protein 1.
FLS-mediated pathology in RA
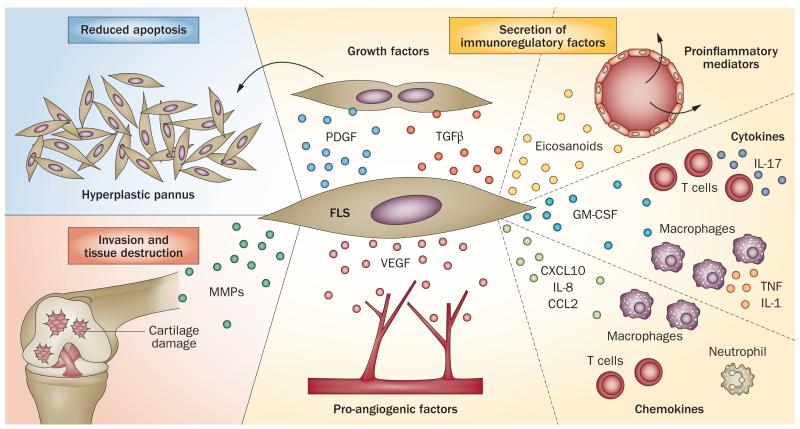
A combination of primary and inflammation-induced changes in FLS promotes pannus growth, inflammation, and cartilage and bone destruction in RA. Indeed, FLS are implicated in many pathophysiological mechanisms of joint destruction in RA (summarized in Figure 1).
Figure 1.
Roles of FLS in RA. FLS play a critical part in many pathogenic events in the RA synovium. They can contribute to pathology through a reduced ability to undergo apoptosis (forming pannus), the production of proteases that degrade the extracellular matrix, and invasion into cartilage. In addition, FLS produce a variety of molecules that modulate growth, inflammation, angiogenesis, and cell recruitment, and induce activation of and cytokine production by immune cells. Abbreviations: CCL2, CC-chemokine ligand 2; CXCL10, CXC-chemokine ligand 10; FLS, fibroblast-like synoviocytes; GM-CSF, granulocyte-macrophage colony-stimulating factor; MMP, matrix metalloproteinase; PDGF, platelet-derived growth factor; RA, rheumatoid arthritis; TGF-β, transforming growth factor β; VEGF, vascular endothelial growth factor.
Cell survival and pannus formation
The hyperplastic rheumatoid pannus is characterized by an overabundance of FLS. This cellular excess stems largely from an imbalance between proliferation and apoptosis of FLS, although increased differentiation and migration of mesenchymal stem cells could also contribute. Studies have failed to find evidence of markedly increased proliferation of FLS in the RA synovium or in cell culture,10 yet clonal expansion of these cells in vivo has been postulated on the basis of the emergence of distinct clonal cell lines in cultured RA FLS.11 Conversely, evidence of reduced apoptosis of FLS in the RA synovium has been shown according to a variety of criteria, including findings from electron microscopy, histochemistry and other assays.12 Interestingly, RA FLS retain their resistance to apoptosis in vitro, suggesting that anomalies persist well after they are removed from the cytokine and growth factor milieu of the RA synovium.13 The autonomous ability of RA FLS to survive in an environment enriched with apoptosis-inducing factors, such as oxygen radicals, nitric oxide and cytokines, resembles the phenotype of some tumours. RA FLS display additional ‘tumour-like’ features, including increased migration, reduced con tact inhibition, reduced attachment-dependent growth,1 and, as already mentioned, the ability to invade and ‘metastasize’ in vivo.2,3
Invasion and tissue destruction
A second important pathologic feature of FLS in RA is an invasive phenotype that favours tissue destruction. Normal FLS control the composition and consistency of the ECM and synovial fluid, secreting a large variety of ECM components, enzymes able to destroy the ECM (such as matrix metalloproteinases [MMPs]), and inhibitors of matrix-degrading enzymes (such as tissue inhibitors of MMPs [TIMPS]). RA FLS produce abundant ECM components, and also secrete collagenases (MMP1 and MMP13), stromelysin-1 (MMP3), aggrecanases (ADAMTS4 and ADAMTS5) and cathepsins, which tips the balance between proteases and their inhibitors towards tissue destruction.14,15 Protease production synergizes with the high expression of adhesion molecules such as cadherin-11 to favour resorption of ECM and cartilage. The importance of FLS in cartilage destruction was confirmed in mice with inflammatory arthritis, because animals deficient in cadherin-11 were protected from cartilage erosion.7 FLS are also putatively important promoters of bone erosion based on their ability to secrete receptor activator of nuclear factor κB ligand (RANKL, also known as TNF ligand superfamily member 11), which promotes osteoclast differentiation.16
FLS invasiveness in patients with RA is partly stimulated by local proinflammatory factors such as IL-1 and TNF, reactive oxygen and nitrogen species whose formation is favoured by local hypoxia, growth factors such as platelet derived growth factor (PDGF), and ECM proteins. However, invasiveness is also a feature retained by RA FLS, and can be detected in ex vivo invasion assays and in RA FLS-cartilage co-implantation assays in mice.2,17 Thus, the invasive phenotype of FLS in RA is dependent on both autonomous and local factors.
Proinflammatory networks
The third important pathologic feature of FLS in patients with RA is their increased ability to secrete a variety of cytokines, chemokines, and proangiogenic factors, compared with FLS in healthy people. RA FLS exert important immunoregulatory functions through cytokine secretion and direct interactions with immune cells. IL-1-stimulated or TNF-stimulated RA FLS are a major source of IL-6, a pathogenic cytokine and a validated target of biologic therapy in RA, in vivo and in vitro.18 IL-6 and some other cytokines that are produced by RA FLS, such as IL-18 and granulocyte–macrophage colony-stimulating factor (GM-CSF), are known to fuel the activation of cells of the innate and adaptive immune systems in the RA joint, whereas other cytokines, such as IL-1 receptor antagonist (IL-1Ra) and transforming growth factor β (TGF-β), exert anti-inflammatory actions. Type 1 interferons, produced by FLS upon stimulation of Toll-like receptors (TLRs) such as TLR3, can have proinflammatory or anti-inflammatory actions.19 RA FLS also produce large amounts of growth factors (such as PDGF), chemokines (such as CC-chemokine ligand 2 [CCL2], also known as monocyte chemoattractant protein 1), short-lived mediators (such as prostaglandins and leukotrienes),14 and proangiogenic factors (such as vascular endothelial growth factor).20
The net effect of these factors is to promote inflammation in the joint by activating FLS through autocrine responses and neighbouring cells through paracrine networks, and by recruiting other cells to the synovium through the elaboration of chemotactic factors. The overall proinflammatory action of FLS in arthritis was demonstrated in the K/BxN serum transfer mouse model of RA by deletion of cadherin-11, which disrupts organization of FLS and which strongly impaired clinical and histologic evidence of joint inflammation in the animals.7
A number of exogenous stimuli promoting survival and invasiveness, such as IL-1 and TNF, also seem to control the proinflammatory actions of FLS in RA. An example described in 2012 involves TNF-stimulated overexpression of the enzyme autotaxin (also called ectonucleotide pyrophosphatase/phosphodiesterase family member 2), which in turn leads to increased levels of an extracellular bioactive lipid (lysophosphatidic acid) in the synovium.21 The role of this FLS pathway was elegantly demonstrated in vivo by showing that selective deletion of autotaxin in FLS led to reduced inflammation and joint destruction in TNF transgenic mice.21 Nevertheless, the extracellular milieu cannot fully explain the proinflammatory phenotype of RA FLS, which continue to produce elevated levels of cytokines for many cell culture passages even in the absence of exogenous stimuli.1 These observations suggest that intrinsic changes, in addition to external mediators, shape the proinflammatory profile of FLS in RA.
Molecular pathology of FLS in RA
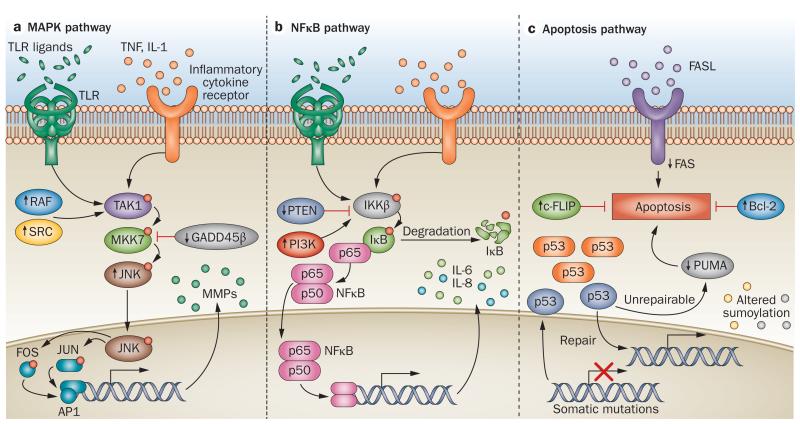
The pathologic behaviour of FLS in patients with RA correlates with several molecular alterations in these cells. Considerable research has implicated a variety of signalling pathways triggered by engagement of FLS surface and cytoplasmic receptors in this pathology, especially those activated by cytokines and TLR ligands. These receptors enable FLS to sense the inflammatory environment of the rheumatoid joint and res pond appropriately. In addition, the same signalling path ways that mediate these responses to exogenous stimuli contain intrinsic anomalies in RA FLS. Thus, the passive responder and imprinted aggressor aspects of FLS behaviour in RA could be mediated, at least in part, by activation of the same signalling machinery. Signal transduction pathways in FLS have been extensively reviewed elsewhere.4,22 Here, we summarize changes in the mitogen-activated protein kinase (MAPK), nuclear factor κB (NFκB), and apoptosis pathways in RA FLS (Figure 2), because these pathways have been the most extensively studied and are among the most convincingly involved in control of RA FLS aggressiveness.
Figure 2.
Molecular pathology of FLS in RA. Upward and downward arrows indicate changed functional levels of the corresponding protein in RA FLS (cultured FLS derived from patients with RA) versus osteoarthritis and/or normal FLS. a | Enhanced MAPK signalling increases production of MMPs and cytokines in RA. The JNK pathway is shown; analogous cascades in the ERK and p38 MAPK pathways are not shown. Ligation of surface receptors activates the MAPK pathway, leading to phosphorylation of JNKs and increased expression of genes such as MMP genes. JNKs are hyperactive in RA FLS, whereas GADD45β expression is reduced; SRC and RAF activity are increased, which further contributes to JNK activation. b | Activation of the NFκB pathway is increased in RA FLS. Stimulation of surface receptors increases IKKβ activation, leading to degradation of IκB. Nuclear translocation of NFκB leads to transcription of proinflammatory genes. Increased activation of NFκB activity in RA is promoted, in part, by overexpression of PI3K and/or decreased expression of PTEN in the RA synovium. c | Damage to key regulators including mitochondrial genes and p53 underlies the reduced tendency of RA FLS to undergo apoptosis. Increased activation and/or expression of c-FLIP and Bcl-2 and altered sumoylation contribute to enhanced survival of RA FLS. Abbreviations: Bcl-2, apoptosis regulator Bcl-2; c-FLIP, cellular FLICE-like inhibitory protein; ERK, extracellular signal-regulated kinase; FASL, FAS ligand; FLS, fibroblast-like synoviocytes; GADD45β, growth arrest and DNA damage-inducible protein GADD45β; IκB, inhibitor of NFκB; IKKβ, IκB kinase β; JNK, c-Jun N-terminal kinase; MAPK, mitogen-activated protein kinase; MKK7, MAPK kinase 7; MMP, matrix metalloproteinase; NFκB, nuclear factor κB; p53, cellular tumour antigen p53; PI3K, phosphoinositide 3-kinase; PTEN, phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase; PUMA, p53 upregulated modulator of apoptosis; RAF, RAF proto-oncogene serine/threonine-protein kinase; SRC, proto-oncogene tyrosine-protein kinase Src; TAK1, transforming growth factor β-activated kinase 1; TLR, Toll-like receptor.
MAPK pathway anomalies
Perhaps the most extensively studied pathway in RA FLS is the MAPK cascade, which is stimulated by cytokine receptor and TLR engagement. MAPK signal transduction involves the activation of three levels of kinases: the top tier includes MAPK kinase kinases (MAP3Ks); the middle level includes MAPK kinases (MKKs); and the distal level comprises three types of MAPK—extracellular-signal regulated kinases (ERKs, such as MAPK 15), p38 MAPKs (such as MAPK 14), and the c-Jun N-terminal kinases (JNKs, such as MAPK 8 and MAPK 10). Once activated through phosphorylation by MKKs, MAPKs can migrate from the cytosol to the nucleus where they phosphorylate and activate important transcription factors.23
One of the most important MAPK-activated mediators of FLS invasiveness is transcription factor AP1, a heterodimer of phosphorylated JUN and FOS subunits, which binds to and activates the promoter region of several MMP genes. Although several kinases can phosphorylate JUN, the JNKs are perhaps the most efficient at doing so. A critical role for JNKs is supported by the observation that a chemical inhibitor of all JNK isoforms, SP600125, decreased MMP production by human FLS and prevented joint destruction in a rat model of RA.24 Knockdown studies have shown that the upstream kinase dual-specificity MKK7 (also known as MAPKK 7) is the most important MKK for activation of JNKs in RA FLS, and the MKK7–JNK–AP1 axis is primarily activated by TGF-β-activated kinase 1 (TAK1, also known as MAP3K 7) in RA FLS.25
Other key mediators of FLS aggressiveness in RA are MAPKs 11–14 (also known as p38 MAPK isoforms β, γ, δ and α, respectively), which are activated in the synovium of patients with RA.26 Pharmacologic inhibition of p38α and p38β MAPKs decreased cytokine secretion by cultured RA FLS in vitro.27 Upstream activation of these kinases is controlled by MKK3 (also known as MAPKK 3) and MKK6 (also known as MAPKK 6), and, to a lesser extent, MKK4 (also known as MAPKK 4).28,29 Introduction of dominant-negative MKK3 or MKK6 constructs in human FLS suppresses cytokine and MMP production.28 The unexpected failure of p38 MAPK inhibitors in the treatment of RA30,31 (see later section on targeting FLS) probably results from inhibitor-induced activation of proinflammatory pathways in macrophages that overcome the anti-inflammatory effects of p38 MAPK inhibition in FLS. Indeed, mice carrying macrophage-selective deletion of p38α MAPK display increased severity in K/BxN serum transfer induced arthritis. Of interest, in the same study deletion of Mkk3 (also known as Map2k3) or Mkk6 (also known as Map2k6) bypassed some of these limitations and produced a more robust therapeutic effect in vivo than pharmacological inhibition of p38α MAPK, suggesting that pharmacological inhibition of MKK3 and/or MKK6 might be a promising option for FLS-targeted therapy of RA.29
Several primary molecular alterations detected in RA FLS promote activation of the MAPK pathway. For example, expression and/or activation levels of proto-oncogene tyrosine-protein kinase Src,32 RAF proto-oncogene serine/threonine-protein kinase,33,34 and RAS-specific guanine nucleotide-releasing factor 135 are increased in RA FLS, favouring cell survival through activation of MAPKs and MMP production through increased phosphorylation of JUN. JNK1 and JNK2 activation is substantially greater in RA FLS compared with cultured FLS derived from patients with osteoarthritis (OA FLS), which largely accounts for persistently higher MMP expression by RA FLS compared with OA FLS.36 Expression of growth-arrest and DNA damage-inducible protein GADD45β, a natural inhibitor of MKK7, is low in RA FLS and RA synovia, which could also contribute to hyperactivity of MKK7 and increased activation of JNKs.37 In mice, deficiency of Gadd45β enhances the severity of K/BxN serum transfer induced arthritis, which is solely dependent on innate immunity and effector mechanisms,37 but decreases arthritis severity in the collagen-induced mouse model of RA, which is dependent on adaptive immunity.38 Thus, the effect of altering Mkk7 activity in mice depends on the relative balance of lymphocytes versus effector cells such as FLS.
NFκB pathway anomalies
The NFκB pathway is a major regulator of proinflammatory cytokine production and is activated by IL-1, TNF, and TLR signalling. In FLS, signalling through the NFκB pathway requires inhibitor of NFκB kinase (IKK) subunit β (IKKβ) in the cytosol and is independent of IKKα.39 Activation of IKKβ leads to phosphorylation of proteins of the inhibitor of NFκB (IκB) family. IκB proteins form complexes with cytosolic subunits of NFκB, maintaining them in an inactive state—after phosphorylation, IκBs are degraded by the proteasome, leaving NFκB free to migrate into the nucleus and initiate gene transcription.40 Inhibiting the NFκB pathway by leaving NFκB sequestered in the cytoplasm, specifically by pharmacologic inhibition of IKKβ41 or by transfecting a dominant-negative IκB construct42, reduced the activation and survival of RA FLS.
Among key alterations of RA FLS that might promote NFκB activation are changes that promote the generation of phosphoinositols. For example, decreased expression of phosphatidylinositol 3,4,5-trisphosphate 3-phosphatase and dual-specificity protein phosphatase PTEN has been detected at sites of cartilage destruction in patients with RA.43 In 2012, pho sphatidylinositol 4,5-bisphosphate 3-kinase (PI3K) catalytic subunit δ isoform (PI3Kδ), which is primarily expressed in bone marrow derived cells, was found to be overexpressed in RA synovium, in comparison with OA synovium.44 Although it remains to be clarified whether PI3Kδ is directly connected to NFκB activation in RA FLS, pharmaco logic inhibition of PI3Kδ affected the balance between survival, death, and proliferation in these cells.44
IKKε and serine/threonine-protein kinase TBK1 (also known as TANK-binding kinase 1) are homologous to IKKα and IKKβ but, rather than phosphorylating IκB proteins, they instead mainly regulate interferon responses to innate immunity stimuli by phosphorylating interferon-regulating factors (IRFs) such as IRF3 and IRF7 in FLS. IKKε is expressed in the synovial intima of patients with RA, and in RA FLS active IKKε can phosphorylate JUN and induce MMP expression independent of JNKs.45 Despite these apparently proinflammatory properties, however, expression of type I interferons, which is promoted by either IKKε or TBK1 in FLS is generally anti-inflammatory in animal models of arthritis.19 It is likely, therefore, that inhibition of IKKε and/or TBK1 would decrease levels of protective type I interferons in the RA synovium. Supporting this notion, mice deficient in Irf7 have markedly increased disease severity in the K/BxN serum transfer model of arthritis.46
Apoptosis pathway anomalies
Molecular mechanisms of RA FLS resistance to apoptosis were reviewed in detail elsewhere in 2009.47 Anomalies in pathways that promote invasiveness and cytokine secretion also favour survival of RA FLS by increasing expression of antiapoptotic molecules, such as apoptosis regulator Bcl-2 (which is over expressed in synovial samples from patients with RA12), and by decreasing or altering the response to ligation of apoptosis-regulating receptors, such as TNF receptor superfamily (TNFRSF) member 6 (also known as CD95 and FAS)13,48 and TNFRSF10A (also known as TRAIL receptor 1).49 Reduced apoptosis can, in part, be secondary to increased expression and engagement of integrins on FLS in the RA synovium.47 Intrinsic deficiencies in apoptosis-specific pathways are also reportedly prominent in RA FLS, especially abnormalities in the p53 pathway.50 After cell damage, the p53 pathway induces either cell-cycle arrest to allow time for DNA repair, or apoptosis if the damage is beyond repair. Although expression of the eponymous protein, cellular tumour antigen p53, is relatively high in synovial samples from patients with RA (as compared with samples from healthy individuals),51 the p53 protein is unable to induce apoptosis of RA FLS, owing to a combination of insensitivity of the p53 downstream signalling machinery and dominant-negative mutations.50 These blockages can be bypassed in vitro by forced expression of the down-stream effector p53 upregulated modulator of apoptosis (PUMA; also known as Bcl-2-binding component 3).52 Interestingly, besides promoting survival, defects of the p53 pathway also lead to increased invasiveness of FLS in vitro and in vivo.17
Another example of an apoptotic-pathway defect in RA FLS is increased expression of the antiapoptotic protein cellular FLICE-like inhibitory protein (c-FLIP; also known as CASP8 and FADD-like apoptosis regulator); mRNA expression of this regulator was also detected at sites of cartilage invasion in patients with RA.53 The same study also demonstrated that expression of c-FLIP in RA FLS is induced by TNF in vitro.53
An overall increase in sumoylation is another abnormality that can cause resistance to apoptosis, and is reportedly intrinsic to RA FLS.13 Sumoylation consists large protein small ubiquitin-like modifier 1 (SUMO-1, also called sentrin), which usually results in conformational and functional changes in the modified protein. The synovial intima of patients with RA is marked by increased expression of SUMO-1,54 whereas RA FLS have decreased expression levels of sentrin-specific protease 1 (SENP1),55 an enzyme that removes SUMO-1. Similar to defects of the p53 pathway, increased sumoylation, besides being antiapoptotic, also affects invasiveness of RA FLS through increased production of MMP-1.55
Imprinted FLS anomalies in RA
As we have discussed, FLS not only act as passive effectors for pathogenic cytokine signalling cascades initiated by immune cells in RA, but also seem to contain intrinsic alterations that confer pathogenic behaviour independent of external stimulation and/or lead to their ‘overexuberant response’ to the inflammatory extracellular milieau. So, what causes FLS to become permanently altered and function as imprinted aggressors in RA pathogenesis?
Somatic mutations
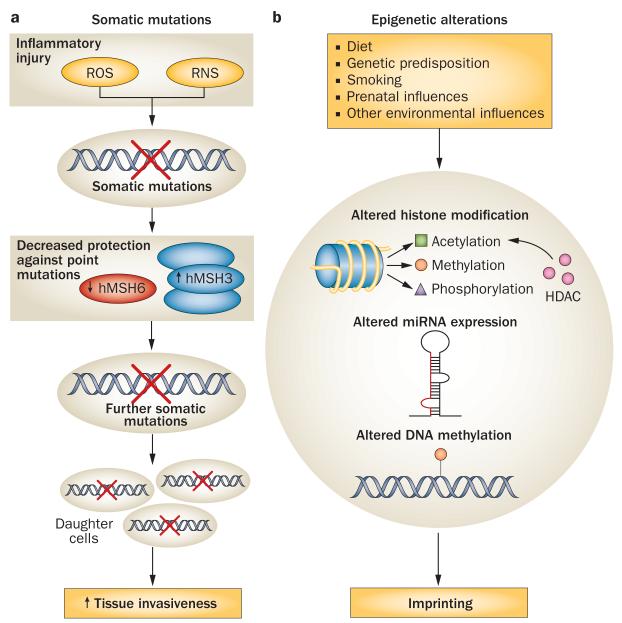
Molecular mechanisms similar or identical to those that cause neoplastic transformation seem to operate in RA FLS.1,56 Somatic mutations in proto-oncogenes and tumour suppressors are commonly found in cancers, and have been identified in RA FLS (Figure 3). For example, primary FLS from synovia of patients with RA as well as RA FLS can carry inactivating mutations in TP53, which encodes cellular tumour antigen p53, that—in some cases—overlap those found in tumours.51,57 When transfected into dermal fibroblasts in vitro, these mutated genes produce proteins with a dominant-negative effect over the endogenous version, promoting cell survival.50 Besides TP53, functional mutations have also been described in other genes, such as RAF1 (encoding RAF proto-oncogene serine/threonine-protein kinase),34 and even in mitochondrial DNA in RA FLS.58 Evidence of increased microsatellite instability has been found in RA synovia compared with OA synovia.59
Figure 3.
Imprinted anomalies of RA FLS (cultured FLS derived from patients with RA). a | Somatic mutations might promote the aggressive characteristics and proliferation of FLS in RA. Inflammatory injury (e.g. from ROS or RNS) is thought to induce an imbalance in the expression of DNA repair proteins, decreasing levels of MSH6, which protects against point mutations, and simultaneously increasing expression of MSH3, which protects against large genomic rearrangements. Further mutations can result, and somatic mutations are inherited by daughter cells. b | Epigenetic modifications of FLS that contribute to pathology in RA can be inherited or induced by environmental factors. Aberrant patterns of histone acetylation alter gene expression in RA FLS; histone methylation and phosphorylation are additional epigenetic mechanisms that have not yet been thoroughly investigated in RA FLS. Altered expression of miRNA can contribute to the aggressive phenotype of RA FLS. Differential DNA methylation leads to altered expression of key genes involved in cell adhesion, cytokine production and proliferation. Abbreviations: FLS, fibroblast-like synoviocytes; HDAC, histone deacetylase; MSH6, DNA mismatch repair protein MSH6; MSH3, DNA mismatch repair protein MSH3; miRNA, microRNA; RA, rheumatoid arthritis; RNS, reactive nitrogen species; ROS, reactive oxygen species.
Although pathogenic roles for somatic mutations in RA FLS remain uncertain, the emergence of such genetic changes in inflamed synovia of patients with RA is similar to the occurrence of cancer at sites of chronic inflammation.60 Stimulation of cultured RA FLS with TNF induces mutations in mitochondrial DNA, and the frequency of mutations in mitochondrial DNA in the synovium of patients with RA correlates with histologic evidence of inflammation and TNF levels.61 Exposure of RA FLS to a nitric oxide donor induces an imbalance in the expression of two genes that control DNA repair, thus possibly explaining their increased microsatellite instability. Interestingly, the expression of DNA mismatch repair protein MSH6, an enzyme that promotes repair of point mutations (the type of mutations found in RA FLS) was decreased in this setting, yet at the same time the nitric oxide donor caused increased expression of DNA mismatch repair protein MSH3 an enzyme that protects against large genomic rearrangements (Figure 3).59 The emergence of multiple distinct clonal cell lines when culturing RA FLS in vitro11 suggests that multiple FLS populations carrying different mutations coexist in the RA synovium, and/or that FLS can undergo sequential mutations in multiple genes, acquiring selective advantages based on an increasingly aggressive phenotype without true neoplastic transformation.
Epigenetic alterations
Epigenetic modifications in multiple cell types are emerging as possible mechanisms of molecular pathology in RA, as reviewed elsewhere in 2011.62 Epigenetic alterations include DNA methylation, histone methylation, histone acetylation, histone phosphorylation, and expression of microRNAs (miRNAs)62 (Figure 3). Such changes affect gene expression and are often transmitted from mother to daughter cells.
As yet, alterations in histone methylation and/or phosphorylation have not been thoroughly investigated in RA FLS. Much work in RA FLS has, however, been focused on methylation of cytosine in position C5 in CpG islands of the genome, which usually leads to repression of gene expression and is mediated by DNA methyl transferase enzymes (DNMTs). Reduced global methylation has been described in RA FLS.62 Decreased methylation was proposed to drive activation of an endogenous retrotransposable element in RA FLS; the expression of the same retrotransposable element positively correlates with markers of activation in RA FLS and in the RA synovium.63 Interestingly, treatment of FLS with inhibitors of DNA methylation partially replicates the activated, tumour-like phenotype of RA FLS.62 In a study published in 2012, a genome-wide survey of DNA methylation in RA FLS and OA FLS did not observe overall differences in global methylation but instead identified 1,859 hypomethylated or hypermethylated loci in RA FLS as compared with OA FLS.64 A 20-locus ‘methylome signature’ distinguished RA FLS from either OA FLS or FLS from healthy controls.64 Hypomethylated loci corresponded to components of focal adhesion pathways, ECM regulation, and cell–ECM interactions, whereas hypermethylated loci were involved in specialized signalling pathways.64
Besides distinct DNA methylation patterns, RA FLS also exhibit alterations of histone acetylation, and treatment of RA FLS with inhibitors of histone deacetylases (enzymes that remove acetyl groups from histones) reduced production of inflammatory cytokines in vitro.65 Furthermore, the sumoylation-related increase in MMP-1 production by RA FLS—responsible, as we have mentioned, for increased invasiveness of these cells—is mediated by increased histone acetylation of the MMP1 promoter region.55
Several studies have reported altered expression of various miRNAs—short non-coding fragments of RNA that regulate gene expression—in RA FLS, particularly miRNAs that regulate proliferation and survival (for example, miR-124a66), invasiveness, and production of proinflammatory cytokines (for example, miR-20367). The role of miRNA in RA was reviewed in 2012.68
Drivers of epigenetic change
Epigenetic changes can arise in FLS at any time in life, or even before birth, and can be driven by genetic or environmental factors, including certain dietary habits or smoking. Ageing is also known to induce specific changes in DNA methylation and histone chemistry. A 2012 study reported a correlation between decreased DNA methylation in RA FLS and increased expression of X-linked genes encoding enzymes that are involved in the synthesis of polyamines and are able to sequester a cofactor essential for DNA methylation.69 The authors postulate that local inflammation is able to trigger this pathogenic pathway, because increased expression of polyamine-synthesizing enzymes can be induced in RA FLS by stimulation with IL-1 in vitro.69
Although it is still difficult to assess whether they are a cause or consequence of disease, epigenetic anomalies constitute a versatile and unifying mechanism that integrates different aetiologic factors into a single model. For example, we speculate that exposure to different environmental factors can lead to differences in epigenetic regulation of key pathogenic genes in FLS, and influence the pairwise concordance rate between identical twins in RA (which reportedly is between 13 and 21%).70,71 On the other hand, epigenetic modifications of FLS imprinted during foetal or early postnatal life might explain a fraction of RA heritability independent of known genetic risk factors.
Targeting FLS in RA
Indications that RA FLS are imprinted in ways that encourage aggressiveness suggest two things: first, that anti-inflammatory and immunosuppressive therapy might not be sufficient to completely halt joint destruction in RA—at least not in well-established RA; and, second, that direct targeting of FLS represents a potentially viable option for alternative or adjuvant therapies aimed at modifying the disease course. The various characteristics of RA FLS that we have discussed form the basis for a range of investigative approaches to targeting FLS in RA, and encompass cadherin-11-mediated adhesion and paracrine signalling, aberrant survival, epigenetic alterations, and pathological intracellular si gnalling pathways, as we discuss in this section.
Work in mice suggests that interfering with cadherin-11 function on the surface of FLS is an appealing approach to controlling their aggressiveness in RA patients.9 Cadherin-11 antagonist antibodies are currently under development for RA therapy. As cadherin-11 is minimally expressed in immune cells, anti-cadherin-11 antibodies would represent a novel— probably nonimmunosuppressive—biologic approach to RA therapy.7
Various components of the MAPK cascade are candidate targets for FLS-directed therapy in RA. JNKs are potential targets in RA, and in vivo efficacy of an inhibitor of all JNK isoforms (in terms of inhibiting radiographic damage and reducing swelling in adjuvant-induced arthritis) in rats was reported in 2001.24 Deletion of Jnk1 (also known as Mapk8) reduces severity of arthritis in the methylated albumin injection mouse model of arthritis by interfering with macrophage migration and function.72,73 However, these same studies challenged the importance of targeting some JNK isoforms in RA FLS because deletion of Jnk2 (also known as Mapk9), which encodes the predominant Jnk isoform expressed in human FLS, did not affect disease in the methylated albumin injection mouse model, and exacerbated disease in the K/BxN mouse model of RA.72,73
Besides JNKs, attention has also turned to the upstream kinase MKK7. Mkk7 antisense oligonucleotides were effective at ameliorating disease in the K/BxN serum transfer mouse model of RA, suggesting that pharmacological inhibition of MKK7 might be a viable approach to decrease RA FLS aggressiveness in patients with RA.74 The upstream kinase MAP3K 7 is another interesting candidate target for RA FLS-directed therapy; however, no validation of this approach in mouse models of arthritis has yet been published.
Inhibitors of two other kinases that indirectly activate the JNK pathway, PI3K isoforms (especially PI3Kδ)44,75 and tyrosine-protein kinase SYK76 (which is also expressed at high levels in several immune cell types), are also considered promising strategies to tame FLS hyper activation in RA. Small-molecule inhibitors of SYK have already demonstrated efficacy in a phase II trial in 457 patients with active RA and an inadequate response to methotrexate.77
After the emergence of several lines of evidence that inhibition of p38α MAPK has anti-inflammatory effects in vitro,78 inhibition of this MAPK was aggressively pursued as a potential therapeutic strategy in RA, but was unfortunately found to be ineffective in several phase II clinical trials. These studies included trials of pamapimod and VX-702—selective inhibitors of p38α MAPK—in 204 and 313 patients, respectively,30,79 and a trial of SCIO-469—an inhibitor with a modest selectivity for p38α over p38β MAPK— in 302 patients31. A probable explanation for this unexpected failure is that p38α MAPK has an anti-inflammatory role in RA macrophages in vivo. Thus, in RA, the proinflammatory effect of inhibiting p38α MAPK in macrophages would obliterate the beneficial effect of inhibiting p38 MAPKs in FLS.25 Targeting the upstream kinases MKK3 or MKK6 represent potential alternative approaches to inhibiting p38 MAPKs directly.29,80
Inhibition of the NFκB pathway has been validated at the preclinical stage in RA using a variety of approaches.42 For example, intraperitoneal administration of a small-molecule inhibitor of NFκB and intra-articular viral transduction of a construct encoding A20, a protein that normally inhibits NFkB signalling, were both effective in reducing severity of collagen-induced arthritis in mice.41,81 Also, intra-articular viral transduction of constructs encoding dominant-negative Ikkβ ameliorated adjuvant-induced arthritis in rats.82 As well as reducing FLS aggressiveness, approaches directed at inhibiting NFκB signalling might have beneficial effects on additional pathogenic mechanisms in RA, owing to the pleiotropic effect of NFκB on immune and other cells. As we discussed earlier, it is difficult to predict the effect of blockade of IKK-related kinases IKKε and/or TBK1 because of the opposing effects of these kinases on the release of MMPs and type I interferons by RA FLS.
Therapies aimed at inducing apoptosis of FLS could also be valuable in RA. Overexpression of PUMA in RA FLS leads to rapid p53-independent death through caspase 3 activation, suggesting that gene therapy with constructs of the gene encoding PUMA, BBC3, could bypass the p53 pathway signalling deficiency in RA FLS.52 Expression of a dominant-negative IκB construct or of an antisense oligonucleotide against a mediator of NFκB-dependent-apoptosis resistance, E3 ubiquitin-protein ligase XIAP, were able to restore RA FLS apoptosis in vitro and in vivo after intra-articular viral transduction in SCID mice with cartilage implants co-cultured with RA FLS.42 Intra-articular viral transduction approaches were also able to enhance RA FLS apoptosis by increasing expression of the proapoptotic factor soluble TNF-related apoptosis-inducing ligand (TRAIL; also known as TNF ligand superfamily member 10).83
Pharmacologic approaches aimed at correcting epigenetic anomalies in FLS are also under intense investigation in preclinical studies. For example, systemic administration of histone deacetylase inhibitors has yielded promising results in mouse and rat models of RA.84 Similar experiments using inhibitors of DNMTs and/or approaches to modify expression or function of specific miRNA in RA FLS are warranted. Finally, inhibitors of autotaxin and/or of lysophosphatidic acid receptors are a promising approach to decrease RA FLS aggressiveness.21 The fact that several inhibitors of these receptors—including orally available molecules—are in advanced stages of preclinical validation for therapy of cancer and other diseases should facilitate the pursuit of the same therapeutic avenue in RA.
Conclusion
FLS have a dual role in RA, by responding appropriately to the inflammatory environment and through aggressive behaviour imprinted during their sojourn through the rheumatoid synovium. Some, but not necessarily all, FLS effector functions can be halted by treatment of RA with current therapeutic agents, which suppress inflammation and cut off the signals that drive passive FLS responses. We predict that combination therapies that simultaneously modify immune responses while reducing FLS aggressiveness will become an option in the near future. Combining an immunosuppressive agent with an FLS-directed therapy might enable more effective control of disease activity, without inducing the crippling effects on defence against infection that can be observed when using multiple biologic agents that target immune responses.
Key points.
-
■
Fibroblast-like synoviocytes (FLS), normally found in the synovial intimal lining of diarthrodial joints, display an aggressive, invasive phenotype in rheumatoid arthritis (RA) and participate in joint destruction
-
■
FLS from patients with RA promote inflammatory cell recruitment and activation, pannus angiogenesis, cartilage degradation, and bone erosion
-
■
The phenotype of FLS from patients with RA is partly a passive response to the inflammatory milieu in vivo, and partly an imprinted feature that persists when the cells are cultured in vitro
-
■
Imprinted anomalies of FLS in RA arise, at least in part, through epigenetic modifications of the genome, such as altered microRNA expression and DNA methylation
-
■
Increased knowledge of the biology of FLS in RA will pave the way to novel FLS-targeted therapies with limited immunosuppressive action
Review criteria.
This Review explains broad concepts of fibroblast-like synoviocyte biology in the context of rheumatoid arthritis. MEDLINE was searched for articles and abstracts published since 1980 using the terms “synoviocyte”, “fibroblast-like synoviocyte” and “synovial fibroblast”. Studies relevant to rheumatoid arthritis were identified using the search term “rheumatoid arthritis”. Non-English-language reports were excluded. Full-length papers were downloaded and reviewed and their reference lists were scanned for further relevant references. The list was last updated in August 2012.
Acknowledgements
The authors are indebted to M. Bottini and S. Stanford for help with preparation of the figures. This work was supported, in part, by Institutional La Jolla Institute of Allergy and Immunology funds (to N. Bottini) and by NIH grants R01AI067752, R01AI070555, and R01AR47825 (to G. S. Firestein). This manuscript is #1556 published from the La Jolla Institute of Allergy and Immunology.
Footnotes
Competing interests
The authors declare no competing interests.
Contributor Information
Nunzio Bottini, Division of Cellular Biology, La Jolla Institute of Allergy and Immunology, 9420 Athena Circle, La Jolla, CA 92037, USA.
Gary S. Firestein, Division of Rheumatology, Allergy and Immunology, UCSD School of Medicine, Mail Code 0602, 9500, Gilman Drive, La Jolla, CA 92014, USA
References
- 1.Firestein GS. Invasive fibroblast-like synoviocytes in rheumatoid arthritis. Passive responders or transformed aggressors? Arthritis Rheum. 1996;39:1781–1790. doi: 10.1002/art.1780391103. [DOI] [PubMed] [Google Scholar]
- 2.Müller-Ladner U, et al. Synovial fibroblasts of patients with rheumatoid arthritis attach to and invade normal human cartilage when engrafted into SCID mice. Am. J. Pathol. 1996;149:1607–1615. [PMC free article] [PubMed] [Google Scholar]
- 3.Lefèvre S, et al. Synovial fibroblasts spread rheumatoid arthritis to unaffected joints. Nat. Med. 2009;15:1414–1420. doi: 10.1038/nm.2050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bartok B, Firestein GS. Fibroblast-like synoviocytes: key effector cells in rheumatoid arthritis. Immunol. Rev. 2010;233:233–255. doi: 10.1111/j.0105-2896.2009.00859.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Edwards JC, Leigh RD, Cambridge G. Expression of molecules involved in B lymphocyte survival and differentiation by synovial fibroblasts. Clin. Exp. Immunol. 1997;108:407–414. doi: 10.1046/j.1365-2249.1997.4061306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Okazaki M, et al. Molecular cloning and characterization of OB-cadherin, a new member of cadherin family expressed in osteoblasts. J. Biol. Chem. 1994;269:12092–12098. [PubMed] [Google Scholar]
- 7.Lee DM, et al. Cadherin-11 in synovial lining formation and pathology in arthritis. Science. 2007;315:1006–1010. doi: 10.1126/science.1137306. [DOI] [PubMed] [Google Scholar]
- 8.Valencia X, et al. Cadherin-11 provides specific cellular adhesion between fibroblast-like synoviocytes. J. Exp. Med. 2004;200:1673–1679. doi: 10.1084/jem.20041545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chang SK, et al. Cadherin-11 regulates fibroblast inflammation. Proc. Natl Acad. Sci. USA. 2011;108:8402–8407. doi: 10.1073/pnas.1019437108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firestein GS, Yeo M, Zvaifler NJ. Apoptosis in rheumatoid arthritis synovium. J. Clin. Invest. 1995;96:1631–1638. doi: 10.1172/JCI118202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Imamura F, et al. Monoclonal expansion of synoviocytes in rheumatoid arthritis. Arthritis Rheum. 1998;41:1979–1986. doi: 10.1002/1529-0131(199811)41:11<1979::AID-ART13>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- 12.Matsumoto S, Müller-Ladner U, Gay RE, Nishioka K, Gay S. Ultrastructural demonstration of apoptosis, Fas and Bcl-2 expression of rheumatoid synovial fibroblasts. J. Rheumatol. 1996;23:1345–1352. [PubMed] [Google Scholar]
- 13.Meinecke I, et al. Modification of nuclear PML protein by SUMO-1 regulates Fas-induced apoptosis in rheumatoid arthritis synovial fibroblasts. Proc. Natl Acad. Sci. USA. 2007;104:5073–5078. doi: 10.1073/pnas.0608773104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kumkumian GK, et al. Platelet-derived growth factor and IL-1 interactions in rheumatoid arthritis. Regulation of synoviocyte proliferation, prostaglandin production, and collagenase transcription. J. Immunol. 1989;143:833–837. [PubMed] [Google Scholar]
- 15.Lotz M, Guerne PA. Interleukin-6 induces the synthesis of tissue inhibitor of metalloproteinases-1/erythroid potentiating activity (TIMP-1/EPA) J. Biol. Chem. 1991;266:2017–2020. [PubMed] [Google Scholar]
- 16.Shigeyama Y, et al. Expression of osteoclast differentiation factor in rheumatoid arthritis. Arthritis Rheum. 2000;43:2523–2530. doi: 10.1002/1529-0131(200011)43:11<2523::AID-ANR20>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 17.Pap T, Aupperle KR, Gay S, Firestein GS, Gay RE. Invasiveness of synovial fibroblasts is regulated by p53 in the SCID mouse in vivo model of cartilage invasion. Arthritis Rheum. 2001;44:676–681. doi: 10.1002/1529-0131(200103)44:3<676::AID-ANR117>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 18.Guerne PA, Zuraw BL, Vaughan JH, Carson DA, Lotz M. Synovium as a source of interleukin 6 in vitro. Contribution to local and systemic manifestations of arthritis. J. Clin. Invest. 1989;83:585–592. doi: 10.1172/JCI113921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Crow MK. Type I interferon in organ-targeted autoimmune and inflammatory diseases. Arthritis Res. Ther. 2010;12(Suppl. 1):S5. doi: 10.1186/ar2886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Palmer CD, Mutch BE, Page TH, Horwood NJ, Foxwell BM. Bmx regulates LPS-induced IL-6 and VEGF production via mRNA stability in rheumatoid synovial fibroblasts. Biochem. Biophys. Res. Commun. 2008;370:599–602. doi: 10.1016/j.bbrc.2008.03.142. [DOI] [PubMed] [Google Scholar]
- 21.Nikitopoulou I, et al. Autotaxin expression from synovial fibroblasts is essential for the pathogenesis of modeled arthritis. J. Exp. Med. 2012;209:925–933. doi: 10.1084/jem.20112012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stanczyk J, Ospelt C, Gay RE, Gay S. Synovial cell activation. Curr. Opin. Rheumatol. 2006;18:262–267. doi: 10.1097/01.bor.0000218947.42730.dd. [DOI] [PubMed] [Google Scholar]
- 23.Johnson GL, Lapadat R. Mitogen-activated protein kinase pathways mediated by ERK, JNK, and p38 protein kinases. Science. 2002;298:1911–1912. doi: 10.1126/science.1072682. [DOI] [PubMed] [Google Scholar]
- 24.Han Z, et al. c-Jun N-terminal kinase is required for metalloproteinase expression and joint destruction in inflammatory arthritis. J. Clin. Invest. 2001;108:73–81. doi: 10.1172/JCI12466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Yoshizawa T, et al. Role of MAPK kinase 6 in arthritis: distinct mechanism of action in inflammation and cytokine expression. J. Immunol. 2009;183:1360–1367. doi: 10.4049/jimmunol.0900483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Korb A, et al. Differential tissue expression and activation of p38 MAPK α, β, γ, and δ isoforms in rheumatoid arthritis. Arthritis Rheum. 2006;54:2745–2756. doi: 10.1002/art.22080. [DOI] [PubMed] [Google Scholar]
- 27.Westra J, Limburg PC, de Boer P, van Rijswijk MH. Effects of RWJ 67657, a p38 mitogen activated protein kinase (MAPK) inhibitor, on the production of inflammatory mediators by rheumatoid synovial fibroblasts. Ann. Rheum. Dis. 2004;63:1453–1459. doi: 10.1136/ard.2003.013011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue T, Hammaker D, Boyle DL, Firestein GS. Regulation of p38 MAPK by MAPK kinases 3 and 6 in fibroblast-like synoviocytes. J. Immunol. 2005;174:4301–4306. doi: 10.4049/jimmunol.174.7.4301. [DOI] [PubMed] [Google Scholar]
- 29.Guma M, et al. Pro- and anti-inflammatory functions of the p38 pathway in rheumatoid arthritis: advantages of targeting upstream kinases MKK3 or MKK6. Arthritis Rheum. 2012;64:2887–2895. doi: 10.1002/art.34489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cohen SB, et al. Evaluation of the efficacy and safety of pamapimod, a p38 MAP kinase inhibitor, in a double-blind, methotrexate-controlled study of patients with active rheumatoid arthritis. Arthritis Rheum. 2009;60:335–344. doi: 10.1002/art.24266. [DOI] [PubMed] [Google Scholar]
- 31.Genovese MC, et al. A 24-week, randomized, double-blind, placebo-controlled, parallel group study of the efficacy of oral SCIO-469, a p38 mitogen-activated protein kinase inhibitor, in patients with active rheumatoid arthritis. J. Rheumatol. 2011;38:846–854. doi: 10.3899/jrheum.100602. [DOI] [PubMed] [Google Scholar]
- 32.Shahrara S, Castro-Rueda HP, Haines GK, Koch AE. Differential expression of the FAK family kinases in rheumatoid arthritis and osteoarthritis synovial tissues. Arthritis Res. Ther. 2007;9:R112. doi: 10.1186/ar2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pap T, et al. Cooperation of Ras- and c-Myc-dependent pathways in regulating the growth and invasiveness of synovial fibroblasts in rheumatoid arthritis. Arthritis Rheum. 2004;50:2794–2802. doi: 10.1002/art.20461. [DOI] [PubMed] [Google Scholar]
- 34.Weisbart RH, et al. BRAF drives synovial fibroblast transformation in rheumatoid arthritis. J. Biol. Chem. 2010;285:34299–34303. doi: 10.1074/jbc.C110.168195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Abreu JR, et al. The Ras guanine nucleotide exchange factor RasGRF1 promotes matrix metalloproteinase-3 production in rheumatoid arthritis synovial tissue. Arthritis Res. Ther. 2009;11:R121. doi: 10.1186/ar2785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Han Z, et al. Jun N-terminal kinase in rheumatoid arthritis. J. Pharmacol. Exp. Ther. 1999;291:124–130. [PubMed] [Google Scholar]
- 37.Svensson CI, et al. Gadd45β deficiency in rheumatoid arthritis: enhanced synovitis through JNK signaling. Arthritis Rheum. 2009;60:3229–3240. doi: 10.1002/art.24887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Luo Y, et al. Suppression of collagen-induced arthritis in growth arrest and DNA damage-inducible protein 45β-deficient mice. Arthritis Rheum. 2011;63:2949–2955. doi: 10.1002/art.30497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aupperle K, et al. NF-κB regulation by IκB kinase-2 in rheumatoid arthritis synoviocytes. J. Immunol. 2001;166:2705–2711. doi: 10.4049/jimmunol.166.4.2705. [DOI] [PubMed] [Google Scholar]
- 40.Oeckinghaus A, Ghosh S. The NF-κB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009;1:a000034. doi: 10.1101/cshperspect.a000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Okazaki Y, et al. Effect of nuclear factor-κB inhibition on rheumatoid fibroblast-like synoviocytes and collagen induced arthritis. J. Rheumatol. 2005;32:1440–1447. [PubMed] [Google Scholar]
- 42.Zhang HG, et al. Gene therapy that inhibits nuclear translocation of nuclear factor κB results in tumor necrosis factor α-induced apoptosis of human synovial fibroblasts. Arthritis Rheum. 2000;43:1094–1105. doi: 10.1002/1529-0131(200005)43:5<1094::AID-ANR20>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 43.Pap T, et al. Activation of synovial fibroblasts in rheumatoid arthritis: lack of expression of the tumour suppressor PTEN at sites of invasive growth and destruction. Arthritis Res. 2000;2:59–64. doi: 10.1186/ar69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bartok B, et al. PI3 kinase δ is a key regulator of synoviocyte function in rheumatoid arthritis. Am. J. Pathol. 2012;180:1906–1916. doi: 10.1016/j.ajpath.2012.01.030. [DOI] [PubMed] [Google Scholar]
- 45.Sweeney SE, Hammaker D, Boyle DL, Firestein GS. Regulation of c-Jun phosphorylation by the IκB kinase-ε complex in fibroblast-like synoviocytes. J. Immunol. 2005;174:6424–6430. doi: 10.4049/jimmunol.174.10.6424. [DOI] [PubMed] [Google Scholar]
- 46.Sweeney SE, Corr M, Kimbler TB. Role of interferon regulatory factor 7 in serum-transfer arthritis: regulation of interferon-β production. Arthritis Rheum. 2012;64:1046–1056. doi: 10.1002/art.33454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Korb A, Pavenstadt H, Pap T. Cell death in rheumatoid arthritis. Apoptosis. 2009;14:447–454. doi: 10.1007/s10495-009-0317-y. [DOI] [PubMed] [Google Scholar]
- 48.Wakisaka S, et al. Modulation by proinflammatory cytokines of Fas/Fas ligand-mediated apoptotic cell death of synovial cells in patients with rheumatoid arthritis (RA) Clin. Exp. Immunol. 1998;114:119–128. doi: 10.1046/j.1365-2249.1998.00701.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Audo R, et al. Mechanisms and clinical relevance of TRAIL-triggered responses in the synovial fibroblasts of patients with rheumatoid arthritis. Arthritis Rheum. 2011;63:904–913. doi: 10.1002/art.30181. [DOI] [PubMed] [Google Scholar]
- 50.Han Z, Boyle DL, Shi Y, Green DR, Firestein GS. Dominant-negative p53 mutations in rheumatoid arthritis. Arthritis Rheum. 1999;42:1088–1092. doi: 10.1002/1529-0131(199906)42:6<1088::AID-ANR4>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 51.Firestein GS, et al. Apoptosis in rheumatoid arthritis: p53 overexpression in rheumatoid arthritis synovium. Am. J. Pathol. 1996;149:2143–2151. [PMC free article] [PubMed] [Google Scholar]
- 52.Cha HS, Rosengren S, Boyle DL, Firestein GS. PUMA regulation and proapoptotic effects in fibroblast-like synoviocytes. Arthritis Rheum. 2006;54:587–592. doi: 10.1002/art.21631. [DOI] [PubMed] [Google Scholar]
- 53.Schedel J, et al. FLICE-inhibitory protein expression in synovial fibroblasts and at sites of cartilage and bone erosion in rheumatoid arthritis. Arthritis Rheum. 2002;46:1512–1518. doi: 10.1002/art.10309. [DOI] [PubMed] [Google Scholar]
- 54.Franz JK, et al. Expression of sentrin, a novel antiapoptotic molecule, at sites of synovial invasion in rheumatoid arthritis. Arthritis Rheum. 2000;43:599–607. doi: 10.1002/1529-0131(200003)43:3<599::AID-ANR17>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 55.Maciejewska-Rodrigues H, et al. Epigenetics and rheumatoid arthritis: the role of SENP1 in the regulation of MMP-1 expression. J. Autoimmun. 2010;35:15–22. doi: 10.1016/j.jaut.2009.12.010. [DOI] [PubMed] [Google Scholar]
- 56.Fassbender HG, Simmling-Annefeld M. The potential aggressiveness of synovial tissue in rheumatoid arthritis. J. Pathol. 1983;139:399–406. doi: 10.1002/path.1711390314. [DOI] [PubMed] [Google Scholar]
- 57.Yamanishi Y, et al. p53 tumor suppressor gene mutations in fibroblast-like synoviocytes from erosion synovium and non-erosion synovium in rheumatoid arthritis. Arthritis Res. Ther. 2005;7:R12–R18. doi: 10.1186/ar1448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Da Sylva TR, Connor A, Mburu Y, Keystone E, Wu GE. Somatic mutations in the mitochondria of rheumatoid arthritis synoviocytes. Arthritis Res. Ther. 2005;7:R844–R851. doi: 10.1186/ar1752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Lee SH, et al. Microsatellite instability and suppressed DNA repair enzyme expression in rheumatoid arthritis. J. Immunol. 2003;170:2214–2220. doi: 10.4049/jimmunol.170.4.2214. [DOI] [PubMed] [Google Scholar]
- 60.Tak PP, Zvaifler NJ, Green DR, Firestein GS. Rheumatoid arthritis and p53: how oxidative stress might alter the course of inflammatory diseases. Immunol. Today. 2000;21:78–82. doi: 10.1016/s0167-5699(99)01552-2. [DOI] [PubMed] [Google Scholar]
- 61.Harty LC, et al. Mitochondrial mutagenesis correlates with the local inflammatory environment in arthritis. Ann. Rheum. Dis. 2012;71:582–588. doi: 10.1136/annrheumdis-2011-200245. [DOI] [PubMed] [Google Scholar]
- 62.Karouzakis E, Gay RE, Gay S, Neidhart M. Epigenetic deregulation in rheumatoid arthritis. Adv. Exp. Med. Biol. 2011;711:137–149. doi: 10.1007/978-1-4419-8216-2_10. [DOI] [PubMed] [Google Scholar]
- 63.Kuchen S, et al. The L1 retroelement-related p40 protein induces p38δ MAP kinase. Autoimmunity. 2004;37:57–65. doi: 10.1080/08916930310001637977. [DOI] [PubMed] [Google Scholar]
- 64.Nakano K, Whitaker JW, Boyle DL, Wang W, Firestein GS. DNA methylome signature in rheumatoid arthritis. Ann. Rheum. Dis. doi: 10.1136/annrheumdis-2012-201526. http://dx.doi.org/10.1136/annrheumdis-2012-201526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Grabiec AM, Korchynskyi O, Tak PP, Reedquist KA. Histone deacetylase inhibitors suppress rheumatoid arthritis fibroblast-like synoviocyte and macrophage IL-6 production by accelerating mRNA decay. Ann. Rheum. Dis. 2012;71:424–431. doi: 10.1136/ard.2011.154211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nakamachi Y, et al. MicroRNA-124a is a key regulator of proliferation and monocyte chemoattractant protein 1 secretion in fibroblast-like synoviocytes from patients with rheumatoid arthritis. Arthritis Rheum. 2009;60:1294–1304. doi: 10.1002/art.24475. [DOI] [PubMed] [Google Scholar]
- 67.Stanczyk J, et al. Altered expression of microRNA-203 in rheumatoid arthritis synovial fibroblasts and its role in fibroblast activation. Arthritis Rheum. 2011;63:373–381. doi: 10.1002/art.30115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Duroux-Richard I, Jorgensen C, Apparailly F. What do microRNAs mean for rheumatoid arthritis? Arthritis Rheum. 2012;64:11–20. doi: 10.1002/art.30651. [DOI] [PubMed] [Google Scholar]
- 69.Karouzakis E, Gay RE, Gay S, Neidhart M. Increased recycling of polyamines is associated with global DNA hypomethylation in rheumatoid arthritis synovial fibroblasts. Arthritis Rheum. 2012;64:1809–1817. doi: 10.1002/art.34340. [DOI] [PubMed] [Google Scholar]
- 70.Ballestar E. An introduction to epigenetics. Adv. Exp. Med. Biol. 2011;711:1–11. doi: 10.1007/978-1-4419-8216-2_1. [DOI] [PubMed] [Google Scholar]
- 71.Bogdanos DP, et al. Twin studies in autoimmune disease: genetics, gender and environment. J. Autoimmun. 2012;38:156–169. doi: 10.1016/j.jaut.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 72.Guma M, Ronacher LM, Firestein GS, Karin M, Corr M. JNK-1 deficiency limits macrophage-mediated antigen-induced arthritis. Arthritis Rheum. 2011;63:1603–1612. doi: 10.1002/art.30271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Denninger K, et al. JNK1, but not JNK2, is required in two mechanistically distinct models of inflammatory arthritis. Am. J. Pathol. 2011;179:1884–1893. doi: 10.1016/j.ajpath.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee SI, Boyle DL, Berdeja A, Firestein GS. Regulation of inflammatory arthritis by the upstream kinase mitogen activated protein kinase kinase 7 in the c-Jun N-terminal kinase pathway. Arthritis Res. Ther. 2012;14:R38. doi: 10.1186/ar3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haruta K, et al. Inhibitory effects of ZSTK474, a phosphatidylinositol 3-kinase inhibitor, on adjuvant-induced arthritis in rats. Inflamm. Res. 2012;61:551–562. doi: 10.1007/s00011-012-0444-8. [DOI] [PubMed] [Google Scholar]
- 76.Cha HS, et al. A novel spleen tyrosine kinase inhibitor blocks c-Jun N-terminal kinase-mediated gene expression in synoviocytes. J. Pharmacol. Exp. Ther. 2006;317:571–578. doi: 10.1124/jpet.105.097436. [DOI] [PubMed] [Google Scholar]
- 77.Weinblatt ME, et al. An oral spleen tyrosine kinase (Syk) inhibitor for rheumatoid arthritis. N. Engl. J. Med. 2010;363:1303–1312. doi: 10.1056/NEJMoa1000500. [DOI] [PubMed] [Google Scholar]
- 78.Schieven GL. The p38α kinase plays a central role in inflammation. Curr. Top. Med. Chem. 2009;9:1038–1048. doi: 10.2174/156802609789630974. [DOI] [PubMed] [Google Scholar]
- 79.Damjanov N, Kauffman RS, Spencer-Green GT. Efficacy, pharmacodynamics, and safety of VX-702, a novel p38 MAPK inhibitor, in rheumatoid arthritis: results of two randomized, double-blind, placebo-controlled clinical studies. Arthritis Rheum. 2009;60:1232–1241. doi: 10.1002/art.24485. [DOI] [PubMed] [Google Scholar]
- 80.Inoue T, et al. Mitogen-activated protein kinase kinase 3 is a pivotal pathway regulating p38 activation in inflammatory arthritis. Proc. Natl Acad. Sci. USA. 2006;103:5484–5489. doi: 10.1073/pnas.0509188103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Hah YS, et al. A20 suppresses inflammatory responses and bone destruction in human fibroblast-like synoviocytes and in mice with collagen-induced arthritis. Arthritis Rheum. 2010;62:2313–2321. doi: 10.1002/art.27545. [DOI] [PubMed] [Google Scholar]
- 82.Tak PP, et al. Inhibitor of nuclear factor κB kinase β is a key regulator of synovial inflammation. Arthritis Rheum. 2001;44:1897–1907. doi: 10.1002/1529-0131(200108)44:8<1897::AID-ART328>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 83.Shi J, et al. Epirubicin potentiates recombinant adeno-associated virus type 2/5-mediated TRAIL expression in fibroblast-like synoviocytes and augments the antiarthritic effects of rAAV2/5-TRAIL. Arthritis Rheum. 2012;64:1345–1354. doi: 10.1002/art.33492. [DOI] [PubMed] [Google Scholar]
- 84.Lin HS, et al. Anti-rheumatic activities of histone deacetylase (HDAC) inhibitors in vivo in collagen-induced arthritis in rodents. Br. J. Pharmacol. 2007;150:862–872. doi: 10.1038/sj.bjp.0707165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Kavanaugh AF, et al. Treatment of refractory rheumatoid arthritis with a monoclonal antibody to intercellular adhesion molecule 1. Arthritis Rheum. 1994;37:992–999. doi: 10.1002/art.1780370703. [DOI] [PubMed] [Google Scholar]