Abstract
Background
Short-chain fatty acids (SCFA) are produced by colonic microbiota from dietary carbohydrates and proteins that reach the colon. It has been suggested that SCFA may promote obesity via increased colonic energy availability. Recent studies suggest obese humans have higher faecal SCFA than lean, but it is unclear if this difference is due to increased SCFA production or reduced absorption.
Objectives
To compare rectal SCFA absorption, dietary intake and faecal microbial profile in lean (LN) versus overweight and obese (OWO) individuals.
Design
Eleven (11) LN and 11 OWO individuals completed a 3-day diet record, provided a fresh faecal sample and had SCFA absorption measured using the rectal dialysis bag method. The procedures were repeated after two weeks.
Results
Age-adjusted faecal SCFA concentration was significantly higher in OWO than LN (81.3 ± 7.4 vs. 64.1 ± 10.4 mmol/kg, P = 0.023). SCFA absorption (24.4 ± 0.8 vs 24.7 ± 1.2%, respectively, P =0.787) and dietary intakes were similar between the groups, except for a higher fat intake in OWO. However, fat intake did not correlate with SCFAs or bacterial abundance. OWO had higher relative Firmicutes abundance (83.1 ± 4.1 vs 69.5 ± 5.8%, respectively, P = 0.008) and a higher Firmicutes:Bacteriodetes ratio (P = 0.023) than LN. There was a positive correlation between Firmicutes and faecal SCFA within the whole group (r =0.507, P =0.044), with a stronger correlation after adjusting for available carbohydrate (r = 0.615, P =0.005).
Conclusions
The higher faecal SCFA in OWO subjects is not due to differences in SCFA absorption or diet. Our results are consistent with the hypothesis that OWO subjects produce more colonic SCFA than LN due to differences in colonic microbiota. However, further studies are needed to prove this.
Keywords: Short Chain Fatty Acid, Obesity, Colonic Absorption, Diet, colonic microbiota
INTRODUCTION
Short-chain fatty acids (SCFA): acetate, propionate and butyrate are anions in human faeces, present in a molar ratio of ~ 60:20:20, respectively.(1) SCFA are produced by the colonic microbiota through anaerobic fermentation. The chief substrates for colonic fermentation are undigested carbohydrates, namely dietary fibre and resistant starch and, in smaller amounts, proteins.(2) Short-chain fatty acids are readily absorbed from the lumen(2) and are used as metabolic precursors; acetate is utilized for lipogenesis in the liver and as a fuel source once it enters the peripheral circulation. Propionate is largely taken up by the liver, and is used as a substrate for hepatic gluconeogenesis. Butyrate is the major fuel source for colonocytes.(3)
Recently, the role of SCFA as a contributor to obesity has been highlighted. Studies in mice suggest that the obese microbiota, characterized by a high Firmicutes to Bacteriodetes ratio (F:B),(4) promotes obesity because of excess SCFA production and thus increased colonic energy availability that may contribute to weight gain.(5) In agreement with this, some obese humans have been found to have higher faecal SCFA concentrations than lean individuals;(6,7) however the results for F:B ratio have been inconsistent.(6,8)
An increase in faecal SCFA concentration could be due to several factors, including reduced colonic SCFA absorption,(9) reduced colonic transit time,(10) or increased SCFA production due to differences in dietary intake(11,12) or colonic microbiota.(13,14) A previous study from our lab found a large range of SCFA absorption rates in lean participants and, across this range, there was an inverse relationship between rectal acetate absorption and faecal acetate concentration.(9) Therefore, a higher SCFA concentration in obese individuals may be a result of lower SCFA colonic absorption. However, this possibility has not been investigated. Moreover, there has been little research examining differences in dietary intakes, microbial profile and gut transit time, factors which may determine the difference in faecal SCFA concentrations between these groups. Therefore, in this pilot observational study, our primary objectives were to compare the rate of SCFA absorption from the rectum in LN and OWO individuals and to examine the relationship between rectal SCFA absorption and faecal SCFA concentrations. Our secondary objectives were to compare dietary intake, microbial profile and gut transit time in LN and OWO subjects, and to see if these variables relate to fecal SCFA.
SUBJECTS AND METHODS
Subjects
Twenty two (22) male or non-pregnant, non-lactating females over the age of 17 years were recruited via advertisements posted around the University of Toronto campus and from a pool of subjects previously involved in studies by our group. Participants were divided into two groups based on their BMI. Eleven participants in the LN group (BMI≤25) and 11 participants in the OWO group (BMI>25). Subjects were excluded for any of the following reasons: regular user of antibiotics (≥1 course per year over the last 5 years), any use of antibiotics, laxatives or other drugs known to influence gastrointestinal function within three months of the study, presence of inflammatory bowel disease, malabsorption, gastrointestinal infection, short bowel, or other condition affecting gastrointestinal function or any recent (3 months prior to the study) illness or surgery requiring hospitalization. Ethical approval was obtained from the Research Ethics Board, University of Toronto. Written informed consent was obtained from all participants.
Eligible participants completed questionnaires that provided demographics, medical history, drug use, and physical activity data(15,16). Their height and weight were measured and they were given instructions on how to complete a 3-day dietary intake history, fill out a 3-day bowel habit diary and collect a faecal sample in a plastic bag (Ziploc, S.C. Johnson &Son, Inc.) using the Fisher brand commode specimen collection system (Fisher Scientific, Ottawa, ON). After this, subjects participated in two 4-day study periods separated by a 2-week washout period.
Study period protocol
For the first 3 days of each study period subjects kept a diet record and filled out the bowel habit diary. On the 3rd day, after picking up a Styrofoam box full of dry ice and faecal-collection kit, subjects collected a fresh stool sample which was immediately placed onto dry ice. Subjects came to the laboratory on the morning (between 8 to 10am) of the 4th day after eating their normal breakfast. After handing in their diet record, bowel habit questionnaire and faecal sample, 2 breath samples were collected as described elsewhere.(19) Subjects then underwent a procedure to measure rectal SCFA absorption using a rectal dialysis bag method that has been validated previously by others(17,18). First, subjects put ~5cm of the end of a piece of Tygon flexible plastic tubing (Norton Performance Plastics, Akron, OH; o.d. 5mm) into their rectum, infused 500mL of water, and emptied their colon; they repeated this step if the return was not clean. Subjects then waited 15 min to ensure that there was no further urge to void, and inserted a dialysis bag containing SCFA solution into their rectum. The bag consisted of dialysis tubing (o.d. 16mm; Dialysis Tubing Cellulose Membrane AVG, Sigma-Aldrich Canada, Ltd) which had been knotted at one end and filled with 10 ml of a solution containing 150mmol/L of SCFA in a molar ratio of 90:30:30 mmol/L AC:PR:BU, respectively. After expelling any air bubbles, the opposite end was tied and the filled dialysis bag weighed. The length of the filled portion of the dialysis bags was 5cm, and an 8–10cm length of empty tubing was left attached to facilitate removal of the tubing from the rectum. After careful instructions, each participant inserted a dialysis bag, covered with a small amount of water-soluble lubricant (K-Y Jelly®), into his or her rectum until the distal end was 2cm from the anal sphincter. After 30 min the dialysis bag was gently removed by pulling the protruding long end and immediately processed for analysis. Approximately, 2 weeks after completing the first 4-day study period, subjects underwent a second study period using the same protocol, including the diet record, bowel habit diary, faecal sample collection and rectal SCFA absorption procedure.
Sample Handling
After retraction from the rectum, the dialysis bag was weighed to detect volume changes and a 3-ml syringe with a 21G1½ needle was then used to collect 1 ml of the solution from the dialysis bag. The rest of the solution was carefully emptied out of the bag and the empty bag was weighed again. The net solution weight was calculated by subtracting the weight of the bag before and after the procedure. The 1 ml samples were immediately stored at −70°C for later analysis. Frozen faecal samples were kept at −20°C prior to analysis.
pH and SCFA analysis
After faecal samples were thawed, they were homogenized. pH was determined by pH meter and faecal SCFA concentrations were determined by gas chromatography (GC) as previously described.(19) After thawing the supernatants from the rectal dialysis bag, they were diluted 1:500 with double distilled water, centrifuged and subsequently analyzed by GC.
Questionnaires
Intake of nutrients was calculated from the 3-day diet records using ESHA Research’s Food Processor® SQL, Version 10.9.0. A self-administered bowel habit questionnaire provided a subjective assessment of bowel function. Participants assessed the following characteristics for each bowel movement during the 3-day diet record: ease of movement (scale: from 0 easy to pass, to 6 difficult to pass); stool consistency (scale: 0-watery, 2-soft, 4-formed, 6-very hard); frequency of flatulence, presence of cramping, presence of bloating (scale: 0-none, 2-mild, 4-moderate, 6-severe).
Analysis of Faecal Microbiota
DNA extraction and Ion Torrent V6 16S rRNA sequencing were performed as described elsewhere.(20)
STATISTICAL ANALYSIS
The percentage of SCFA absorption was calculated as the mmol of SCFA which disappeared from the dialysis bag divided by the mmol of SCFA present at baseline (SCFAbl) multiplied by 100:
A Mixed-models approach was used for all statistical tests to account for the repeated measurements (two visits for each participant) performed by SAS Version 9.3. Residuals from final models were assessed to ensure that model assumptions were met. Differences in faecal SCFA concentration, SCFA absorption and bacterial composition between the LN and OWO groups were assessed in 3 models: without adjustment, with adjustments for age and with adjustments for both age and carbohydrate intake. The effect of faecal SCFA concentration on SCFA absorption and the effect modification of the group were tested.
To examine our secondary objective, dietary intakes and microbial profile were used as predictors for faecal SCFA concentration using mixed-models analysis. For microbial relative abundance, dietary intakes were used as predictors. A combination of dietary intakes and microbial abundance were used to test for a better model, and presented only if the model was still significant (i.e., firmicutes and CHO predicted faecal SCFA better than firmicutes alone). The correlation coefficients between the SCFA, dietary intake and bacterial relative abundance were calculated by a Pearson’s r test (IBM SPSS Statistics version 21) using the average value of the two study periods. The P-value of the correlation was calculated by SAS Proc-Mixed to control for the repeated measurements. In all the correlation tests, inclusion of the effect modification of the group tested our hypothesis, because a significant interaction provides evidence for a different pattern of effect between the LN and the OWO groups. Only significant interactions are presented. Differences with P-values ≤0.05 (2-tailed) were considered to be statistically significant. The results are expressed as means ±SEM.
To examine whether changes in energy, fat, carbohydrate and fibre intakes were associated with similar changes in faecal SCFA concentrations in LN vs OWO participants, we compared fecal SCFA concentrations after the diet period with the lower intake in each subject with fecal SCFA after the diet period with the higher intake.
Sequencing Analysis
The weighted UniFrac distances were calculated in QIIME(21) by using a phylogenetic tree of OTU sequences built with FastTree(22) and based on an OTU sequence alignment with MUSCLE.(23) The QIIME pipeline was also used to calculate Shannon’s diversity index (logarithms with base 2). A modified version of the ALDEx R package(24) was used to compare relative abundances of ribotypes at different taxonomic levels between the LN and OWO groups. Microbiome data are best represented as compositional distributions (SI Fig. 1) and are thus not independent of each other.(24,25) The correct interpretation of data of these types is to state that the relative abundance of A is greater or less than B.(26) Therefore to compare relative abundances we used the ALDEx R package that estimates the technical variation inherent in high-throughput sequencing by Monte-Carlo sampling from a Dirichlet distribution.(27) The Monte-Carlo replicates are transformed using the centred log-ratio transformation(26) that takes the base2 logarithm of the Monte-Carlo estimates of organism abundances in each sample divided by the per-sample geometric mean organism abundance. This transformation has several desirable properties that do not exist in proportional data; notably subcomposition coherence and linear sample independence. Data transformed in this way permit the use of standard statistical tests to determine significance.(26) We used the median centred log-ratio value output by ALDEx2 to conduct statistical significance for these comparisons and for the F:B ratio using a SAS Proc-Mixed with p<0.05.(28)
RESULTS
Participant characteristics (Table 1)
Table 1.
Characteristics of the study participants
| LN (n = 11) | OWO (n = 11) | P-value | |
|---|---|---|---|
| BMI, kg/m2 | 22.6 ± 0.6 | 30.1 ± 0.8 | <0.0001 |
| Gender, Male: Female | 6:5 | 6:5 | 0.669 |
| Age, y | 35.8 ± 4.2 | 42.5 ± 3.9 | 0.262 |
| Ethnicity, C:O | 7:4 | 6:5 | 0.665 |
| Activity Level, METs | 27.0 ± 8.1 | 29.7 ± 10.4 | 0.838 |
| Faecal consistency1 | 3.3 ± 0.3 | 3.0 ± 0.2 | 0.317 |
| Bowel Movements per day1 | 1.3 ± 0.2 | 1.5 ± 0.2 | 0.425 |
| Breath methane, ppm | 24.1 ± 10.5 | 1.3 ± 7.5 | 0.043 |
| Breath Hydrogen, ppm | 3.9 ± 1.7 | 6.5 ± 1.2 | 0.129 |
Abbreviations: BMI, body mass index; C, Caucasian; O, others; METs, Metabolic Equivalents. Values are means ± SEM.
calculated from Bowel Habit Diary (see Methods). Independent t-test, test was used to calculate differences between the groups, χ2 was used for categorical variables. SAS Proc-Mixed was used to control for repeated measurements for faecal consistency, breath methane and breath hydrogen.
OWO subjects had significantly higher BMI (P<0.0001) and breath methane (P=0.043) than LN, but there was no significant difference between the two groups in age (P=0.262), gender (χ2=0.669), ethnicity (χ2=0.665), physical activity (P=0.838), faecal consistency (P=0.317), frequency of evacuation (p=0.425), and breath hydrogen (P=0.129).
Fecal SCFA and Microbiota
The OWO group had significantly higher total faecal acetate, butyrate and total SCFA concentrations compared to the LN group after adjusting for age (Table 2). Without age adjustment, the differences were not significant (Table 2). No significant differences were seen in iso-butyrate, iso-valerate and valerate concentrations between the groups. Mean SCFA absorption from the dialysis bags was similar between the OWO and the LN groups (Table 2). Among all bacterial phyla, only the relative abundance of the Firmicutes phylum was significantly different between the LN and the OWO group with the aggregated relative abundance of Firmicutes being increased 2.8 fold in OWO compared to LN (P=0.008) (Table 2). There was a 5 fold difference in the ratio between the relative abundances of Firmicutes and Bacteroidetes (P=0.023, or P=0.0098 when adjusted for age)(table 2).
Table 2.
Differences in faecal SCFA concentrations, SCFA absorption, faecal bacterial composition and faecal pH in LN and OWO groups (n=22)
| LN | OW | P-value | P-value1 | P-value2 | |
|---|---|---|---|---|---|
| SCFA absorption, % | 24.7 ± 1.2 | 24.4 ± 0.8 | 0.787 | 0.639 | 0.745 |
| tSCFA, mmol/kg wet wt | 64.1 ± 10.4 | 81.3 ± 7.4 | 0.114 | 0.023 | 0.011 |
| AC, mmol/kg wet wt | 35.1 ± 6.1 | 45.3 ± 4.3 | 0.109 | 0.048 | 0.016 |
| PR, mmol/kg wet wt | 12.7 ± 2.8 | 15.4 ± 2.0 | 0.350 | 0.103 | 0.083 |
| BU, mmol/kg wet wt | 11.1 ± 2.4 | 15.4 ± 1.7 | 0.089 | 0.017 | 0.015 |
| Iso-BU, mmol/kg wet wt | 1.5 ± 0.5 | 1.4 ± 0.3 | 0.833 | 0.946 | 0.970 |
| Iso-VA, mmol/kg wet wt | 2.1 ± 0.7 | 2.0 ± 0.5 | 0.889 | 0.909 | 0.945 |
| VA, mmol/kg wet wt | 1.6 ± 0.5 | 1.9 ± 0.4 | 0.627 | 0.283 | 0.295 |
| Faecal pH | 6.7 ± 0.1 | 6.4 ± 0.1 | 0.037 | 0.011 | 0.005 |
| Firmicutes, % | 69.5 ± 5.8 | 83.1 ± 4.1 | 0.008 | 0.0007 | 0.001 |
| Bacteroidetes, % | 19.4 ± 6.1 | 6.4 ± 4.3 | 0.335 | 0.329 | 0.335 |
| F:B Ratio3 | 6.8 ± 1.0 | 34.3 ± 1.6 | 0.023 | 0.010 | 0.010 |
Abbreviations: AC, Acetate; BU, Butyrate; F:B, Firmicutes to Bacteriodetes; PR, Propionate; tSCFA, total short chain fatty acids; VA, Valerate. Values are means ± SEM.
adjusted for age;
adjusted for age and available carbohydrate (g/day). All calculated by SAS Proc-Mixed to account for repeated measurements.
The F:B ratio is expressed as a base 2 logarithm derived from the median centre log-ratio transformed values of each sample.
Dietary intake
The average consumption of all dietary nutrients did not differ significantly between the groups, except that fat intake (g/d, but not % energy) was higher in the OWO group (P=0.014). Available carbohydrate intake was similar between the groups when expressed as g/d, but when expressed as % energy was higher in the LN group (P=0.028) (Supplemental Table 1).
Group interactions
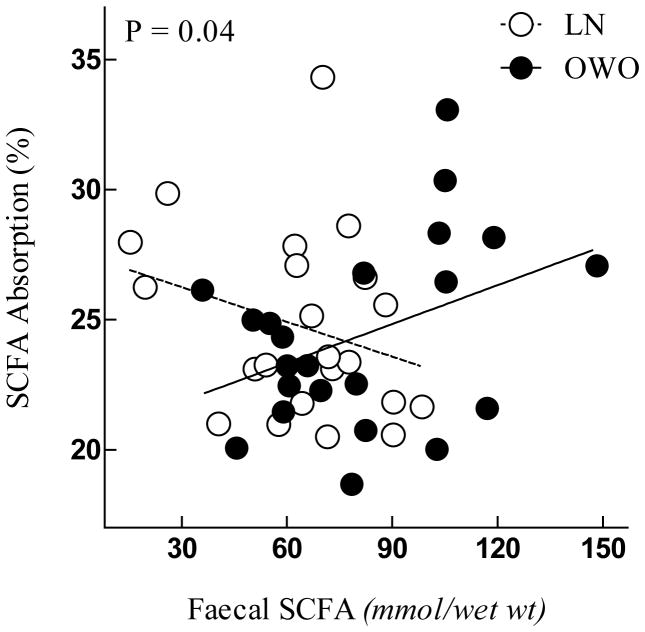
The model for SCFA absorption showed a significant interaction between faecal SCFA concentration and group (P=0.04), indicating a general trend for OWO individuals to absorb more SCFA from the dialysis bag when their faecal SCFA concentrations are higher (Figure 1).
Figure 1.
Faecal SCFA concentration by Group interaction with SCFA absorption.
P value for the interaction calculated by SAS Proc-Mixed model to account for repeated measurements. Each point represents one visit.
The mean changes within participants in faecal acetate concentration associated with increases in dietary intakes (energy, dietary fibre and fat) differed significantly between LN and OWO subjects. In the OWO group the diet period with the higher energy intake (2430±123 vs 2030±82kcal/d) was associated with higher faecal acetate (50.8±5.5 vs 39.8±4.7mmol/kg) while in the LN group the diet period with the higher energy intake (2120±117 vs 1870±126kcal/d) was associated with lower faecal acetate (33.6±3.8 vs 36.5±33.6mmol/kg; diet×group interaction, p=0.009) (Figure 2A). Similarly, in the OWO group the diet period with the higher dietary fibre intake (23.7±1.7 vs 19.7±1.9g/d) was associated with higher faecal acetate (48.5±5.3 vs 42.1±5.0mmol/kg) while in the LN group the diet period with the higher dietary fibre intake (25.4±3.4 vs 22.1±3.5g/d) was associated with lower faecal acetate (32.1±4.0 vs 38.1±4.0mmol/kg; diet×group interaction, P=0.03) (Figure 2B). Finally, in the OWO group the diet period with the higher fat intake (106.2±5.0 vs 84.5±4.3g/d) was associated with higher faecal acetate (49.7±5,7 vs 41.0±4.8mmol/kg) while in the LN group the diet period with the higher fat intake (82.6±5.8 vs 69.7±6.2g/d) was associated with the lower faecal acetate (32.2±4.1 vs 37.9±3.9mmol/kg; diet×group interaction, P=0.03) (Figure 2C).
Figure 2.
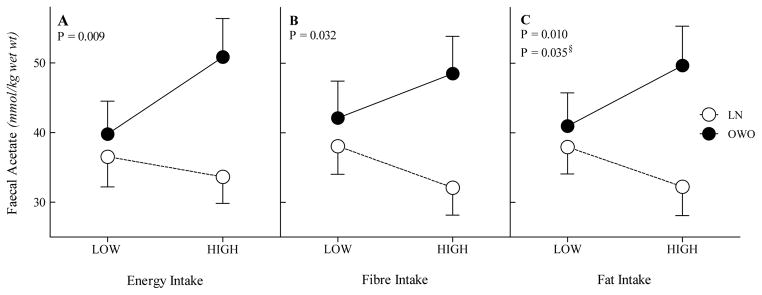
Group and dietary components interactions with faecal acetate concentration; Group by Energy intake interaction with faecal acetate concentration (A), Group by fibre intake with faecal acetate concentration (B), Group by fat intake interaction with faecal acetate concentration (C). Low, visit with lower intake; High, visit with higher intake. §adjusted for energy.
Correlations between Faecal SCFA, microbiota and dietary intakes
Significant predictors for faecal SCFA concentrations and for Firmicutes abundance are presented in table 3. Significant predictors for faecal SCFA were protein (g/day) (r=0.408, P=0.004) and the Firmicutes relative abundance (r=0.507, P=0.044) and the result was more significant after adjusting for available carbohydrate (r=0.615, P=0.005). Significant predictors for the Firmicutes relative abundance were available carbohydrate (r= −0.185, P=0.008), Fibre (r= −0.477, P=0.015) and BMI (r= 0.502, P=0.017).
Table 3.
Pearson correlation coefficients among faecal SCFA (mmol/kg wet wt), microbial composition, F:B ratio and their predictors (n=22)
| Total SCFA | Acetate | Propionate | Butyrate | Firmicutes | F:B | ||
|---|---|---|---|---|---|---|---|
| Calories, Kcal | Correlation | 0.370 | 0.416 | 0.333 | 0.251 | 0.293 | 0.128 |
| P value | 0.053 | 0.028 | 0.079 | ns | 0.089 | Ns | |
| Fat, g/d | Correlation | 0.139 | 0.147 | 0.183 | 0.139 | 0.567 | 0.259 |
| P value | ns | ns | ns | ns | ns | Ns | |
| Available CHO, g/d | Correlation | 0.306 | 0.445 | 0.141 | 0.131 | −0.185 | −0.262 |
| P value | ns | 0.066 | ns | ns | 0.008 | Ns | |
| Fibres, g/d | Correlation | −0.295 | −0.297 | −0.243 | −0.249 | −0.477 | −0.356 |
| P value | ns | ns | 0.088 | ns | 0.015 | Ns | |
| Protein, g/d | Correlation | 0.408 | 0.300 | 0.511 | 0.310 | 0.370 | 0.335 |
| P value | 0.004 | 0.023 | 0.002 | 0.015 | ns | Ns | |
| Age | Correlation | −0.383 | −0.237 | −0.437 | −0.370 | −0.293 | −0.168 |
| P value | 0.079 | ns | 0.042 | 0.091 | ns | Ns | |
| BMI | Correlation | 0.292 | 0.288 | 0.182 | 0.347 | 0.502 | 0.510 |
| P value | ns | ns | ns | ns | 0.017 | 0.015 | |
| Firmicutes | Correlation | 0.507 | 0.471 | 0.454 | 0.468 | ------ | ------ |
| P value | 0.044 | 0.057 | 0.065 | 0.053 | ------ | ------ | |
| Firmicutes1 | Correlation | 0.615 | 0.646 | 0.501 | 0.511 | ------ | ------ |
| P value | 0.005 | 0.002 | 0.020 | 0.032 | ------ | ------ | |
| F:B Ratio | Correlation | 0.244 | 0.105 | 0.277 | 0.331 | ------ | ------ |
| P value | ns | ns | ns | ns | ------ | ------ |
Abbreviations: AC, Acetate; BMI, body mass index; BU, Butyrate; CHO, carbohydrate; F:B, Firmicutes to Bacteriodetes; ns, non significant (P > 0.1); PR, Propionate; tSCFA, total short chain fatty acids. Correlations are Pearson’s Correlations Coefficient; P Values calculated by SAS Proc-Mixed to account for repeated measurements;
adjusted for available carbohydrate (g/day).
DISCUSSION
Our findings suggest that the OWO have higher faecal SCFA than LN, with no difference in rectal SCFA absorption. Therefore, the higher faecal SCFA concentration in the OWO group is unlikely to be due to lower colonic SCFA absorption. The higher SCFA concentration is also not likely to be due to different dietary intake. Although fat intake was higher in the OWO individuals compared to the LN individuals, no significant correlations were observed between fat intake and faecal SCFA concentration. We found a higher relative abundance of Firmicutes and F:B ratio in the OWO group. These findings, together with the positive correlation between Firmicutes and faecal SCFA, are consistent with the hypothesis that OWO have higher faecal SCFA concentrations due to the obese microbial profile which is more efficient in fermenting substrates than the lean microbial profile.
Consistent with other studies,(6,7,29) the OWO individuals in the present study had a higher faecal SCFA concentration compared to their LN counterparts after adjusting for age. We adjusted for age because a larger study from our lab revealed a significant negative correlation between age and fecal SCFAs (Fernandes et al. submitted manuscript). The current results are consistent with this in demonstrating a negative trend between the individual and total SCFAs and age (p<0.05 for propionate and valerate, p<0.09 for total SCFA and butyrate). Moreover, a decrease in faecal SCFA with age was also found in mice,(30) dogs,(31) as well as in human serum.(32)
Faecal SCFA are typically measured to reflect the colonic production of the SCFA; however, they are also a surrogate measurement of the SCFA absorption from the colon. The similar capability of the LN and OWO groups to absorb SCFA in this study suggests that reduced SCFA absorption is not the reason why OWO subjects have a higher faecal SCFA concentration than LN individuals. This conclusion was supported by the finding that there was no association between faecal SCFA and SCFA absorption within the whole study group. In our study, the total and individual SCFA were absorbed in proportion to their concentration inside the dialysis bag (data not shown). The concentration-dependent absorption points to passive diffusion as a predominant mechanism, as has been previously suggested.(33,34) However, since the pH of the lumen is approximately 5.6 to 6.6(ref #(35)) (6.6 in our participants), more than 95% of the SCFAs are in the anionic form,(1) and anionic SCFA cannot be absorbed via simple passive diffusion but rather by a facilitated passive diffusion. The Monocarboxylate Transporters (MCT1) and the sodium-coupled MCT (SMCT), were suggested to facilitate the transport of SCFA from the lumen into the colonocytes and blood.(36,37) A possible explanation for the group × faecal SCFA interaction seen for SCFA absorption could be the ability of the colonic SCFAs to up-regulate the MCT gene expression and function.(11,38,39) Within the OWO group, individuals with a higher colonic SCFA concentration had more SCFA molecules to up-regulate more transporters in their rectum, and consequently had higher SCFA absorption than OWO individuals with lower faecal SCFA concentration. While within the LN group, the SCFA concentration may not be enough to up-regulate as many transporters, and therefore this association did not exist. Vogt and Wolever(9) concluded from rectal infusion studies using a SCFA concentration of 100mmol/L that a mechanism other than simple diffusion might play a role in the absorption of colonic SCFA. This finding is comparable with our suggestion that the higher range of colonic SCFA concentration may result in a more efficient absorption.
A weakness of our study is that we measured SCFA absorption from the rectum which may not represent that in the proximal colon where most SCFA are absorbed. However, the rectum is the continuation of the colon and was previously shown to contain the same SCFA transporters as the rest of the colon.(37)Moreover, we have shown that rectal infusion of SCFA increases serum SCFA concentrations(40) Thus, although differences in the transport rate between the cecum and the rectum might occur, there is a biological plausibility to extend our findings to the rest of the colon. Another weakness is that the validity of our results depends on the assumption that the disappearance of the SCFA from the dialysis bag represents rectal absorption, which could be criticized. However, studies that validated the technique to measure rectal mucosal transport in rats and humans, although not proven for SCFA, showed that only 5–9% of the labeled sodium that disappeared from the bag was recovered from the human luman, they also showed that at least 90% of the bag’s solution was absorbed.(17,18)
Dietary macronutrients may affect SCFA concentration either directly, as substrates for fermentation or indirectly, through their influence on colonic microbiota and thereby on the SCFA concentration.(41)Consistent with another study,(48) we found protein intake to be positively associated with faecal SCFAs. However, since protein intake was similar in the LN and OWO groups, it does not explain the higher SCFA in the OWO group. The only difference in dietary intake between groups was that fat intake (g/d) was higher in the OWO group. Fat is not a substrate for bacterial fermentation although a small amount of dietary fat can reach the colon(42) and animal studies suggest that high fat diets are associated with alteration in microflora, though not necessarily with altered SCFA concentration.(30,43–45) However, we found no correlations between fat intake and bacterial phyla or between fat intake and SCFA concentration suggesting that fat did not determine the higher faecal SCFA concentration in the OWO compared to the LN group. The findings of Teixeira et al. were consistent with ours, showing no correlations between fat intake and faecal SCFA in lean and obese women.(7) Fava et al. showed in overweight individuals that a high monounsaturated fat diets did not change faecal SCFA, but that a high saturated fatty acid diet increased SCFA concentration.(46) By contrast, Brinkworth et al. found that an energy-restricted high-fat diet decreased the faecal SCFA concentrations in overweight participants compared to an energy-restricted low fat diet.(47) However, in the latter study carbohydrate and fibre intakes were extremely low in the high fat diet which may make the results difficult to compare.
Our study design, which included two visits for each individual, allowed us to investigate how changes within individuals’ diets affect their faecal SCFA concentration. Interestingly, an increase in individual’s energy, fibre or fat intakes from visit to visit was correlated with an increase in acetate within OWO individuals, but a decrease within LN individuals. These interactions suggest that LN and OWO individuals respond to changes in habitual diets differently, possibly due to differences in colonic bacteria between the groups. Different bacteria employ different metabolic pathways which may result in altered colonic SCFA.(48) However, there was no correlation between the bacterial changes and the SCFA changes (data not shown). Jumpertz et al. also demonstrated that lean and obese individuals have differences in faecal energy losses when increasing their energy intake.(49) The higher relative abundance of the Firmicutes was observed to be due largely to a higher relative abundance of the aggregated abundance of members of the family Erysipelotrichaceae (data not shown). Notably, members of this family are found in increased abundance in mice fed a high fat diet.(50) The higher relative Firmicutes abundance and the higher F:B ratio we observed in the OWO group compared to LN, together with the positive correlation between Firmicutes and faecal SCFA (CHO-adjusted), and with the similar dietary intakes between the groups support the early hypothesis that the obese-microbiome is more efficient in harvesting energy (SCFA) from the diet.(4,5,30,51,52) We adjusted for carbohydrate because carbohydrates had strong negative association with Firmicutes. Though dietary fibre also had a strong association with Firmicutes, fibre was not a significant predictor when added to this model.
Gut transit time can influence faecal SCFA concentration and colonic microbiota.(10,53–55) A reasonably accurate qualitative assessment of gut transit time is faecal consistency(56), and since faecal consistency was similar between the groups, we assume that differences in gut transit time did not contribute to the differences in faecal SCFA concentrations and colonic bacteria between the groups.
It is of note that measures of fecal SCFA and microbiota, represent the status in the rectum and not other regions of the colon where most SCFA are produced. Thus, our results need to be interpreted with caution. It is possible that fecal SCFA and microbiota may differ in OWO subjects due to factors we did not measure such as differences in the small intestinal absorption of nutrients, or in the rate of fermentation in the proximal colon resulting in different substrates reaching the distal colon, or other unknown causes.
We conclude that the higher faecal SCFA concentration seen in OWO compared to LN individuals is unlikely a result of differences in SCFA absorption or dietary intakes. The fact that increases in energy, dietary fibre and fat intakes within individuals were associated with an increased faecal acetate in OWO but a decrease LN subjects may be explained by higher Firmicutes abundance and higher F:B ratio in OWO compared LN subjects. These differences, together with the positive correlation between Firmicutes abundance and faecal SCFA concentration, are consistent with the hypothesis that OWO subjects produce more colonic SCFA than LN due to differences in colonic microbiota. However, larger studies looking at the effect of diet and colonic flora on faecal SCFA in LN and OWO participants are warranted.
Supplementary Material
Acknowledgments
We are thankful to Jean M Macklaim for support in statistical analysis of microbial data. Supported by grant no.OOP-64648 from the Canadian Institutes for Health Research (CIHR), Institute of Nutrition, Metabolism and Diabetes.
This study was funded by a Canadian Institutes of Health Research grant #486906
Abbreviations Used
- BMI
body mass index
- F:B
Firmicutes:Bacteroidetes
- LN
lean
- OWO
overweight and obese
- SCFA
short chain fatty acid
Footnotes
S.R. Rozenbloom, J. Fernandes, G. B. Gloor, and T.M.S. Wolever have no conflicts of interest.
Conflict of interest: The authors declared no conflict of interest.
Supplementary information is available at International Journal of Obesity’s website.
References
- 1.Cummings JH. Short chain fatty acids in the human colon. Gut. 1981;22:763–779. doi: 10.1136/gut.22.9.763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Topping DL, Clifton PM. Short-chain fatty acids and human colonic function: Roles of resistant starch and nonstarch polysaccharides. Physiol Rev. 2001;81:1031–1064. doi: 10.1152/physrev.2001.81.3.1031. [DOI] [PubMed] [Google Scholar]
- 3.Wong JMW, De Souza R, Kendall CWC, Emam A, Jenkins DJA. Colonic health: Fermentation and short chain fatty acids. J Clin Gastroenterol. 2006;40:235–243. doi: 10.1097/00004836-200603000-00015. [DOI] [PubMed] [Google Scholar]
- 4.Ley RE, Bäckhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci U S A. 2005;102:11070–11075. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 6.Schwiertz A, Taras D, Schäfer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity. 2010;18:190–195. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 7.Teixeira TFS, Grze kowiak L, Franceschini SCC, Bressan J, Ferreira CLLF, Peluzio MCG. Higher level of faecal SCFA in women correlates with metabolic syndrome risk factors. Br J Nutr. 2013;109:914–919. doi: 10.1017/S0007114512002723. [DOI] [PubMed] [Google Scholar]
- 8.Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obes. 2008;32:1720–1724. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 9.Vogt JA, Wolever TMS. Fecal acetate is inversely related to acetate absorption from the human rectum and distal colon. J Nutr. 2003;133:3145–3148. doi: 10.1093/jn/133.10.3145. [DOI] [PubMed] [Google Scholar]
- 10.El Oufir L, Barry JL, Flourié B, Cherbut C, Cloarec D, Bornet F, et al. Relationships between transit time in man and in vitro fermentation of dietary fiber by fecal bacteria. Eur J Clin Nutr. 2000;54:603–609. doi: 10.1038/sj.ejcn.1600687. [DOI] [PubMed] [Google Scholar]
- 11.Haenen D, Zhang J, da Silva CS, Bosch G, van der Meer IM, van Arkel J, et al. A diet high in resistant starch modulates microbiota composition, SCFA concentrations, and gene expression in pig intestine1–3. J Nutr. 2013;143:274–283. doi: 10.3945/jn.112.169672. [DOI] [PubMed] [Google Scholar]
- 12.Cummings JH, Beatty ER, Kingman SM, Bingham SA, Englyst HN. Digestion and physiological properties of resistant starch in the human large bowel. Br J Nutr. 1996;75:733–747. doi: 10.1079/bjn19960177. [DOI] [PubMed] [Google Scholar]
- 13.Duncan SH, Belenguer A, Holtrop G, Johnstone AM, Flint HJ, Lobley GE. Reduced dietary intake of carbohydrates by obese subjects results in decreased concentrations of butyrate and butyrate-producing bacteria in feces. Appl Environ Microbiol. 2007;73:1073–1078. doi: 10.1128/AEM.02340-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bourriaud C, Robins RJ, Martin L, Kozlowski F, Tenailleau E, Cherbut C, et al. Lactate is mainly fermented to butyrate by human intestinal microfloras but inter-individual variation is evident. J Appl Microbiol. 2005;99:201–212. doi: 10.1111/j.1365-2672.2005.02605.x. [DOI] [PubMed] [Google Scholar]
- 15.Ainsworth BE, Haskell WL, Leon AS, Jacobs DR, Jr, Montoye HJ, Sallis JF, et al. Compendium of physical activities: Classification of energy costs of human physical activities. Med Sci Sports Exerc. 1993;25:71–80. doi: 10.1249/00005768-199301000-00011. [DOI] [PubMed] [Google Scholar]
- 16.Modifiable Activity Questionnaire. Med Sci Sports Exerc. 1997;29:S73–S78. doi: 10.1097/00005768-199710000-00011. [DOI] [PubMed] [Google Scholar]
- 17.Edmonds CJ. Absorption of sodium and water by human rectum measured by a dialysis method. Gut. 1971;12:356–362. doi: 10.1136/gut.12.5.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McNeil NI, Cummings JH, James WPT. Short chain fatty acid absorption by the human large intestine. Gut. 1978;19:819–822. doi: 10.1136/gut.19.9.819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernandes J, Wang A, Su W, Rozenbloom SR, Taibi A, Comelli EM, et al. Age, dietary fiber, breath methane, and fecal short chain Fatty acids are interrelated in archaea-positive humans. J Nutr. 2013;143:1269–75. doi: 10.3945/jn.112.170894. [DOI] [PubMed] [Google Scholar]
- 20.Petrof EO, Gloor GB, Vanner SJ, Weese SJ, Carter D, Daigneault MC, et al. Stool substitute transplant therapy for the eradication of Clostridium difficile infection: ‘RePOOPulating’ the gut. Microbiome. 2013;1:3. doi: 10.1186/2049-2618-1-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bittinger K, Bushman FD, Caporaso JG, Costello EK, Fierer N, Goodrich JK, et al. QIIME allows analysis of high-throughput community sequencing data. Nature Methods. 2010;7:335. doi: 10.1038/nmeth.f.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Price MN, Dehal PS, Arkin AP. FastTree: computing large minimum evolution trees with profiles instead of a distance matrix. Molecular Biology & Evolution. 2009;26:1641–1650. doi: 10.1093/molbev/msp077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Edgar RC. MUSCLE: a multiple sequence alignment method with reduced time and space complexity. BMC Bioinformatics. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fernandes AD, Macklaim JM, Linn TG, Reid G, Gloor GB. ANOVA-Like Differential Expression (ALDEx) Analysis for Mixed Population RNA-Seq. Plos One. 2013;8:e67019. doi: 10.1371/journal.pone.0067019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedman J, Alm EJ. Inferring Correlation Networks from Genomic Survey Data. Plos Computational Biology. 2012;8:e1002687. doi: 10.1371/journal.pcbi.1002687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aitchison J. The statistical analysis of compositional data. New York: Chapman and Hall; 1986. [Google Scholar]
- 27.Jaynes ET. Probability theory : the logic of science. New York, NY: Cambridge University Press; 2003. [Google Scholar]
- 28.Strimmer K. fdrtool: a versatile R package for estimating local and tail area-based false discovery rates. Bioinformatics. 2008;24:1461–1462. doi: 10.1093/bioinformatics/btn209. [DOI] [PubMed] [Google Scholar]
- 29.Ppatil D, Pdhotre D, Gchavan S, Sultan A, Jain DS, Lanjekar VB, et al. Molecular analysis of gut microbiota in obesity among Indian individuals. J Biosci. 2012;37:647–657. doi: 10.1007/s12038-012-9244-0. [DOI] [PubMed] [Google Scholar]
- 30.Murphy EF, Cotter PD, Healy S, Marques TM, O’Sullivan O, Fouhy F, et al. Composition and energy harvesting capacity of the gut microbiota: Relationship to diet, obesity and time in mouse models. Gut. 2010;59:1635–1642. doi: 10.1136/gut.2010.215665. [DOI] [PubMed] [Google Scholar]
- 31.Gomes MO, Beraldo MC, Putarov TC, Brunetto MA, Zaine L, Glória MB, et al. Old beagle dogs have lower faecal concentrations of some fermentation products and lower peripheral lymphocyte counts than young adult beagles. Br J Nutr. 2011;106 (Suppl 1):S187–190. doi: 10.1017/S0007114511002960. [DOI] [PubMed] [Google Scholar]
- 32.Wolever TMS, Fernandes J, Rao AV. Serum acetate:propionate ratio is related to serum cholesterol in men but not women. J Nutr. 1996;126:2790–2797. doi: 10.1093/jn/126.11.2790. [DOI] [PubMed] [Google Scholar]
- 33.Stein J, Zores M, Schröder O. Short-chain fatty acid (SCFA) uptake into Caco-2 cells, by a pH- dependent and carrier mediated transport mechanism. Eur J Nutr. 2000;39:121–125. doi: 10.1007/s003940070028. [DOI] [PubMed] [Google Scholar]
- 34.Fleming SE, Choi SY, Fitch MD. Absorption of short-chain fatty acids from the rat cecum in vivo. J Nutr. 1991;121:1787–1797. doi: 10.1093/jn/121.11.1787. [DOI] [PubMed] [Google Scholar]
- 35.Cummings JH, Pomare EW, Branch WJ, Naylor CPE, Macfarlane GT. Short chain fatty acids in human large intestine, portal, hepatic and venous blood. Gut. 1987;28:1221–1227. doi: 10.1136/gut.28.10.1221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gill RK, Saksena S, Alrefai WA, Sarwar Z, Goldstein JL, Carroll RE, et al. Expression and membrane localization of MCT isoforms along the length of the human intestine. American Journal of Physiology - Cell Physiology. 2005;289:C846–C852. doi: 10.1152/ajpcell.00112.2005. [DOI] [PubMed] [Google Scholar]
- 37.Iwanaga T, Takebe K, Kato I, Karaki S, Kuwahara A. Cellular expression of monocarboxylate transporters (MCT) in the digestive tract of the mouse, rat, and humans, with special reference to slc5a8. Biomed Res. 2006;27:243–254. doi: 10.2220/biomedres.27.243. [DOI] [PubMed] [Google Scholar]
- 38.Borthakur A, Priyamvada S, Kumar A, Natarajan AA, Gill RK, Alrefai WA, et al. A novel nutrient sensing mechanism underlies substrate-induced regulation of monocarboxylate transporter-1. American Journal of Physiology - Gastrointestinal and Liver Physiology. 2012;303:G1126–G1133. doi: 10.1152/ajpgi.00308.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou J, Hegsted M, McCutcheon KL, Keenan MJ, Xi A, Raggio AM, et al. Peptide YY and proglucagon mRNA expression patterns and regulation in the gut. Obesity. 2006;14:683–689. doi: 10.1038/oby.2006.77. [DOI] [PubMed] [Google Scholar]
- 40.Freeland KR, Wolever TMS. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br J Nutr. 2010;103:460–466. doi: 10.1017/S0007114509991863. [DOI] [PubMed] [Google Scholar]
- 41.De Filippo C, Cavalieri D, Di Paola M, Ramazzotti M, Poullet JB, Massart S, et al. Impact of diet in shaping gut microbiota revealed by a comparative study in children from Europe and rural Africa. Proc Natl Acad Sci U S A. 2010;107:14691–14696. doi: 10.1073/pnas.1005963107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gabert L, Vors C, Louche-Pélissier C, Sauvinet V, Lambert-Porcheron S, Drai J, et al. 13C tracer recovery in human stools after digestion of a fat-rich meal labelled with [1,1,1-13C3]tripalmitin and [1,1,1-13C3]triolein. Rapid Communications in Mass Spectrometry. 2011;25:2697–2703. doi: 10.1002/rcm.5067. [DOI] [PubMed] [Google Scholar]
- 43.Hildebrandt MA, Hoffmann C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen Y, et al. High-Fat Diet Determines the Composition of the Murine Gut Microbiome Independently of Obesity. Gastroenterology. 2009;137:1716–1724. e2. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim K, Gu W, Lee I, Joh E, Kim D. High Fat Diet-Induced Gut Microbiota Exacerbates Inflammation and Obesity in Mice via the TLR4 Signaling Pathway. PLoS ONE. 2012:7. doi: 10.1371/journal.pone.0047713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mujico JR, Baccan GC, Gheorghe A, Díaz LE, Marcos A. Changes in gut microbiota due to supplemented fatty acids in diet-induced obese mice. Br J Nutr. 2013;110:711–720. doi: 10.1017/S0007114512005612. [DOI] [PubMed] [Google Scholar]
- 46.Fava F, Gitau R, Griffin BA, Gibson GR, Tuohy KM, Lovegrove JA. The type and quantity of dietary fat and carbohydrate alter faecal microbiome and short-chain fatty acid excretion in a metabolic syndrome ‘at-risk’ population. Int J Obes. 2013;37:216–223. doi: 10.1038/ijo.2012.33. [DOI] [PubMed] [Google Scholar]
- 47.Brinkworth GD, Noakes M, Clifton PM, Bird AR. Comparative effects of very low-carbohydrate, high-fat and high-carbohydrate, low-fat weight-loss diets on bowel habit and faecal short-chain fatty acids and bacterial populations. Br J Nutr. 2009;101:1493–1502. doi: 10.1017/S0007114508094658. [DOI] [PubMed] [Google Scholar]
- 48.Mahowald MA, Rey FE, Seedorf H, Turnbaugh PJ, Fulton RS, Wollam A, et al. Characterizing a model human gut microbiota composed of members of its two dominant bacterial phyla. Proc Natl Acad Sci U S A. 2009;106:5859–5864. doi: 10.1073/pnas.0901529106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jumpertz R, Le DS, Turnbaugh PJ, Trinidad C, Bogardus C, Gordon JI, et al. Energy-balance studies reveal associations between gut microbes, caloric load, and nutrient absorption in humans. Am J Clin Nutr. 2011;94:58–65. doi: 10.3945/ajcn.110.010132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang C, Zhang M, Wang S, Han R, Cao Y, Hua W, et al. Interactions between gut microbiota, host genetics and diet relevant to development of metabolic syndromes in mice. ISME Journal. 2010;4:232–241. doi: 10.1038/ismej.2009.112. [DOI] [PubMed] [Google Scholar]
- 51.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–1023. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 52.Zhang H, DiBaise JK, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci U S A. 2009;106:2365–2370. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lewis SJ, Heaton KW. Increasing butyrate concentration in the distal colon by accelerating intestinal transit. Gut. 1997;41:245–251. doi: 10.1136/gut.41.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.El Oufir L, Flourié B, Bruley Des Varannes S, Barry JL, Cloarec D, Bornet F, et al. Relations between transit time, fermentation products, and hydrogen consuming flora in healthy humans. Gut. 1996;38:870–877. doi: 10.1136/gut.38.6.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stephen AM, Wiggins HS, Cummings JH. Effect of changing transit time on colonic microbial metabolism in man. Gut. 1987;28:601–609. doi: 10.1136/gut.28.5.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Saad RJ, Rao SSC, Koch KL, Kuo B, Parkman HP, McCallum RW, et al. Do stool form and frequency correlate with whole-gut and colonic transit results from a multicenter study in constipated individuals and healthy controls. Am J Gastroenterol. 2010;105:403–411. doi: 10.1038/ajg.2009.612. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.