Abstract
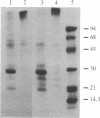
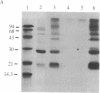
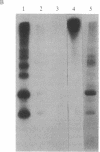
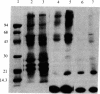
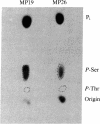
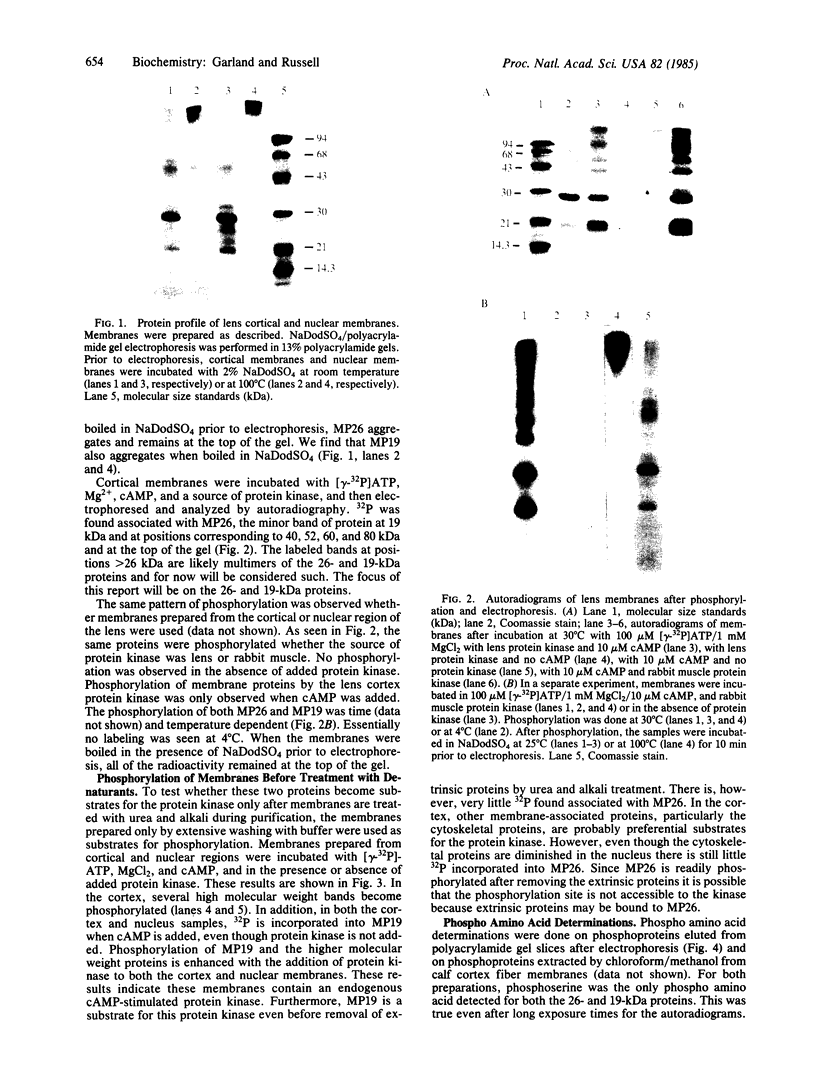
Two intrinsic membrane proteins of calf lens fiber cells can be phosphorylated by a soluble bovine lens cAMP-dependent protein kinase and rabbit muscle cAMP-dependent protein kinase. After electrophoresis of the phosphorylated membranes, 32P comigrates with the lens main intrinsic protein at 26-27 kDa and with a minor band of protein that migrates at 19-20 kDa. 32P is also found with proteins that, based on the molecular sizes, are likely multimers of the 19-kDa and 26-kDa proteins. Upon boiling in NaDodSO4, all the radioactivity is found at the top of the gel, suggesting that both phosphoproteins are intrinsic membrane proteins. Serine is the only phospho amino acid detected in both proteins regardless of the source of protein kinase. The phosphorylation sites of both proteins are lost upon cleavage with trypsin and chymotrypsin. The smaller phosphoprotein is likely not a crystallin, because antibodies directed against alpha-, beta-, or gamma-crystallins do not cross-react with the 19-kDa protein. The 19-kDa 32P-labeled protein does not migrate coincident with calf alpha-crystallin.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcalá J., Lieska N., Maisel H. Protein composition of bovine lens cortical fiber cell membranes. Exp Eye Res. 1975 Dec;21(6):581–595. doi: 10.1016/0014-4835(75)90040-8. [DOI] [PubMed] [Google Scholar]
- Alcalá J., Valentine J., Maisel H. Human lens fiber cell plasma membranes. I. Isolation, polypeptide composition and changes associated with ageing. Exp Eye Res. 1980 Jun;30(6):659–677. doi: 10.1016/0014-4835(80)90065-2. [DOI] [PubMed] [Google Scholar]
- Benedetti E. L., Dunia I., Bentzel C. J., Vermorken A. J., Kibbelaar M., Bloemendal H. A portrait of plasma membrane specializations in eye lens epithelium and fibers. Biochim Biophys Acta. 1976 Dec 14;457(3-4):353–384. doi: 10.1016/0304-4157(76)90004-6. [DOI] [PubMed] [Google Scholar]
- Bernardini G., Peracchia C., Venosa R. A. Healing-over in rat crystalline lens. J Physiol. 1981 Nov;320:187–192. doi: 10.1113/jphysiol.1981.sp013943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloemendal H., Hermsen T., Dunia I., Benedetti E. L. Association of crystallins with the plasma membrane. Exp Eye Res. 1982 Jul;35(1):61–67. doi: 10.1016/s0014-4835(82)80023-7. [DOI] [PubMed] [Google Scholar]
- Bloemendal H. Lens proteins. CRC Crit Rev Biochem. 1982;12(1):1–38. doi: 10.3109/10409238209105849. [DOI] [PubMed] [Google Scholar]
- Bloemendal H., Zweers A., Vermorken F., Dunia I., Benedetti E. L. The plasma membranes of eye lens fibres. Biochemical and structural characterization. Cell Differ. 1972 Jun;1(2):91–106. doi: 10.1016/0045-6039(72)90032-2. [DOI] [PubMed] [Google Scholar]
- Bok D., Dockstader J., Horwitz J. Immunocytochemical localization of the lens main intrinsic polypeptide (MIP26) in communicating junctions. J Cell Biol. 1982 Jan;92(1):213–220. doi: 10.1083/jcb.92.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracchi P. G., Carta F., Fasella P., Maraini G. Selective binding of aged alpha-crystallin to lens fibre ghosts. Exp Eye Res. 1971 Jul;12(1):151–154. doi: 10.1016/0014-4835(71)90140-0. [DOI] [PubMed] [Google Scholar]
- Broekhuyse R. M., Kuhlmann E. D. Lens membranes 1. Composition of urea-treated plasma membranes from calf lens. Exp Eye Res. 1974 Sep;19(3):297–302. doi: 10.1016/0014-4835(74)90148-1. [DOI] [PubMed] [Google Scholar]
- Broekhuyse R. M., Kuhlmann E. D. Lens membranes. IV. Preparative isolation and characterization of membranes and various membrane proteins from calf lens. Exp Eye Res. 1978 Mar;26(3):305–320. doi: 10.1016/0014-4835(78)90077-5. [DOI] [PubMed] [Google Scholar]
- Broekhuyse R. M., Kuhlmann E. D., Stols A. L. Lens membranes II. Isolation and characterization of the main intrinsic polypeptide (MIP) of bovine lens fiber membranes. Exp Eye Res. 1976 Sep;23(3):365–371. doi: 10.1016/0014-4835(76)90135-4. [DOI] [PubMed] [Google Scholar]
- De Mello W. C. Effect of intracellular injection of calcium and strontium on cell communication in heart. J Physiol. 1975 Sep;250(2):231–245. doi: 10.1113/jphysiol.1975.sp011051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dilley K. J., Bron A. J., Habgood J. O. Anterior polar and posterior subcapsular cataract in a patient with retinitis pigmentosa: a light-microscopic and ultrastructural study. Exp Eye Res. 1976 Feb;22(2):155–167. doi: 10.1016/0014-4835(76)90042-7. [DOI] [PubMed] [Google Scholar]
- Délèze J., Loewenstein W. R. Permeability of a cell junction during intracellular injection of divalent cations. J Membr Biol. 1976 Aug 27;28(1):71–86. doi: 10.1007/BF01869691. [DOI] [PubMed] [Google Scholar]
- Farnsworth P. N., Burke P. A., Wagner B. J., Fu S. C., Regan T. J. Diabetic cataracts in the rhesus monkey lens. Metab Pediatr Ophthalmol. 1980;4(1):31–42. [PubMed] [Google Scholar]
- Fitzgerald P. G., Bok D., Horwitz J. Immunocytochemical localization of the main intrinsic polypeptide (MIP) in ultrathin frozen sections of rat lens. J Cell Biol. 1983 Nov;97(5 Pt 1):1491–1499. doi: 10.1083/jcb.97.5.1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friedlander M. Immunological approaches to the study of myogenesis and lens fiber junction formation. Curr Top Dev Biol. 1980;14(Pt 2):321–358. doi: 10.1016/s0070-2153(08)60200-8. [DOI] [PubMed] [Google Scholar]
- Garner M. H., Roy D., Rosenfeld L., Garner W. H., Spector A. Biochemical evidence for membrane disintegration in human cataracts. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1892–1895. doi: 10.1073/pnas.78.3.1892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A. Lens gap junctions: a structural hypothesis for nonregulated low-resistance intercellular pathways. Invest Ophthalmol Vis Sci. 1979 Nov;18(11):1104–1122. [PubMed] [Google Scholar]
- Gorin M. B., Yancey S. B., Cline J., Revel J. P., Horwitz J. The major intrinsic protein (MIP) of the bovine lens fiber membrane: characterization and structure based on cDNA cloning. Cell. 1984 Nov;39(1):49–59. doi: 10.1016/0092-8674(84)90190-9. [DOI] [PubMed] [Google Scholar]
- Hawkes R., Niday E., Gordon J. A dot-immunobinding assay for monoclonal and other antibodies. Anal Biochem. 1982 Jan 1;119(1):142–147. doi: 10.1016/0003-2697(82)90677-7. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L., Gilula N. B. Liver gap junctions and lens fiber junctions: comparative analysis and calmodulin interaction. Cold Spring Harb Symp Quant Biol. 1982;46(Pt 2):639–645. doi: 10.1101/sqb.1982.046.01.060. [DOI] [PubMed] [Google Scholar]
- Horwitz J., Wong M. M. Peptide mapping by limited proteolysis in sodium dodecyl sulfate of the main intrinsic polypeptides isolated from human and bovine lens plasma membranes. Biochim Biophys Acta. 1980 Mar 26;622(1):134–143. doi: 10.1016/0005-2795(80)90165-8. [DOI] [PubMed] [Google Scholar]
- Kawaba T., Takamura R., Matsuda H., Hayashi S. [Studies on cyclic nucleotides and protein kinase of lens. II. Endogenous substrate proteins for cyclic AMP dependent protein kinase in bovine lens]. Nippon Ganka Gakkai Zasshi. 1982;86(6):578–586. [PubMed] [Google Scholar]
- Keeling P., Johnson K., Sas D., Klukas K., Donahue P., Johnson R. Arrangement of MP26 in lens junctional membranes: analysis with proteases and antibodies. J Membr Biol. 1983;74(3):217–228. doi: 10.1007/BF02332125. [DOI] [PubMed] [Google Scholar]
- Kemp B. E., Graves D. J., Benjamini E., Krebs E. G. Role of multiple basic residues in determining the substrate specificity of cyclic AMP-dependent protein kinase. J Biol Chem. 1977 Jul 25;252(14):4888–4894. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lasser A., Balazs E. A. Biochemical and fine structure studies on the water-insoluble components of the calf lens. Exp Eye Res. 1972 May;13(3):292–308. doi: 10.1016/0014-4835(72)90111-x. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Cell surface membranes in close contact. Role of calcium and magnesium ions. J Colloid Interface Sci. 1967 Sep;25(1):34–46. doi: 10.1016/0021-9797(67)90007-0. [DOI] [PubMed] [Google Scholar]
- Loewenstein W. R. Permeable junctions. Cold Spring Harb Symp Quant Biol. 1976;40:49–63. doi: 10.1101/sqb.1976.040.01.008. [DOI] [PubMed] [Google Scholar]
- Paul D. L., Goodenough D. A. Preparation, characterization, and localization of antisera against bovine MP26, an integral protein from lens fiber plasma membrane. J Cell Biol. 1983 Mar;96(3):625–632. doi: 10.1083/jcb.96.3.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson B. Changes in the lens related to the reduction of transparency. Exp Eye Res. 1973 Jun;16(1):29–39. doi: 10.1016/0014-4835(73)90234-0. [DOI] [PubMed] [Google Scholar]
- Ramaekers F. C., Selten-Versteegen A. M., Bloemendal H. Interaction of newly synthesized alpha-crystallin with isolated lens plasma membranes. Biochim Biophys Acta. 1980 Feb 15;596(1):57–63. doi: 10.1016/0005-2736(80)90170-4. [DOI] [PubMed] [Google Scholar]
- Rose B., Loewenstein W. R. Permeability of cell junction depends on local cytoplasmic calcium activity. Nature. 1975 Mar 20;254(5497):250–252. doi: 10.1038/254250a0. [DOI] [PubMed] [Google Scholar]
- Roy D., Rosenfeld L., Spector A. Lens plasma membrane: isolation and biochemical characterization. Exp Eye Res. 1982 Aug;35(2):113–129. doi: 10.1016/s0014-4835(82)80060-2. [DOI] [PubMed] [Google Scholar]
- Russell P., Robison W. G., Jr, Kinoshita J. H. A new method for rapid isolation of the intrinsic membrane proteins from lens. Exp Eye Res. 1981 Apr;32(4):511–516. doi: 10.1016/s0014-4835(81)80030-9. [DOI] [PubMed] [Google Scholar]
- Unakar N. J., Genyea C., Reddan J. R., Reddy V. N. Ultrastructural changes during the development and reversal of galactose cataracts. Exp Eye Res. 1978 Feb;26(2):123–133. doi: 10.1016/0014-4835(78)90109-4. [DOI] [PubMed] [Google Scholar]
- Welsh M. J., Aster J. C., Ireland M., Alcala J., Maisel H. Calmodulin binds to chick lens gap junction protein in a calcium-independent manner. Science. 1982 May 7;216(4546):642–644. doi: 10.1126/science.6280283. [DOI] [PubMed] [Google Scholar]
- Wong M. M., Robertson N. P., Horwitz J. Heat induced aggregation of the sodium dodecyl sulfate-solubilized main intrinsic polypeptide isolated from bovine lens plasma membrane. Biochem Biophys Res Commun. 1978 Sep 14;84(1):158–165. doi: 10.1016/0006-291x(78)90277-2. [DOI] [PubMed] [Google Scholar]