Abstract
Objective
The genetic factors leading to a predisposition to otitis media are not well understood. The objective of the current study was to develop a tag-single nucleotide polymorphism (SNP) panel to determine if there is an association between candidate gene polymorphisms and the development of chronic otitis media with effusion.
Study Design
A 1:1 case/control design of 100 cases and 100 controls was used. The study was limited to the chronic otitis media with effusion phenotype to increase the population homogeneity.
Methods
A panel of 192 tag-SNPs was selected. Saliva for DNA extraction was collected from 100 chronic otitis media with effusion cases and 100 controls. After quality control, 100 case and 79 control samples were available for hybridization. Genomic DNA from each subject was hybridized to the single nucleotide polymorphism probes, and genotypes were generated. Quality control across all samples and SNPs reduced the final SNPs used for analysis to 170. Each single nucleotide polymorphism was then analyzed for statistical association with chronic otitis media with effusion.
Results
Eight single nucleotide polymorphisms from 4 genes had an unadjusted p-value of <0.05 for association with the chronic otitis media with effusion phenotype (TLR4, MUC5B, SMAD2, SMAD4); five of these polymorphisms were in the TLR4 gene.
Conclusion
While these results need to be replicated in a novel population, the presence of 5 single nucleotide polymorphisms in the TLR4 gene having association with chronic otitis media with effusion in our study population lends evidence for the possible role of this gene in the susceptibility to otitis media.
Keywords: otitis media, genetics, single nucleotide polymorphisms, innate immune system
Introduction
Background
Otitis media (OM) is the most common medical problem affecting infants and children of any age1. Most children will experience one or more episodes of acute OM (AOM) in the first two years of life1-2 and ninety-three percent of all children will have at least one episode of AOM during childhood1. Recurrent OM (ROM) occurs in 20-30% of the pediatric population3-5. Chronic otitis media with effusion (COME) is defined as middle ear effusion lasting at least three months, which occurs in up to 10% of children with a point prevalence of 20%6. Of school-aged children who develop otitis media with effusion, 15-25% will develop chronic effusions lasting more than three months6. OM can lead to serious complications6-7 and the societal costs attributable to OM approach $5 billion annually8. COME may lead to hearing loss and speech and language delays6.
Despite well-known environmental risk factors, host genetic factors are increasingly implicated in susceptibility to OM9-11. As one review of the subject stated12, “There is incontrovertible evidence from epidemiological, anatomical, physiological, and immunological studies that susceptibility to recurrent acute otitis media and persistent otitis media with effusion is genetically determined.” In another study, a positive family history was present in more than 50% of patients with ROM13. In siblings and parents, as much as 60-70% of OM liability is due to genetic background14-15. Some of the strongest evidence for a genetic predisposition comes from twin and triplet studies that demonstrated a heritability of 57% for acute and 72% for chronic ear infections 11. There is a also higher correlation for ROM in monozygotic twins as compared with dizygotic twins15.
The specific genetic and immunologic factors underlying these findings are still unclear. Because the pathogenesis of OM is multi-factorial, with contributions from immunity, inflammatory regulation, and Eustachian tube function, the genetics of OM are likely to be complex. Thus, the identification of OM susceptibility genes could potentially allow for the development of molecular assays for stratification of OM risk in individual children. Such knowledge could impact clinical follow-up and decision-making in treatment of children with OM.
The genetic factors associated with the otitis media-prone state have focused on twin and familial studies9 and genes identified via GWAS studies16-18. Studies of the following OM phenotypes have been published: RAOM/COME16,17, 19-24, RAOM25, and COM26.
Gene Studies
A number of genes are implicated in OM pathogenesis. Analysis of the expression levels of single genes has been performed. For example, differences in mucin gene MUC5AC expression were noted in mucopurulent versus serous middle ear effusions27. Candidate gene studies have been published on the following genes19, 28: TLR4, interleukins, TNFα, F-Box Only protein 11, mucins, mannose-binding lectin 2, surfactant protein A. Genome-wide association studies have identified the following chromosomes as putative susceptibility loci: 10q22.3, 3p25.3, 10q26.3, 17q12 and 19q13.43 16-17, 18.
Innate immune response genes have been studied for their role in OM predisposition, yet conflicting results exist in the literature. Emonts et al. showed an association for polymorphisms in TLR4, among other innate immune response genes with ROM25. Our previous genetic association analysis comparing DNA extracted from otitis-prone patients (RAOM and COME phenotype) undergoing tympanostomy tube placement to control patients undergoing non-otologic surgery did not reveal isolated SNPs in TLR4, TLR2, TLR9, and CD14 genes to be associated with COME29. Heterogeneity in controls could have reduced the power to have detected an association.
TLR4 Animal Model
Animal models exist that help point to etiologic and genetic factors in OM, including the Jeff mouse (FBXO11 knockout) and the C3H/HeJ mouse (TLR4 deficient)30-31. The TLR4 mouse strain has a single amino acid substitution in the extracellular region of TLR4 that renders the receptor insensitive to endotoxin. Interestingly, 50% of C3H/HeJ mice develop chronic otitis media spontaneously30 suggesting a key role for defective TLR signaling in OM pathogenesis. Because similar TLR4 mutations cause reduced responsiveness to endotoxin in humans, this C3H/HeJ mouse model of otitis media has significant translational potential for research concerned with the pathogenesis and treatment of COME32-34. With this understanding of the TLR4 – COM mechanism in the mouse model, we have focused on the COME phenotype in order to improve our chances of identifying SNPs of interest in the TLR4 gene and others.
Tag Single Nucleotide Polymorphism (SNP) approach
Tag SNPs are representative SNPs in a region of a gene that are in high linkage disequilibrium (LD) with other SNPs and therefore, these SNPs “tag” other SNPs in the region. Using tag SNPs, it is possible to identify genetic variation without genotyping every SNP in a chromosomal region. This powerful approach allows association of SNPs that are “tagged” to be inferred. These tag SNPs are then analyzed for association to identify genes potentially increasing an individual's susceptibility to disease. The correlation structure of SNPs within a gene allows for the identification of a subset of SNPs that will “tag” regions of the gene for association with a phenotype. These SNPs associated with the phenotype of interest are not necessarily causative, but point to a gene region of association with the phenotype of interest.
The eight genes used to select a panel of tag SNPS were chosen based on the functional evidence in the C3H/HeJ mouse for the TLR4 gene and from the literature review: TLR4, FBXO11, MUC2, MUC5AC/B, SCN1B, SMAD2, SMAD4 and SFTPD. Both TLR4 and SFTPD play important roles in the host defense against infectious microorganisms and in regulating the innate immune response to a variety of pathogen-associated molecular patterns. SFTPD encodes surfactant protein D. FBXO11, a member of the FBOX-only family is a mediator of the TGF-β pathway. The SMAD genes are also mediators of the TGF-β pathway and regulate cell proliferation, apoptosis and cell differentiation. The mucin genes (MUC2, 5) are important in the production of mucin in OM and COME21. Lastly, the SCN1B gene is a Na channel regulator and has been shown to have some association with the OM-prone state in children24.
Materials and Methods
Study Design
A case-control design was used for the study. Cases included children ages 18 months to eighteen years undergoing tympanostomy tube placement for COME. For study purposes COME was defined as a middle ear effusion persisting for at least three months. These criteria are in line with the practice guidelines of the American Academy of Otolaryngology, American Academy of Pediatrics, and American Academy of Family Practice32-33. Diagnosis of COME was based on patient history, otomicroscopic examination and tympanometry. A control group met the following inclusion criteria: Children age 18 months to eighteen years of age seen in the pediatric otolaryngology clinic for a nonotologic, non-adenotonsillar condition, without a history of any other chronic infection (chronic sinusitis) or other inflammatory indication (recurrent parotitis, neck abscess, chronic infectious state).
Exclusion criteria for both cases and controls included patients with Down's syndrome, cleft lip and/or cleft palate, velocardiofacial syndrome (VCFS, 22q deletion syndrome), genetic disorders with known otologic sequelae, prior otologic surgery, cholesteatoma or history of cholesteatoma, known immunodeficiency, and/or known inflammatory disease. Exclusion criteria for controls included current AOM or COME, history of COME, history of ROM, or tympanostomy tube insertion. An additional exclusion criterion was non-Caucasian ethnicity due to the varying incidence of SNPs in different ethnic populations. Analysis of the possible differences in the two subject groups was performed by a two-tailed t test for age and by the Z test for demographic covariates (family history of ear disease, daycare attendance, environmental or food allergies, asthma, male/female distribution).
To calculate the sample size needed to detect a relative risk (RR) of 3, we assumed an additive genetic model, that the underlying level of linkage disequilibrium (LD) was 0.8, that the prevalence of the disease is 0.2 (see Introduction) and a level of significance of 0.0533. For a 1:1 case/control study design, and 80% power to detect an effect of this magnitude (RR=3), a sample size of 100 cases and 100 controls would be required using SNPs with allele frequency of at least 0.3.
Tag SNP selection
Genetic association studies leverage the ability of a subset of SNP markers to serve as proxies for the uncollected, unknown causal genetic variation. The correlation structure of SNPs within a gene allows for the identification of a subset of SNPs that will “tag” regions of the gene for association with a phenotype34. Using the HapMap reference panel for a Caucasian population (The International HapMap Consortium 200535), we utilized this method of “indirect association” to identify a list of SNPs across each of the eight genes with allele frequencies 0.25-0.33 that allowed detection of association of the “tagged” gene regions with the subjects with COME. Genes to be tested were determined through a combination of literature search of previous association studies of OM and related phenotypes and expert clinical knowledge of the phenotype from the investigator. SNPs to be tested were chosen by several methods: either previous association or through a bioinformatic search of dbSNP® using SNPs within the gene region that had heterozygosity >0.15. SNPs falling within putative functional features, such as exons, promoter and 3'UTR region were preferentially chosen. Tag SNP and LD structure across the gene regions were determined using Haploview36. All SNPs were analyzed for suitability of probe creation using the Illumina, Inc. software with the help of iGenix® bioinformatic staff. The final SNP list contained 8 genes (TLR4, FBXO11, MUC2, MUC5AC/B, SCN1B, SMAD2, SMAD4 and SFTPD) and 192 SNPs.
DNA Collection and Extraction
A sample of the patient's saliva was obtained in clinic using the Oragene® DNA Self-Collection Kit, in which the patient provides 1-2ml of saliva into the kit. The kit is then closed and the saliva is mixed with a preserving fluid to stabilize the DNA at room temperature. Genomic DNA was extracted from the saliva using the Oragene® protocol (http://www.dnagenotek.com/ROW/pdf/PD-PR-006.pdf). The samples were then treated with RNase to digest contaminating RNA and the DNA precipitated with ethanol. DNA was assessed for purity and concentration using absorbance (OD) via Nanodrop, aiming for an A260/A280 ratio of greater than or equal to 1.8.
Genotyping
Custom genotyping technology allows the user to integrate large numbers of samples for a select panel of defined SNPs in a 96-well plate format. A total of 192 SNPs were selected in order to match this format. Tag SNPs were selected and the information sent to iGenix, Inc. where probes corresponding to each SNP were generated. Genomic DNA (250 ng) from each subject was hybridized to the SNP probes in the 96- well plates utilizing standard Illumina custom genotyping technology (http://www.illumina.com/pages.ilmn?ID=5). Genotypes for each sample were generated from the intensity values from each SNP in the Illumina software Genome Studio (Illumina, Inc.). All other analyses were performed using custom scripts in the R statistical programming language (version 2.15.0, http://www.r-project.org/).
SNP association analysis
Exploratory data analysis was performed on the cohort data to identify outliers and differences in distributions. SNPs were assessed for genotyping quality and heterozygosity. Quality control across all SNPs and across all samples resulted in the removal of 22 SNPs: poorly formed genotyping clusters (6); homozygous SNPs (7);low call rate (78%)(1); minor allele frequency (MAF) <1% (4); out of Hardy Weinberg Equilibrium (HWE) in controls (4). This leaves 170 markers for the analysis (see Supplemental Table 1). The statistical association of each individual SNP to case/control status was determined using a score test as implemented in R statistical genetics package GenABEL37. Because of the number of SNPs tested, the p-values were corrected for multiple testing through permutation testing.
Approval from the OHSU Institutional Review Board was obtained for this study.
Results
Demographic Data
Two hundred saliva samples were collected; 15 samples were lost in processing or did not have sufficient DNA in the collected saliva, leaving a remaining 185 subjects in the study (102 cases, 83 controls). Subject samples were removed for the following reasons: duplicate sample (1), call rate <97% (3) and related subjects as identified by Identity by Descent (IBS)>95% (2). This leaves 179 subjects for analysis, 100 cases, 79 controls.
Phenotype Data
Cases had an average duration of 7 months of middle ear effusions. Cases and controls were examined for differences in age or sex distributions: distribution of age was similar for cases and controls (p = .585) as well as evenly distributed for sex (p = 0.608)(Table 1). There was no statistically significant difference in smoke exposure in the home, environmental/food allergies, daycare attendance or asthma between the two study populations. However, there was a significantly higher proportion of COME phenotype with a family history of otitis media (Table 1). Diagnoses bringing control patients into the otolaryngology clinic included: hemangioma or vascular malformation (18), lymphadenopathy (4), dysphagia (6), branchial cleft anomaly or thyroglossal duct cyst (5), sensorineural hearing loss (4), neck mass (7), vocal cord nodules (3), other (32).
Table 1.
Phenotype Data
| Case n = 100 | Control N = 79 | Z score | P value | |
|---|---|---|---|---|
| Age, years (s.d.) | 6.51 (3.47) | 6.83 (4.20) | 0.585* | |
| Male | 57 | 42 | 0.512 | 0.6083 |
| Female | 43 | 37 | 0.512 | 0.6083 |
| OM Family hx | 65/93 | 33/79 | 3.712 | 0.0002 |
| Daycare | 42/98 | 23/79 | 1.886 | 0.0594 |
| Smoking | 16/99 | 11/79 | 0.413 | 0.6793 |
| Allergies | 9/98 | 11/79 | 0.990 | 0.3220 |
| Asthma | 9/99 | 12/79 | 1.253 | 0.2101 |
from two-tailed t test
SNP association Analysis
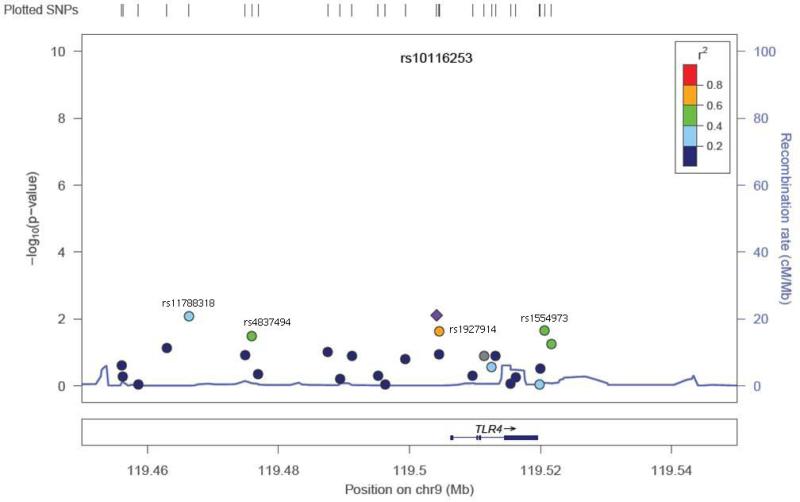
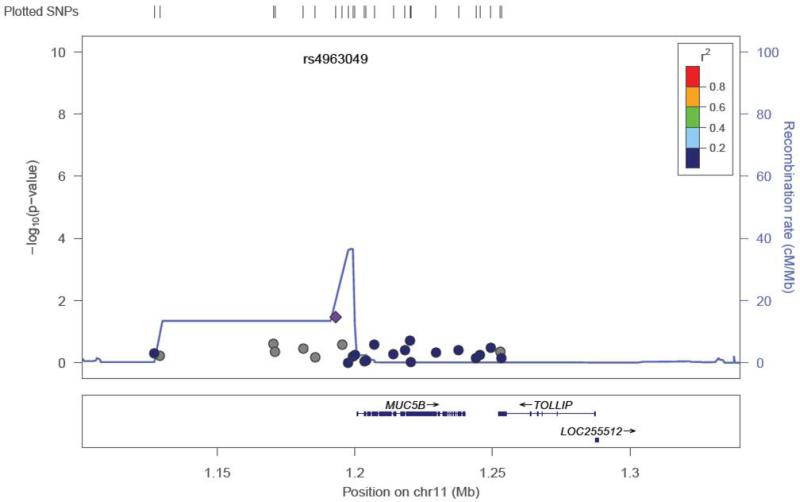
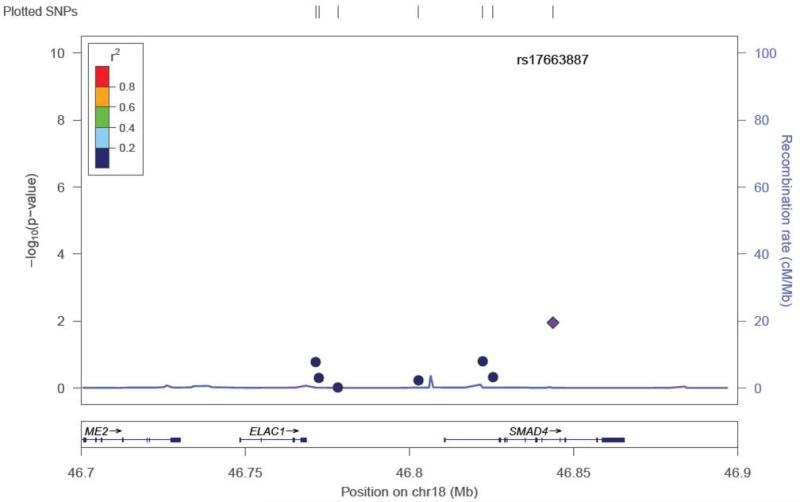
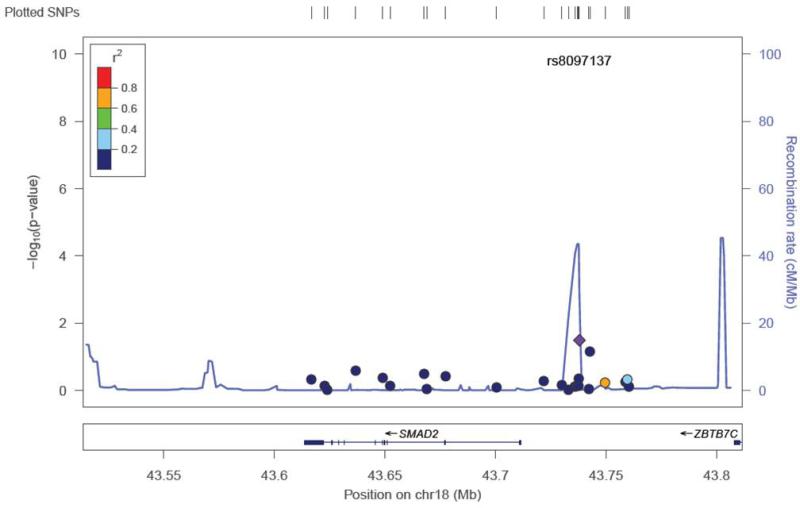
Twenty-two SNPs were removed from the final dataset (see Methods). Of the selected SNPs, a total of 13 were previously reported as associated with the otitis media population (dbSNP® database). None of the SNPs suggestive for association in our study were from this group of SNPs (Supplemental Table 1). The mean heterozygosity for a SNP was 0.294 +/− 0.163. The mean heterozygosity for a subject was 0.294 +/− 0.050. Because of the number of SNPs tested, the p-values were corrected for multiple testing through permutation testing (Table 2). After multiple testing correction with permutation testing, the 8 SNPs markers failed to hold their p values (0.655 – 0.99; Table 2). There were 8 SNP markers located in 4 genes that had an unadjusted p-value <0.05 (Table 2). These SNPs were found in the TLR4 gene (5), MUC5AC/B (1), SMAD2 (1), and SMAD4 (1) (Table 2). Five of the 8 SNPs were in the TLR4 gene and range in linkage disequilibrium from 0.4-0.8 (TLR4plot Figure 1). This level of LD indicates that these SNPs are most likely capturing one association signal for TLR4. Effect size for the associated SNPs ranged from 2.212 – 0.346. The TLR4 SNP plot (Figure 1) shows that the SNP rs10116253 possibly lies in the promoter region of the TLR4 gene. The MUC5AC/B SNP plot shows the SNP rs4963049 lies in an area of recombination (Figure 2). The SMAD4 SNP (Figure 3) lies within the gene region itself. The SMAD2 SNP lies in between the SMAD2 gene and the ZBTB7C gene (Fig 4). The SNP appears to lie in an area of recombination. Whether the SNP truly belongs to SMAD2 or ZBTB7C could depend on the annotation of the data base used. In our case, the dbSNP® database annotates this SNP to the SMAD2 gene.
TABLE 2.
SNP Association Analysis
| SNP | gene | Chromosome | MAF Caucasian | Location | p value (unadjusted) | p value (adjusted)+ |
|---|---|---|---|---|---|---|
| rs11788318 | TLR4 | 9 | NA | INTERGENIC | 0.008 | 0.655 |
| rs4837494 | TLR4 | 9 | 0.158 | INTERGENIC | 0.031 | 0.98 |
| rs10116253 | TLR4 | 9 | 0.25 | INTERGENIC | 0.007 | 0.625 |
| rs1927914 | TLR4 | 9 | NA | INTERGENIC | 0.023 | 0.94 |
| rs1554973 | TLR4 | 9 | 0.2 | INTERGENIC | 0.021 | 0.93 |
| rs4963049 | MUC5AC/B | 11 | 0.133 | INTERGENIC | 0.033 | 0.99 |
| rs8097137 | SMAD2 | 18 | 0.217 | INTERGENIC | 0.032 | 0.985 |
| rs17663887 | SMAD4 | 18 | NA | INTRON | 0.011 | 0.756 |
MAF- minor allele frequency from HapMap-CEU (CEPH:Utah Residents with Northern and Western European Ancestry)
adjusted p-values corrected for multiple testing with permutation testing
Figure 1.
SNP locus plot for the TLR4 gene SNPs tested(27). The TLR4 gene resides on chromosome 9. Five of the 27 SNPs tested (labeled on figure) had significant p values (see Table 2). The SNP rs10116253 (marked by purple diamond) may lie in a promoter region.
Figure 2.
SNP locus plot for the MUC5AC/B gene SNPs tested (25). The MUC5AC/B gene resides on chromosome 11. One of the SNPs tested had a significant p value (see Table 2), rs4963049 (marked by purple diamond). In the area of this SNP, there is a lot of recombination occurring.
Figure 3.
SNP locus plot for the SMAD 4 gene SNPs tested (7). The SMAD 4 gene resides on chromosome 18. One of the SNPs tested had a significant p value (see Table 2), rs17663887 (marked by purple diamond).
Figure 4.
SNP locus plot for the SMAD2 gene SNPs tested (24). The SMAD 2 gene resides on chromosome 18. One of the SNPs tested had a significant p value (see Table 2), rs8097137 (marked by purple diamond). This SNP lies in an area of recombination.
Discussion
In order to increase the chances of encountering SNPs of interest, it was our intent to study a very narrow population of genetically and phenotypically similar OM patients. Previously published studies on the genetics of OM have either studied the ROM phenotype or the combined ROM/COME phenotype together. Otolaryngologists are well aware that the COME phenotype is distinct from ROM in terms of age of presentation and character of fluid present in the middle ear. While there is substantial overlap with the AOM phenotype, we believe that the phenotypes are distinct. By careful screening, we were able to study the distinct COME phenotype and avoid the problems of a diluted or broad phenotype sample.
The two sample populations, case and control, were similar in terms of age, sex distribution, smoke exposure, allergy history and asthma history. The exception to their similarity is that more subjects in the case group had a family history of OM. The increased family history in the case group makes intuitive sense if a predisposition to OM has a genetic contribution, and, a familial association with the otitis-prone child has been shown in the past.
One limitation of the current study is the small number of genes and SNPs studied. However, the genes were chosen based on the experience with the C3H/HeJ TLR4 deficient mouse and its COM phenotype, as well as an extensive review of the literature for candidate genes. In addition, the sample size was relatively small for a tag-SNP study, but we limited the number of genes studied based on the power analysis performed. Lastly, when correction for multiple testing was performed, the SNPs with a significant association with COME did fail to retain their significance. Due to the multiple testing burden associated with the number of SNPs tested, it is possible that this study did not have the power to see an association. A replication study powered to test for the association of these 8 SNPS with the COME phenotype is planned. Undoubtedly, the inheritance of OM is multifactorial and complex.
These 8 SNPs associated with the COME phenotype are from genes governing immune response (TLR4), mucin production (MUC5B), and activation of TGF-β transcription/mediation of signaling pathways for cell proliferation/apoptosis and cell differentiation (SMAD2, SMAD4), all of which could play a role in the predisposition to OM. The confirmation of the phenotype in the mouse model (C3H/HeJ), lend weight to the TLR4 gene's possible role in COME.
Future studies are planned to confirm the 8 SNPs in a novel population of COME children. Eventual genome wide association studies (GWAS) will need to be performed on carefully screened COME patients to look for association with other genes of interest.
Conclusion
Eight candidate SNPs were found with unadjusted p values of <0.05, five of them in the TLR4 gene. Due to the confirmation of COME in the TLR4 deficient mouse and the likely association of the other genes showing association with SNPs in this study with a predisposition to COME, these eight SNPs merit confirmation in a replication population. The innate immune response gene TLR4, among others, may confer susceptibility to COME.
Supplementary Material
Acknowledgments
This work was supported by the National Organization for Hearing Research Foundation and NIH-NIDCD R01 DC009455.
Financial Disclosures: MacArthur – Stockholder, 13Therapeutics; Trune - Stockholder, 13Therapeutics; Wang, Schuller, Lighthall, Wilmot – none
Footnotes
Presented at Triologic Society Meeting, Orlando, Florida, USA. April 12, 2013
REFERENCES
- 1.Teele DQLJ, Rosner B. The Greater Boston Otitis Media Study Group: Epidemiology of otitis media during the first seven years of life in children in Greater Boston: a prospective cohort study. J Infect Dis. 1989;160:83–94. doi: 10.1093/infdis/160.1.83. [DOI] [PubMed] [Google Scholar]
- 2.Godinho RN, Goncalves TM, Nunes FB, et al. Prevalence and impact of chronic otitis media in school age children in Brazil. First epidemiologic study concerning chronic otitis media in Latin America. Int J Pediatr Otorhinolaryngol. 2001 Dec 1;61(3):223–232. doi: 10.1016/s0165-5876(01)00579-1. [DOI] [PubMed] [Google Scholar]
- 3.Pichichero ME. Recurrent and persistent otitis media. Pediatr Infect Dis J. 2000 Sep;19(9):911–916. doi: 10.1097/00006454-200009000-00034. [DOI] [PubMed] [Google Scholar]
- 4.Klein JO. The burden of otitis media. Vaccine. 2000 Dec 8;19(Suppl 1):S2–8. doi: 10.1016/s0264-410x(00)00271-1. [DOI] [PubMed] [Google Scholar]
- 5.Kogan MD, Overpeck MD, Hoffman HJ, Casselbrant ML. Factors associated with tympanostomy tube insertion among preschool-aged children in the United States. Am J Public Health. 2000 Feb;90(2):245–250. doi: 10.2105/ajph.90.2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leskinen K. Complications of acute otitis media in children. Curr Allergy Asthma Rep. 2005 Jul;5(4):308–312. doi: 10.1007/s11882-005-0071-3. [DOI] [PubMed] [Google Scholar]
- 7.Oestreicher-Kedem Y, Raveh E, Kornreich L, Popovtzer A, Buller N, Nageris B. Complications of mastoiditis in children at the onset of a new millennium. The Annals of otology, rhinology, and laryngology. 2005 Feb;114(2):147–152. doi: 10.1177/000348940511400212. [DOI] [PubMed] [Google Scholar]
- 8.Gates GA. Cost-effectiveness considerations in otitis media treatment. Otolaryngol Head Neck Surg. 1996 Apr;114(4):525–530. doi: 10.1016/S0194-59989670243-7. [DOI] [PubMed] [Google Scholar]
- 9.Casselbrant ML, Mandel EM, Rockette HE, et al. The genetic component of middle ear disease in the first 5 years of life. Archives of otolaryngology--head & neck surgery. 2004 Mar;130(3):273–278. doi: 10.1001/archotol.130.3.273. [DOI] [PubMed] [Google Scholar]
- 10.Kvaerner KJ, Tambs K, Harris JR, Magnus P. Distribution and heritability of recurrent ear infections. The Annals of otology, rhinology, and laryngology. 1997 Aug;106(8):624–632. doi: 10.1177/000348949710600802. [DOI] [PubMed] [Google Scholar]
- 11.Kvaerner KJ, Tambs K, Harris JR, Mair IW, Magnus P. Otitis media: relationship to tonsillitis, sinusitis and atopic diseases. Int J Pediatr Otorhinolaryngol. 1996 Apr;35(2):127–141. doi: 10.1016/0165-5876(95)01299-0. [DOI] [PubMed] [Google Scholar]
- 12.Casselbrant ML, Mandel EM. Genetic susceptibility to otitis media. Curr Opin Allergy Clin Immunol. 2005 Feb;5(1):1–4. doi: 10.1097/00130832-200502000-00002. [DOI] [PubMed] [Google Scholar]
- 13.Gebhart DE. Tympanostomy tubes in the otitis media prone child. The Laryngoscope. 1981 Jun;91(6):849–866. doi: 10.1288/00005537-198106000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Bluestone CD KJ. Otitis Media in Infants and Children. 3rd Edition Saunders; Philadelpha: 2001. [Google Scholar]
- 15.Casselbrant ML, Mandel EM, Fall PA, et al. The heritability of otitis media: a twin and triplet study. JAMA. 1999 Dec 8;282(22):2125–2130. doi: 10.1001/jama.282.22.2125. [DOI] [PubMed] [Google Scholar]
- 16.Casselbrant ML, Mandel EM, Jung J, et al. Otitis media: a genome-wide linkage scan with evidence of susceptibility loci within the 17q12 and 10q22.3 regions. BMC Med Genet. 2009;10:85. doi: 10.1186/1471-2350-10-85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen WM, Allen EK, Mychaleckyj JC, et al. Significant linkage at chromosome 19q for otitis media with effusion and/or recurrent otitis media (COME/ROM). BMC Med Genet. 2011;12:124. doi: 10.1186/1471-2350-12-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Daly KA, Brown WM, Segade F, et al. Chronic and recurrent otitis media: a genome scan for susceptibility loci. Am J Hum Genet. 2004 Dec;75(6):988–997. doi: 10.1086/426061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rye MS, Wiertsema SP, Scaman ES, et al. FBXO11, a regulator of the TGFbeta pathway, is associated with severe otitis media in Western Australian children. Genes Immun. 2011 Jul;12(5):352–359. doi: 10.1038/gene.2011.2. [DOI] [PubMed] [Google Scholar]
- 20.McCormick DP, Grady JJ, Diego A, et al. Acute otitis media severity: Association with cytokine gene polymorphisms and other risk factors. Int J Pediatr Otorhinolaryngol. 2011 Mar 25; doi: 10.1016/j.ijporl.2011.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ubell ML, Khampang P, Kerschner JE. Mucin gene polymorphisms in otitis media patients. Laryngoscope. 2010 Jan;120(1):132–138. doi: 10.1002/lary.20688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alper CM, Winther B, Hendley JO, Doyle WJ. Cytokine polymorphisms predict the frequency of otitis media as a complication of rhinovirus and RSV infections in children. Eur Arch Otorhinolaryngol. 2009 Feb;266(2):199–205. doi: 10.1007/s00405-008-0729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Segade F, Daly KA, Allred D, et al. Association of the FBXO11 gene with chronic otitis media with effusion and recurrent otitis media: the Minnesota COME/ROM Family Study. Arch Otolaryngol Head Neck Surg. 2006 Jul;132(7):729–733. doi: 10.1001/archotol.132.7.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sale MM, Chen WM, Weeks DE, et al. Evaluation of 15 functional candidate genes for association with chronic otitis media with effusion and/or recurrent otitis media (COME/ROM). PLoS One. 2011;6(8):e22297. doi: 10.1371/journal.pone.0022297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Emonts M, Veenhoven RH, Wiertsema SP, et al. Genetic polymorphisms in immunoresponse genes TNFA, IL6, IL10, and TLR4 are associated with recurrent acute otitis media. Pediatrics. 2007 Oct;120(4):814–823. doi: 10.1542/peds.2007-0524. [DOI] [PubMed] [Google Scholar]
- 26.Post JC. Genetics of otitis media. Adv Otorhinolaryngol. 2011;70:135–140. doi: 10.1159/000322489. [DOI] [PubMed] [Google Scholar]
- 27.Takeuchi K, Yagawa M, Ishinaga H, Kishioka C, Harada T, Majima Y. Mucin gene expression in the effusions of otitis media with effusion. Int J Pediatr Otorhinolaryngol. 2003 Jan;67(1):53–58. doi: 10.1016/s0165-5876(02)00361-0. [DOI] [PubMed] [Google Scholar]
- 28.Rye MS, Blackwell JM, Jamieson SE. Genetic susceptibility to otitis media in childhood. Laryngoscope. 2012 Mar;122(3):665–675. doi: 10.1002/lary.22506. [DOI] [PubMed] [Google Scholar]
- 29.Carroll SR, Zald PB, Soler ZM, Milczuk HA, Trune DR, MacArthur CJ. Innate immunity gene single nucleotide polymorphisms and otitis media. Int J Pediatr Otorhinolaryngol. 2012 Jul;76(7):976–979. doi: 10.1016/j.ijporl.2012.03.011. [DOI] [PubMed] [Google Scholar]
- 30.MacArthur CJ, Hefeneider SH, Kempton JB, Trune DR. C3H/HeJ mouse model for spontaneous chronic otitis media. The Laryngoscope. 2006 Jul;116(7):1071–1079. doi: 10.1097/01.mlg.0000224527.41288.c4. [DOI] [PubMed] [Google Scholar]
- 31.MacArthur CJ, Trune DR. Mouse models of otitis media. Curr Opin Otolaryngol Head Neck Surg. 2006 Oct;14(5):341–346. doi: 10.1097/01.moo.0000244193.97301.d7. [DOI] [PubMed] [Google Scholar]
- 32.Rosenfeld RM, Culpepper L, Doyle KJ, et al. Clinical practice guideline: Otitis media with effusion. Otolaryngol Head Neck Surg. 2004 May;130(5 Suppl):S95–118. doi: 10.1016/j.otohns.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 33.Gordon D, Haynes C, Blumenfeld J, Finch SJ. PAWE-3D: visualizing power for association with error in case-control genetic studies of complex traits. Bioinformatics. 2005 Oct 15;21(20):3935–3937. doi: 10.1093/bioinformatics/bti643. [DOI] [PubMed] [Google Scholar]
- 34.Carlson CS, Eberle MA, Rieder MJ, Yi Q, Kruglyak L, Nickerson DA. Selecting a maximally informative set of single-nucleotide polymorphisms for association analyses using linkage disequilibrium. Am J Hum Genet. 2004 Jan;74(1):106–120. doi: 10.1086/381000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.A haplotype map of the human genome. Nature. 2005 Oct 27;437(7063):1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Barrett JC, Fry B, Maller J, Daly MJ. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics. 2005 Jan 15;21(2):263–265. doi: 10.1093/bioinformatics/bth457. [DOI] [PubMed] [Google Scholar]
- 37.Aulchenko YS, Ripke S, Isaacs A, van Duijn CM. GenABEL: an R library for genome-wide association analysis. Bioinformatics. 2007 May 15;23(10):1294–1296. doi: 10.1093/bioinformatics/btm108. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.