Abstract
GS-9219, a novel prodrug of the nucleotide analogue 9-(2-phosphonylmethoxyethyl) guanine (PMEG) has significant activity as monotherapy in dogs with non-Hodgkin's lymphoma. Phase I trials have been initiated in humans based on the encouraging activity observed in canine lymphoma. Two new analogues of GS-9219 (GS-343074 and GS-424044) were recently produced for evaluation as potential novel antineoplastic agents against solid tumours. As a preclinical step, effect of GS-343074 and GS-424044 were evaluated against ten canine cancer cell lines for antiproliferative effect. Both analogues displayed antiproliferative activity against multiple canine cancer cell lines, although GS-343074 was more potent and of broader spectrum compared to GS-424044. Flow cytometric analysis of cells that experienced growth inhibition support apoptotic death as a mechanism of action for both analogues. On the basis of in vitro results described here, GS-343074 and GS-424044 show promise as novel anticancer agents in canine cancer.
Keywords: chemotherapy, comparative oncology, in vitro models, oncology, small animal
Introduction
The selection of drugs for treating specific types of cancer is predominantly based on the results of previous clinical trials and is often empirical in nature. Chemotherapy is used in a number of clinical settings, including primary induction treatment for advanced disease or cancers with no other effective therapy, neoadjuvant treatment for patients with localized disease in which surgery and/or radiation therapy is inadequate, adjuvant treatment to local therapy and direct instillation into sanctuary sites.1 In veterinary oncology, chemotherapy is employed following surgery and/or radiation therapy for diseases considered at risk for metastasis, such as canine osteosarcoma, hemangiosarcoma and high grade sarcomas and carcinomas. In some cases, chemotherapy has been shown to prolong survival time over local therapy alone, such as with osteosarcoma.2–6
In general, the objective response rate of most solid tumours to chemotherapy alone is low, due to the fact that most measurable tumours follow Gompertzian growth and cytotoxic drugs follow logarithmic cell kill kinetics (fractional cell kill).1,7,8 Therefore, new strategies and drug development pathways are needed to investigate drugs with improved efficacy and selectivity for neoplastic cells, as well as an acceptable toxicity profile. Preclinical aspects of drug discovery typically involve the discovery or synthetic development of new agents that are subject to anticancer screening in vitro.9 Active compounds are then selected for further testing depending on a demonstration of cellular cytotoxicity or cytostasis, disease specificity and potencyamongotheraspectsdependingonthegoal.
A novel prodrug, GS-9219, of the nucleotide analogue 9-(2-phosphonylmethoxyethyl)guanine (PMEG) was recently developed which forms an active phosphorylated metabolite, PMEG diphosphate (PMEGpp), in cells following enzymatic modification in the cytosol. PMEGpp causes cytotoxicity in dividing cells due to potent inhibition of the nuclear DNA polymerases α, δ and ε by causing DNA chain termination, resulting in inhibition of DNA synthesis.10 GS-9219 was synthesized to produce a PMEG analogue with improved permeability and selectivity for peripheral blood mononuclear cells (PBMC) and was shown to have antiproliferative activity in vitro against a panel of haematopoietic cell lines.10 On the basis of these encouraging in vitro results, GS-9219 was selected for further evaluation in pet dogs with non-Hodgkin's lymphoma (NHL) and objective response rates in phase I/II clinical trials were 79–100%.11 Following these promising findings, phase I trials with GS-9219 were initiated in people, and GS-9219 is currently being developed as a canine lymphoma therapeutic. While GS-9219 has demonstrated significant activity against haematopoietic tumour cell lines and NHL, during the initial PMEG analogue development and screening process two additional analogues (GS-343074 and GS-424044) were identified, which appear to have superior in vitro antiproliferative activity against nonhaematopoietic solid cancer cell lines. The purpose of this study was to determine the antiproliferative and pro-apoptotic activity of these new analogues in various canine tumour cell lines prior to initiating phase I clinical trials in pet dogs with cancer.
Materials and methods
Cell lines
Ten canine cell lines were used, including osteosarcoma (D17 and Abrams), hemangiosarcoma (DEN-HSA), mammary carcinoma (CMT-12 and CMT-27), thyroid carcinoma (C-TAC), melanoma (CML-1, CML-6M and 17-CM98) and 1771 [previously reported as a B-cell lymphoma cell line12; however, our further characterizations indicate this to be a monocyte cell line; HLA Dr+, CD14+, CD21−, polymerase chain reaction (PCR) immunoglobulin rearrangement negative with monocyte/mixed macrophage light scatter (data not shown)]. Three of these lines were developed at the University of Wisconsin-Madison School of Veterinary Medicine (Abrams, DEN-HSA and 17CM98), 2 were obtained through the American Type Culture Collection (D17 and CTAC), 4 were provided by Dr Lauren Wolf at Auburn University (CMT-12, CMT-27, CML-1 and CML-6M) and 1771 were provided by Dr Ann Jeglum (University of Pennsylvania, Philadelphia, PA). All cells were grown in continuous culture with minimum essential media (MEM, Gibco BRL, Grand Island, NY, USA) supplemented with 10% foetal bovine serum (FBS) (C/10) and 5% carbon dioxide at 37°C. Confluent cells were subcultured every 3–4 days (d) after detaching the cells with 0.1% trypsin, 0.02% ethylenediamine tetraacetic acid (EDTA) in phosphate-buffered saline (PBS).
GS-343074 and GS-424044
GS-343074 and GS-424044 were synthesized at Gilead Sciences and provided in 10 nM stock solutions. Stock solutions were aliquoted into single use volumes and stored at −20°C until use.
Growth inhibition assays
The effect of GS-343074 and GS-424044 on anchorage-dependent cell proliferation was assessed using a standard tetrazolium-based colorimetric assay (MTS, CellTiter AQeous One, Promega, Madison, WI, USA). In this assay, the tetrazolium salt, MTS, is bioreduced by cells into a coloured formazan product that is soluble in tissue culture media. The quantity of formazan product as measured by the absorbance at 490 nm is directly proportional to the number of metabolically active cells in culture. IC50 and IC25 values were defined as the concentrations of drug that inhibited cell growth by 50 and 25%, respectively. Approximately 2500–10 000 tumour cells/well were plated in quadruplicate in 96-well microtitre plates and incubated for 24 h at 37°C. After 24 h, varying concentrations of GS-343074 or GS-424044 were added, in either C/10 or 1% FBS-supplemented MEM (C/1). Relative viable cell number was assessed 72 h after drug administration spectrophotometrically at 490 nM with a VERSAmax microplate reader (Molecular Devices, Sunnyvale, CA, USA) and SOFTMAX PRO software (Molecular Devices). Relative viable cell number was standardized to that of cells incubated in C/10 or C/1 alone. In addition to standard 72 h proliferation assays, 5-d drug exposure assays were also performed in representative cell lines with starting cell numbers reduced to 1000–2500 cells/well.
Apoptosis analysis
The effects of GS-343074 and GS-424044 on tumour cell apoptosis were assessed using Annexin V-fluorescein isothiocyanate (FITC) and propidium iodide (PI) staining with flow cytometry. One million cells were plated on 60 mm tissue culture dishes and incubated for 24 h in C/10 at 37°C. After 24 h, varying concentrations of GS-343074 or GS-424044 in C/10 or C/1 were added. Cells were evaluated by flow cytometry 48 h, 72 h and 5 d post drug administration. Briefly, 1 × 105 cells in each condition were stained with FITC-conjugated annexin-V and PI (BD Biosciences, San Diego, CA, USA), and subjected to flow cytometric analysis to assess the proportion of cells undergoing apoptosis. Data was acquired using a FACScan flow cytometer (BD Bioscences) and analysed using FloJo software (Tree Star, Ashland, OR, USA). Early and late apoptosis was defined as annexin-V(+)/PI(−) and annexin-V(+)/PI(+), respectively. Total apoptosis was defined as the sum of the early and late apoptosis.
Statistical analysis
Changes in the proportion of cells undergoing apoptosis, as measured by PI and annexin-V flow cytometric analysis were compared using two-way Fisher's exact test (95% CI). Statistical analyses were performed using a commercially available computer software programme (PRISM 4, GraphPad Software, La Jolla, CA). P-values ≤ 0.05 were considered significant.
Results
GS-343074 exerts growth inhibition on multiple canine cancer lines in vitro
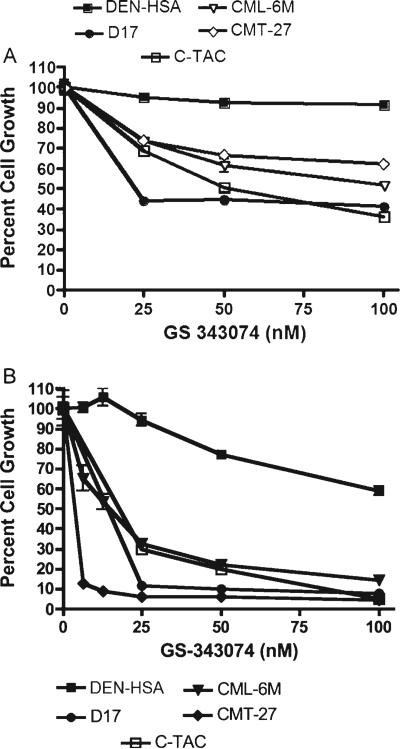
GS-343074 demonstrated antiproliferative activity in a dose-dependent manner against all canine cancer cell lines evaluated except for one hemangiosarcoma line (DEN-HSA). This analogue was most potent against thyroid carcinoma (C-TAC), melanoma (CML-6M), mammary carcinoma (CMT-27) and osteosarcoma (D-17 and Abrams) (Fig. 1A). The antiproliferative effect was greater in growth conditions using C/1, as expected. IC50 values for GS-343074 in C/10 and C/1 were in the range of 30.8–201.4 and 11.9–90.9 nM, respectively (Table 1). Two thirds of the cells evaluated did not achieve 50% growth inhibition following drug application in C/10. IC25 values in C/10 and C/1 were in the range of 9.2–34.8 and 5.6–36.0 nM, respectively. All cell lines evaluated achieved 25% growth inhibition in C/10.
Figure 1.
Growth inhibition of representative canine tumour cell lines [including thyroid carcinoma (C-TAC), melanoma (CML-6M), mammary carcinoma (CMT-27) and osteosarcoma (D-17)] following treatment with GS-343074 in C/10. Dividing cells were exposed for 72 h (A) or 5 d (B) to increasing concentrations of GS-343074. Cell viability was determined using the MTS assay. The percentage of growth was normalized to the control cells exposed to C/10. Error bars represent the standard deviations calculated from four replicate experiments.
Table 1.
Calculated IC50 and IC25 values in ten canine neoplastic cell lines following 72h of treatment with GS-343074 or GS-424044
| IC50/IC25 (nmol/L) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Drug | C-TAC | CML-1 | CML-6M | 17-CM98 | CMT-12 | CMT-27 | DEN-HSA | Abrams | D17 | 1771 |
| GS-343074 (C/1) | 21.4/8.6 | NA/36.0 | 16.8/7.0 | 90.9/10.8 | 16.4/7.4 | 11.9/5.6 | NA/NA | 55.7/12.7 | 20.0/8.2 | 18.0/7.5 |
| GS-343074 (C/10) | 48.4/17.6 | 35.9/9.2 | 146.8/26.2 | NA/24.0 | NA/34.8 | NA/21.2 | NA/28.6 | 201.4/17.2 | 30.8/13.5 | 34.5/15.0 |
| GS-424044 (C/1) | NA/NA | NA/14.2 | 236.5/68.0 | NA/15.0 | 45.4/12.6 | 27.4/37.6 | NA/NA | 324.0/112.2 | 23.3/12.0 | 106.5/16.5 |
| GS-424044 (C/10) | NA/NA | 75.1/12.0 | 336.0/155.8 | 51.0/15.0 | 261.0/62.3 | NA/149.0 | NA/NA | NA/101.8 | 54.8/14.2 | 81.0/24.0 |
NA, not achieved.
GS analogue was administered to cells in MEM supplemented with 1% (C/1) or 10% (C/10) FBS.
Lower concentrations of GS-343074 exerted antiproliferative activity against several canine cancer cell lines in vitro when drug exposure was maintained for 5 d compared to 72 h. GS-343074 showed significant inhibition of cell growth with the 5 d assay for multiple cancer cell lines, consistent with earlier data. Among the most responsive cell lines were canine osteosarcoma (D-17 and Abrams), canine thyroid carcinoma (C-TAC) and canine mammary tumour (CMT-12 and CMT-27) (Fig. 1B). Results of the 5 d assay were comparable to those of the initial 72 h proliferation assays, although IC50 and IC25 values were at lower concentrations. Additionally, all cell lines were susceptible to GS-343074, even those that did not achieve 50% inhibition following 72 h exposure. Values for IC50 ranged from 3.7 to 180.0 nM in the eight cell lines evaluated with 5 d proliferation assays (Table 2). Values for IC25 ranged from 1.7 to 56.4 nM on the eight cell lines susceptible to the drug.
Table 2.
Calculated IC50 and IC25 values in seven canine neoplastic cell lines following 5 d of treatment with GS-343074 or GS-424044
| IC50/IC25 (nmol/L) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Drug | C-TAC | CML-1 | CML-6M | CMT-12 | CMT-27 | DEN-HSA | Abrams | D17 |
| 343074 | 19.0/10.4 | 51.4/7.3 | 55.3/5.1 | 4.6/2.0 | 3.7/1.7 | 180.0/56.4 | 6.4/2.8 | 10.0/5.3 |
| 424044 | NA/NA | 78.6/22.8 | 61.6/24.0 | 78.8/42.6 | 35.6/21.5 | NA/NA | 104.1/73.3 | 10.7/4.4 |
NA, not achieved.
GS analogue was administered to cells in MEM supplemented with 10% (C/10) FBS.
GS-424044 exerts growth inhibition on multiple canine cancer lines in vitro
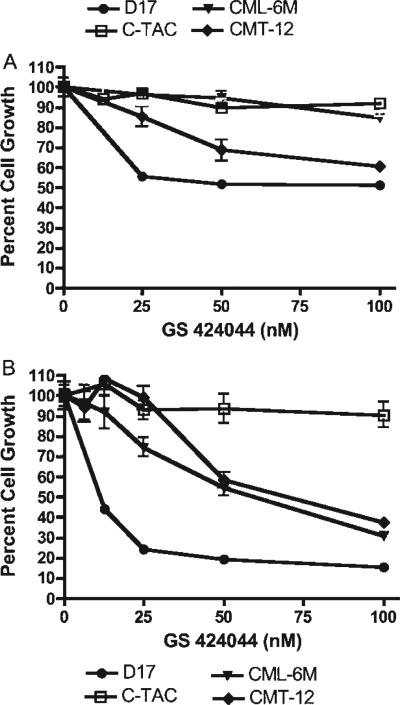
GS-424044 demonstrated antiproliferative activity in a dose-dependent manner against several canine lines, including mammary carcinoma (CMT-12 and CMT-27), melanoma (CML-1, CML-6M and 17CM98), osteosarcoma (D-17 and Abrams) and 1771 cells (Fig. 2A). However, IC50 and IC25 values were higher with GS-424044 compared to GS-343074. IC50 values for GS-424044 in C/10 and C/1 were in the range of 51.0–336.0 and 23.2–324.0 nM, respectively (Table 1). Over half the cell lines evaluated in both C/10 and C/1 did not achieve 50% growth inhibition following administration of drug. IC25 values in C/10 and C/1 were in the range of 12.0–155.8 nM and 12.0–112.2 nM, respectively. Only 2 cell lines (C-TAC, DEN-HSA) of the 10 evaluated did not achieve 25% growth inhibition in either C/10 or C/1.
Figure 2.
Growth inhibition of canine neoplastic cell lines [including thyroid carcinoma (C-TAC), melanoma (CML-6M), mammary carcinoma (CMT-12), and osteosarcoma (D-17] following treatment with GS-424044 in C/10. Dividing cells were exposed for 72 h (A) or 5 d (B) to increasing concentrations of GS-424044. Cell viability was determined using the MTS assay. The percentage of growth was normalized to the control cells exposed to only C/10. Error bars represent the standard deviations calculated from four replicate experiments.
Similar to GS-343074, GS-424044 demonstrated superior antiproliferative activity with relatively low concentrations in vitro when assayed over 5 d (Fig. 2B; Table 2). GS-424044 appeared particularly effective against the canine osteosarcoma cell line D-17, as evidenced by reduced viable cell numbers to less than 20% of the untreated cells (Fig. 2B).
CML-1 cells demonstrated increased sensitivity to growth inhibition when grown in C/10 compared to C/1
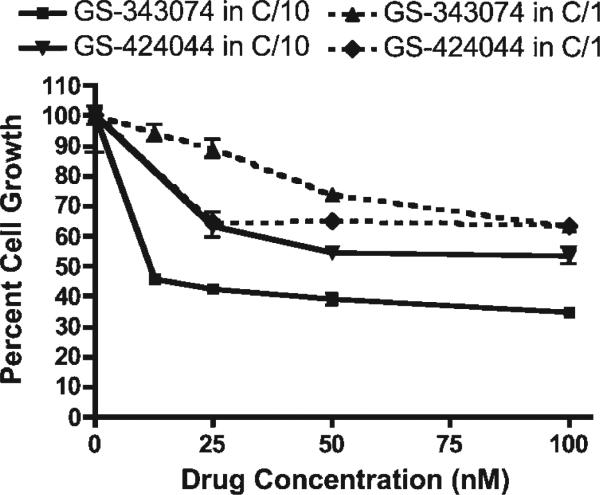
In contrast to other cell lines assayed, CML-1 melanoma cells consistently displayed increased sensitivity to growth inhibition for both GS analogues administered in C/10 compared to C/1 (Fig. 3). One additional melanoma cell line (17-CM98) also demonstrated increased growth inhibition in C/10, however this result occurred only with GS-424044. The third melanoma cell line (CML-6M) displayed more typical growth characteristics, with improved growth in C/10 media compared to C/1.
Figure 3.
Growth inhibition of the canine melanoma cell line (CML-1) following exposure to various concentrations of GS-343074 or GS-424044 in C/10 and C/1. CML-1 cells were inhibited more by the addition of drug in C/10 as opposed to C/1, contrary to expected results.
Both GS-343074 and GS-424044 exert a pro-apoptotic effect on several canine cancer cell lines
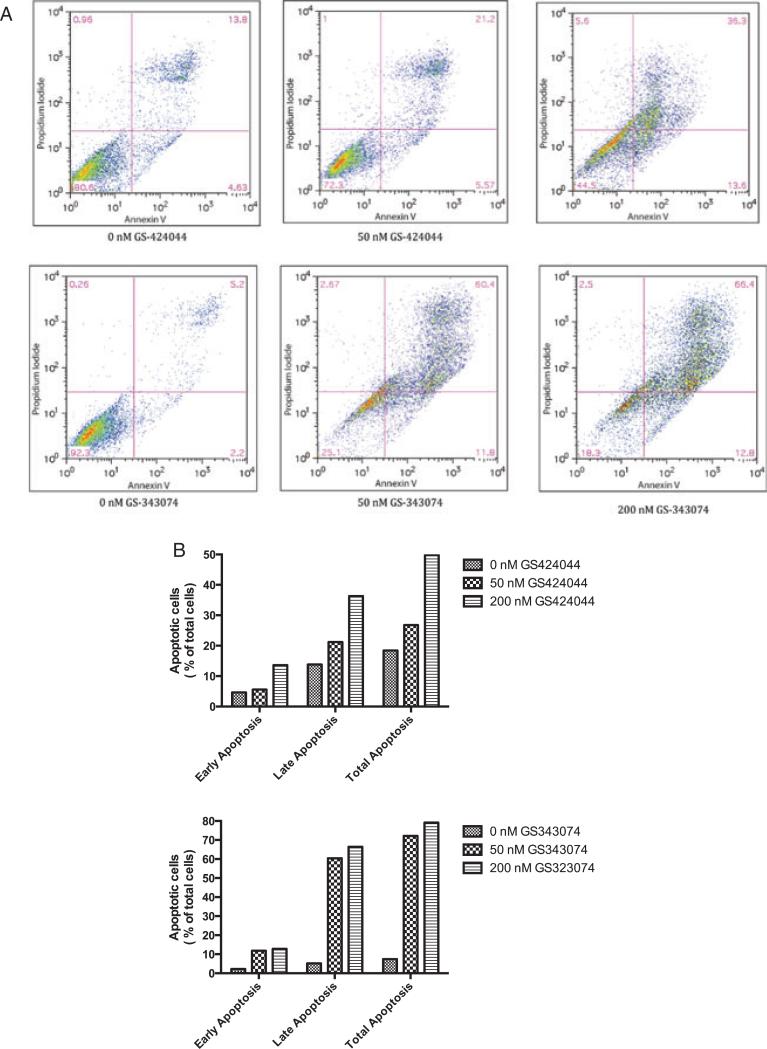
In view of the growth-inhibitory results obtained with the tetrazolium-based assays, experiments were performed to determine whether the observed reduction in cell number occurred as a consequence of induced apoptosis. Cells that displayed growth inhibition on initial assays were selected for apoptosis analysis and a dose-dependent increase in apoptosis was observed in these cell lines following treatment with GS-343074 and GS-424044 (P < 0.001). Representative results of flow cytometric cell-cycle analysis for the canine osteosarcoma cell line, Abrams, treated for 48 h with varying concentrations of GS-343074 or GS-424044 are shown (Fig. 4). Similar results were seen in all cell lines used in this study.
Figure 4.
GS-424044 and GS-343074 induce apoptosis in canine tumour cells. A representative cell line, Abrams, was treated with increasing concentrations of GS-424044 and GS-343074 for 48 h. (A) The percentage of apoptotic cells was detected by flow cytometry using annexin V/PI double staining. (B) Apoptosis was assessed by counting the percentage of annexin V-positive cells. Early apoptosis, annexin-V(+), PI(−); late apoptosis, annexin-V(+), PI(+); total apoptosis, early apoptosis + late apoptosis. Similar were seen in all cell lines used in this study.
Discussion
Significant in vitro activity against haematopoietic tumour cell lines noted for the prodrug GS-9219 led to successful clinical trials in pet dogs with NHL. Responses were noted in 79% of dogs treated with GS-9219 monotherapy and clinical trials in people with haematopoietic tumours have now been initiated.10,11 On the basis of this successful drug development path, there was an interest in evaluating, by a similar integrated approach, the anticancer activity of other PMEG prodrug analogues designed to have more favourable results in non-haematopoietic solid tumours. Evaluation of the in vitro activity of these new analogues in canine-specific solid tumour cell lines was a logical first preclinical step towards this end.
Exposure of a wide array of canine cancer cell lines to GS-343074 induced significant growth inhibition as measured by standard proliferation assays. A dose-dependent decrease in proliferative capabilities was noted, although a plateau occurred at concentrations above approximately 100 nM of the drug for most cell lines evaluated. While IC50 concentrations for GS-343074 varied considerably between cell lines, they were ≤ 50 nM for C-TAC, CML-1, 1771 and D17, indicating potency of this particular GS analogue. While GS-424044 also induced growth inhibition in a dose-dependent fashion, fewer cell lines were inhibited by this analogue and it generally appeared to have less in vitro activity than GS-343074.
Due in part to the slow growth rates of some of the cell lines employed and our prior experience using five consecutive day infusions of GS-9219 in vivo, we also explored proliferation assays with 5 d drug exposures.11 Exposure of the most sensitive canine cell lines to 5 d of GS-343074 induced further growth inhibition in a dose-dependent manner, with a plateau in inhibition at drug concentrations ranging from 20 to 50 nM. CMT-12 and CMT-27 were particularly sensitive to prolonged exposure of the drug, with IC50 concentrations less than 5 nM. These two cell lines did not reach 50% growth inhibition with exposure to only 72 h of drug. Similarly, when cells were exposed to 5 d of GS-424044 at varying concentrations, D17, Abrams, CMT-12, CMT-27, CML-1 and CML-6M had dramatically increased inhibition of growth compared to prior evaluations in which drug exposure was limited to 72 h. In addition, lower doses of the GS-424044 induced improved responses with the prolonged exposure times. IC50 concentrations for cell lines sustaining 50% inhibition ranged from 11 to 104 nM.
One cell line, CMT-12, demonstrated an initial increase in growth at low concentrations of GS-343074 and GS-424044 in 5 d proliferation assays, before growth inhibition was documented at higher drug concentrations. In this case, cells did not overgrow on visual assessment, and it is possible that low doses maximally stimulated repair mechanisms such that cells proliferate above baseline; this phenomenon, termed hormesis, has been previously documented with low doses of radiation therapy and natural anticancer compounds.13,14 This effect would lead to cell proliferation despite the addition of drug at low concentrations followed by growth inhibition once cellular repair was unable to match damage incurred by the drug.
As expected, cell growth was greater for all cell lines when plated in C/10 compared to C/1, with the exception of the canine melanoma cell line CML-1. This was an unexpected finding and suggests that a component of FBS exerts antiproliferative effects on this particular melanoma cell line. Similar results were seen with another melanoma cell line 17-CM98 when GS-424044 was assessed. It may be warranted to evaluate specific factors that inhibit cell growth, as a novel mechanism may be characterized.
The results of flow cytometry indicate that apoptosis is a contributory mechanism responsible for drug-induced reduction in cell number in these assays. Similar drug concentrations appeared to be responsible for the induction of growth inhibition as well as apoptosis. These findings suggest the analogues evaluated exert a cytotoxic rather than a cytostatic effect in vitro in the cell lines evaluated.
It remains to be seen whether concentrations of GS-343074 and GS-424044 required to induce inhibition of cell growth and induction of apoptosis in canine cancer cells in vitro are achievable in vivo. In our previous work in dogs receiving GS-9219, concentrations necessary for in vitro activity were easily achievable in PBMC and lymphoid tumour cells following systemic dosing, in part due to activation of the prodrug in the cytosol and subsequent concentration of active metabolites within the cell. Additionally, a long intracellular half life of the active metabolite was evident whether a single high dose or five daily low doses of GS-9219 were used, and both dosing schedules resulted in similar high intracellular levels and antitumour activity.10,11 If similar pharmacokinetics are established for the new analogues in vivo, then drug concentrations, including those shown to be superior with 5 d of drug exposure in vitro, should be achievable.
The in vitro data presented here suggest that the novel nucleotide analogues GS-343074 and GS-424044 hold promise for the treatment of canine, and possibly human solid tumours and further investigation is warranted. A phase I dose-finding trial was initiated evaluating the safety, pharmacokinetic and pharmacodynamic parameters of these two analogues in canine cancer patients and results are pending. Such information is necessary prior to initiation of phase II activity trials in dogs and should also serve to inform future trials in people should the results be promising. Such an integrated translational approach to drug development, as evidenced with the prior analogue (GS-9219), has received attention recently as a means of potentially accelerating the development pathway for these and other novel anticancer agents in both veterinary species and people.15
Supplementary Material
Acknowledgement
This project was funded by Gilead Science, Inc.
Footnotes
Conflicts of interest
D. B. T. and G. B. are employed by Gilead Sciences, Inc. D. M. V. received a research grant from Gilead Science, Inc.
References
- 1.Chu E, DeVita VT. Principles of medical oncology. In: Devita VT, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg's Cancer: Principles and Practice of Oncology. 8th edn. Vol. 1. Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 337–350. [Google Scholar]
- 2.Spodnick GJ, Berg J, Rand WM, Schelling SH, Couto G, Harvey J, Henderson RA, MacEwen G, Mauldin N, McCaw DL, Moore AS, Morrison W, Norris AM, O'Brandovich J, O'Keefe DA, Page R, Ruslander D, Klausner J, Straw RC, Thompson JP, Withrow SJ. Prognosis for dogs with appendicular osteosarcoma treated by amputation alone: 162 cases (1978–1988). Journal of the American Veterinary Medical Association. 1992;200:995–999. [PubMed] [Google Scholar]
- 3.Thompson JP, Fugent MJ. Evaluation of survival times after limb amputation with and without subsequent administration of cisplatin for treatment of appendicular osteosarcoma in dogs: 30 cases (1979–1990). Journal of the American Veterinary Medical Association. 1992;200:531–533. [PubMed] [Google Scholar]
- 4.Straw RC, Withrow SJ, Richter SL, Powers BE, Klein MK, Postorino NC, LaRue SM, Ogilvie GK, Vail DM, Morrison WB, McGee M, Dickinson K. Amputation and cisplatin for treatment of canine osteosarcoma. Journal of Veterinary Internal Medicine. 1991;5:205–210. doi: 10.1111/j.1939-1676.1991.tb00950.x. [DOI] [PubMed] [Google Scholar]
- 5.Bergman PJ, MacEwen EG, Kurzman ID, Henry CJ, Hammer AS, Knapp DW, Hale A, Kruth SA, Klein MK, Klausner J, Norris AM, McCaw D, Straw RC, Withrow SJ. Amputation and carboplatin for treatment of dogs with osteosarcoma: 48 cases (1991 to 1993). Journal of Veterinary Internal Medicine. 1996;10:76–81. doi: 10.1111/j.1939-1676.1996.tb02031.x. [DOI] [PubMed] [Google Scholar]
- 6.Bacon NJ, Ehrhart NP, Dernell WS, Lafferty M, Withrow SJ. Use of alternating administration of carboplatin and doxorubicin in dogs with microscopic metastases after amputation for appendicular osteosarcoma:50 cases (1999–2006). Journal of the American Veterinary Medical Association. 2008;232:1504–1510. doi: 10.2460/javma.232.10.1504. [DOI] [PubMed] [Google Scholar]
- 7.Chabner BA. Clinical strategies for cancer treatment: the role of drugs. In: Chabner BA, Longo DL, editors. Cancer Chemotherapy & Biotherapy Principles and Practice. 4th edn. Lippincott Williams & Wilkins; Philadelphia: 2006. pp. 1–14. [Google Scholar]
- 8.Chun R, Garrett LD, Vail DM. Cancer chemotherapy. In: Withrow SJ, Vail DM, editors. Withrow & MacEwen's Small Animal Clinical Oncology. 4th edn. Saunders Elsevier; St. Louis: 2007. pp. 163–192. [Google Scholar]
- 9.Chu E. Pharmacology of cancer chemotherapy. In: Devita VT, Lawrence TS, Rosenberg SA, editors. DeVita, Hellman, and Rosenberg's Cancer: Principles and Practice of Oncology. 8th edn. Vol. 1. Lippincott Williams & Wilkins; Philadelphia: 2008. pp. 385–406. [Google Scholar]
- 10.Reiser H, Wang J, Chong L, Watkins WJ, Ray AS, Shibata R, Birkus G, Cihlar T, Wu S, Liu X, Henne IN, Wolfgang GHI, Desai M, Rhodes GR, Fridland A, Lee WA, Plunkett W, Vail DM, Thamm DH, Jeraj R, Tumas DB. GS-9219- a novel acyclic nucleotide analogue with potent antineoplastic activity in dogs with spontaneous non-Hodgkin's lymphoma. Clinical Cancer Research. 2008;14:2824–2832. doi: 10.1158/1078-0432.CCR-07-2061. [DOI] [PubMed] [Google Scholar]
- 11.Vail DM, Thamm DH, Reiser H, Ray AS, Grushenka WHI, Lee WA, Watkins WJ, Babusis D, Henne IN, Kurzman ID, Jeraj R, Vanderhoek M, Plaza S, Anderson C, Wessel WA, Robat C, Lawrence J, Tumas DB. Assessment of GS-9219 in a pet dog model of non-Hodgkin's lymphoma. Clinical Cancer Research. 2009;15:3503–3510. doi: 10.1158/1078-0432.CCR-08-3113. [DOI] [PubMed] [Google Scholar]
- 12.Rosales C, Jeglum KA, Obracka M, Steplewski Z. Cytolytic activity of murine anti-dog lymphoma monoclonal antibodies with canine effector cells and complement. Cellular Immunology. 1988;115:420–428. doi: 10.1016/0008-8749(88)90194-3. [DOI] [PubMed] [Google Scholar]
- 13.Mattson MP. Hormesis defined. Ageing Research Reviews. 2008;7:1–7. doi: 10.1016/j.arr.2007.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott BR. It's time for a new low-dose-radiation risk assessment paradigm-one that acknowledges hormesis. Dose-Response. 2008;6:333–351. doi: 10.2203/dose-response.07-005.Scott. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Paoloni M, Khanna C. Translation of new cancer treatments from pet dogs to humans. Nature Reviews. Cancer. 2008;8:147–156. doi: 10.1038/nrc2273. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.