Abstract
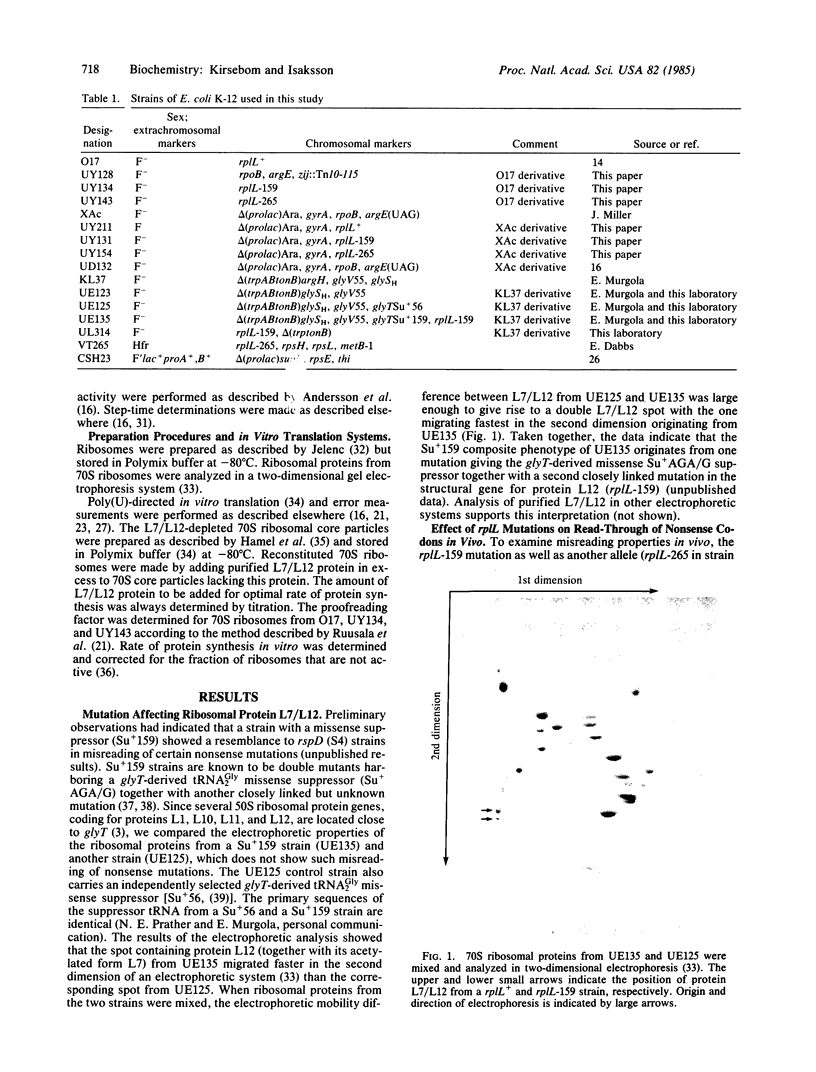
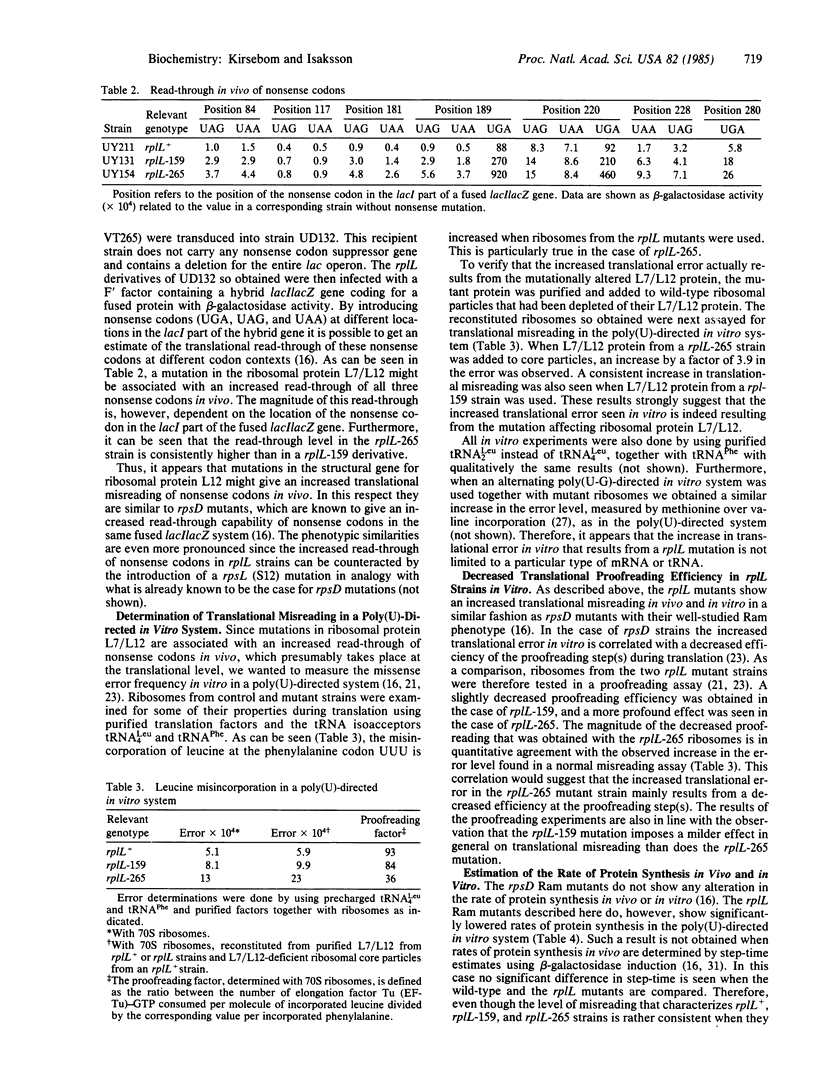
The effects of two mutations, which map at the rplL locus and both give a changed 50S ribosomal protein L7/L12, were studied. Both mutations are associated with an increased misreading of all three nonsense codons in vivo and ribosomes from the mutants give an increased misreading of the phenylalanine codon UUU by tRNALeu in vitro. The rplL-associated misreading in vitro is not limited to a particular type of mRNA or tRNA. Results from a translational proofreading assay, using mutant ribosomes, suggest that protein L7/L12 is involved in the control of translational accuracy by contributing to the efficiency of a translational proofreading step(s).
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersson D. I., Bohman K., Isaksson L. A., Kurland C. G. Translation rates and misreading characteristics of rpsD mutants in Escherichia coli. Mol Gen Genet. 1982;187(3):467–472. doi: 10.1007/BF00332630. [DOI] [PubMed] [Google Scholar]
- Andersson D. I., Kurland C. G. Ram ribosomes are defective proofreaders. Mol Gen Genet. 1983;191(3):378–381. doi: 10.1007/BF00425749. [DOI] [PubMed] [Google Scholar]
- Andersson S. G., Buckingham R. H., Kurland C. G. Does codon composition influence ribosome function? EMBO J. 1984 Jan;3(1):91–94. doi: 10.1002/j.1460-2075.1984.tb01766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann B. J. Linkage map of Escherichia coli K-12, edition 7. Microbiol Rev. 1983 Jun;47(2):180–230. doi: 10.1128/mr.47.2.180-230.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollen A., Cabezón T., de Wilde M., Villarroel R., Herzog A. Alteration of ribosomal protein S17 by mutation linked to neamine resistance in Escherichia coli. I. General properties of neaA mutants. J Mol Biol. 1975 Dec 25;99(4):795–806. doi: 10.1016/s0022-2836(75)80185-9. [DOI] [PubMed] [Google Scholar]
- Cabezón T., Herzog A., De Wilde M., Villarroel R., Bollen A. Cooperative control of translational fidelity by ribosomal proteins in Escherichia coli. III. A ram mutation in the structural gene for protein S5 (rpx E). Mol Gen Genet. 1976 Feb 27;144(1):59–62. doi: 10.1007/BF00277305. [DOI] [PubMed] [Google Scholar]
- Dabbs E. R. Escherichia coli kasugamycin dependence arising from mutation at the rpsI locus. J Bacteriol. 1983 Feb;153(2):709–715. doi: 10.1128/jb.153.2.709-715.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dabbs E. R., Hasenbank R., Kastner B., Rak K. H., Wartusch B., Stöffler G. Immunological studies of Escherichia coli mutants lacking one or two ribosomal proteins. Mol Gen Genet. 1983;192(3):301–308. doi: 10.1007/BF00392166. [DOI] [PubMed] [Google Scholar]
- Galas D. J., Branscomb E. W. Ribosome slowed by mutation to streptomycin resistance. Nature. 1976 Aug 12;262(5569):617–619. doi: 10.1038/262617b0. [DOI] [PubMed] [Google Scholar]
- Hamel E., Koka M., Nakamoto T. Requirement of an Escherichia coli 50 S ribosomal protein component for effective interaction of the ribosome with T and G factors and with guanosine triphosphate. J Biol Chem. 1972 Feb 10;247(3):805–814. [PubMed] [Google Scholar]
- Hill C. W., Foulds J., Soll L., Berg P. Instability of a missense suppressor resulting from a duplication of genetic material. J Mol Biol. 1969 Feb 14;39(3):563–581. doi: 10.1016/0022-2836(69)90146-6. [DOI] [PubMed] [Google Scholar]
- Hopfield J. J. Kinetic proofreading: a new mechanism for reducing errors in biosynthetic processes requiring high specificity. Proc Natl Acad Sci U S A. 1974 Oct;71(10):4135–4139. doi: 10.1073/pnas.71.10.4135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Isaksson L. A., Sköld S. E., Skjöldebrand J., Takata R. A procedure for isolation of spontaneous mutants with temperature sensitive of RNA and/or protein. Mol Gen Genet. 1977 Nov 18;156(3):233–237. doi: 10.1007/BF00267177. [DOI] [PubMed] [Google Scholar]
- Isono S., Isono K. Mutations affecting the structural genes and the genes coding for modifying enzymes for ribosomal proteins in Escherichia coli. Mol Gen Genet. 1978 Sep 20;165(1):15–20. doi: 10.1007/BF00270371. [DOI] [PubMed] [Google Scholar]
- Jelenc P. C., Kurland C. G. Nucleoside triphosphate regeneration decreases the frequency of translation errors. Proc Natl Acad Sci U S A. 1979 Jul;76(7):3174–3178. doi: 10.1073/pnas.76.7.3174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jelenc P. C. Rapid purification of highly active ribosomes from Escherichia coli. Anal Biochem. 1980 Jul 1;105(2):369–374. doi: 10.1016/0003-2697(80)90472-8. [DOI] [PubMed] [Google Scholar]
- Kalnins A., Otto K., Rüther U., Müller-Hill B. Sequence of the lacZ gene of Escherichia coli. EMBO J. 1983;2(4):593–597. doi: 10.1002/j.1460-2075.1983.tb01468.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kühberger R., Piepersberg W., Petzet A., Buckel P., Böck A. Alteration of ribosomal protein L6 in gentamicin-resistant strains of Escherichia coli. Effects on fidelity of protein synthesis. Biochemistry. 1979 Jan 9;18(1):187–193. doi: 10.1021/bi00568a028. [DOI] [PubMed] [Google Scholar]
- Leijonmarck M., Eriksson S., Liljas A. Crystal structure of a ribosomal component at 2.6 A resolution. Nature. 1980 Aug 21;286(5775):824–826. doi: 10.1038/286824a0. [DOI] [PubMed] [Google Scholar]
- Liljas A. Structural studies of ribosomes. Prog Biophys Mol Biol. 1982;40(3):161–228. doi: 10.1016/0079-6107(82)90013-x. [DOI] [PubMed] [Google Scholar]
- Madjar J. J., Michel S., Cozzone A. J., Reboud J. P. A method to identify individual proteins in four different two-dimensional gel electrophoresis systems: application to Escherichia coli ribosomal proteins. Anal Biochem. 1979 Jan 1;92(1):174–182. doi: 10.1016/0003-2697(79)90641-9. [DOI] [PubMed] [Google Scholar]
- Miller J. H., Coulondre C., Farabaugh P. J. Correlation of nonsense sites in the lacI gene with specific codons in the nucleotide sequence. Nature. 1978 Aug 24;274(5673):770–775. doi: 10.1038/274770a0. [DOI] [PubMed] [Google Scholar]
- Murgola E. J., Pagel F. T. Codon recognition by glycine transfer RNAs of Escherichia coli in vivo. J Mol Biol. 1980 Apr 25;138(4):833–844. doi: 10.1016/0022-2836(80)90067-4. [DOI] [PubMed] [Google Scholar]
- Neidhardt F. C., Bloch P. L., Smith D. F. Culture medium for enterobacteria. J Bacteriol. 1974 Sep;119(3):736–747. doi: 10.1128/jb.119.3.736-747.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ninio J. Kinetic amplification of enzyme discrimination. Biochimie. 1975;57(5):587–595. doi: 10.1016/s0300-9084(75)80139-8. [DOI] [PubMed] [Google Scholar]
- Olsson M. O., Isaksson L. A. Analysis of rpsD mutations in Escherichia coli. I. Comparison of mutants with various alterations in ribosomal protein S4. Mol Gen Genet. 1979 Feb 1;169(3):251–257. doi: 10.1007/BF00382271. [DOI] [PubMed] [Google Scholar]
- Olsson M. O., Isaksson L. A. Analysis of rpsD mutations in Escherichia coli. IV. Accumulation of minor forms of protein S7(K) in ribosomes of rpsD mutant strains due to translational read-through. Mol Gen Genet. 1980 Feb;177(3):485–491. doi: 10.1007/BF00271488. [DOI] [PubMed] [Google Scholar]
- Piepersberg W., Böck A., Wittmann H. G. Effect of different mutations in ribosomal protein S5 of Escherichia coli on translational fidelity. Mol Gen Genet. 1975 Sep 29;140(2):91–100. doi: 10.1007/BF00329777. [DOI] [PubMed] [Google Scholar]
- Piepersberg W., Noseda V., Böck A. Bacterial ribosomes with two ambiguity mutations: effects of translational fidelity, on the response to aminoglycosides and on the rate of protein synthesis. Mol Gen Genet. 1979 Mar 9;171(1):23–34. doi: 10.1007/BF00274011. [DOI] [PubMed] [Google Scholar]
- Prince J. B., Taylor B. H., Thurlow D. L., Ofengand J., Zimmermann R. A. Covalent crosslinking of tRNA1Val to 16S RNA at the ribosomal P site: identification of crosslinked residues. Proc Natl Acad Sci U S A. 1982 Sep;79(18):5450–5454. doi: 10.1073/pnas.79.18.5450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruusala T., Ehrenberg M., Kurland C. G. Is there proofreading during polypeptide synthesis? EMBO J. 1982;1(6):741–745. doi: 10.1002/j.1460-2075.1982.tb01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleif R., Hess W., Finkelstein S., Ellis D. Induction kinetics of the L-arabinose operon of Escherichia coli. J Bacteriol. 1973 Jul;115(1):9–14. doi: 10.1128/jb.115.1.9-14.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson R. C., Karim A. M. The accuracy of protein biosynthesis is limited by its speed: high fidelity selection by ribosomes of aminoacyl-tRNA ternary complexes containing GTP[gamma S]. Proc Natl Acad Sci U S A. 1982 Aug;79(16):4922–4926. doi: 10.1073/pnas.79.16.4922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagner E. G., Jelenc P. C., Ehrenberg M., Kurland C. G. Rate of elongation of polyphenylalanine in vitro. Eur J Biochem. 1982 Feb;122(1):193–197. doi: 10.1111/j.1432-1033.1982.tb05866.x. [DOI] [PubMed] [Google Scholar]
- Watson R. J., Parker J., Fiil N. P., Flaks J. G., Friesen J. D. New chromosomal location for structural genes of ribosomal proteins. Proc Natl Acad Sci U S A. 1975 Jul;72(7):2765–2769. doi: 10.1073/pnas.72.7.2765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yates J. L. Role of ribosomal protein S12 in discrimination of aminoacyl-tRNA. J Biol Chem. 1979 Nov 25;254(22):11550–11554. [PubMed] [Google Scholar]
- Zengel J. M., Young R., Dennis P. P., Nomura M. Role of ribosomal protein S12 in peptide chain elongation: analysis of pleiotropic, streptomycin-resistant mutants of Escherichia coli. J Bacteriol. 1977 Mar;129(3):1320–1329. doi: 10.1128/jb.129.3.1320-1329.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]




