Abstract
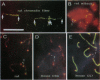
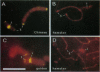
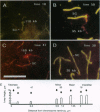
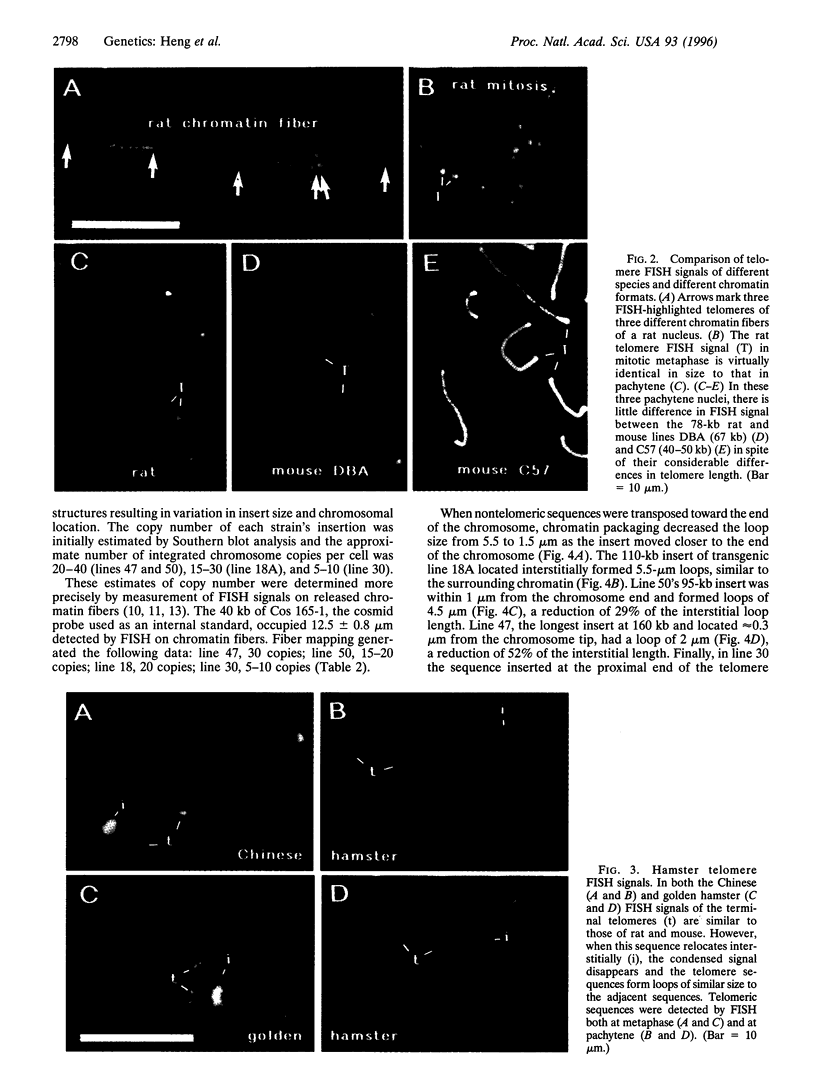
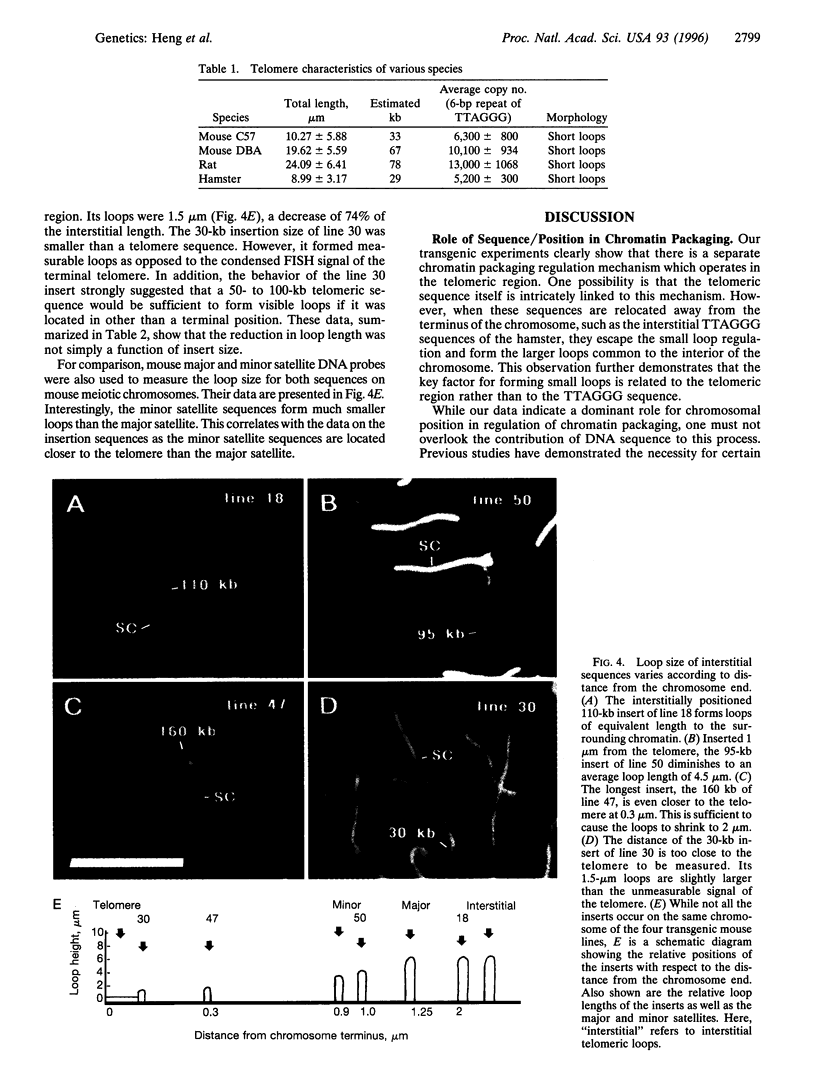
At meiotic prophase, chromatin loops around a proteinaceous core, with the sizes of these loops varying between species. Comparison of the morphology of sequence-related inserts at different sites in transgenic mice demonstrates that loop size also varies with chromosomal geography. Similarly, chromatin loop lengths differ dramatically for interstitially and terminally located hamster telomeric sequences. Sequences, telomeric or otherwise, located at chromosome termini, closely associate with the meiotic proteinaceous core, forming shorter loops than identical interstitial sequences. Thus, we present evidence that different chromatin packaging mechanisms exist for interstitial versus terminal chromosomal regions, which act separately from those operating at the level of the DNA sequence. Chromosomal position plays the dominant role in chromatin packaging.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashley T. Mammalian meiotic recombination: a reexamination. Hum Genet. 1994 Dec;94(6):587–593. doi: 10.1007/BF00206950. [DOI] [PubMed] [Google Scholar]
- Blackburn E. H. Telomeres: no end in sight. Cell. 1994 Jun 3;77(5):621–623. doi: 10.1016/0092-8674(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Chamberlain J. W., Nolan J. A., Conrad P. J., Vasavada H. A., Vasavada H. H., Ploegh H. L., Ganguly S., Janeway C. A., Jr, Weissman S. M. Tissue-specific and cell surface expression of human major histocompatibility complex class I heavy (HLA-B7) and light (beta 2-microglobulin) chain genes in transgenic mice. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7690–7694. doi: 10.1073/pnas.85.20.7690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Counce S. J., Meyer G. F. Differentiation of the synaptonemal complex and the kinetochore in Locusta spermatocytes studied by whole mount electron microscopy. Chromosoma. 1973 Nov 21;44(2):231–253. doi: 10.1007/BF00329119. [DOI] [PubMed] [Google Scholar]
- Dobson M. J., Pearlman R. E., Karaiskakis A., Spyropoulos B., Moens P. B. Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J Cell Sci. 1994 Oct;107(Pt 10):2749–2760. doi: 10.1242/jcs.107.10.2749. [DOI] [PubMed] [Google Scholar]
- Fidlerová H., Senger G., Kost M., Sanseau P., Sheer D. Two simple procedures for releasing chromatin from routinely fixed cells for fluorescence in situ hybridization. Cytogenet Cell Genet. 1994;65(3):203–205. doi: 10.1159/000133632. [DOI] [PubMed] [Google Scholar]
- Gottschling D. E. Telomere-proximal DNA in Saccharomyces cerevisiae is refractory to methyltransferase activity in vivo. Proc Natl Acad Sci U S A. 1992 May 1;89(9):4062–4065. doi: 10.1073/pnas.89.9.4062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng H. H., Squire J., Tsui L. C. High-resolution mapping of mammalian genes by in situ hybridization to free chromatin. Proc Natl Acad Sci U S A. 1992 Oct 15;89(20):9509–9513. doi: 10.1073/pnas.89.20.9509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng H. H., Tsui L. C. Modes of DAPI banding and simultaneous in situ hybridization. Chromosoma. 1993 May;102(5):325–332. doi: 10.1007/BF00661275. [DOI] [PubMed] [Google Scholar]
- Heng H. H., Tsui L. C., Moens P. B. Organization of heterologous DNA inserts on the mouse meiotic chromosome core. Chromosoma. 1994 Oct;103(6):401–407. doi: 10.1007/BF00362284. [DOI] [PubMed] [Google Scholar]
- Kipling D., Cooke H. J. Hypervariable ultra-long telomeres in mice. Nature. 1990 Sep 27;347(6291):400–402. doi: 10.1038/347400a0. [DOI] [PubMed] [Google Scholar]
- Li S., Meistrich M. L., Brock W. A., Hsu T. C., Kuo M. T. Isolation and preliminary characterization of the synaptonemal complex from rat pachytene spermatocytes. Exp Cell Res. 1983 Mar;144(1):63–72. doi: 10.1016/0014-4827(83)90442-1. [DOI] [PubMed] [Google Scholar]
- Makarov V. L., Lejnine S., Bedoyan J., Langmore J. P. Nucleosomal organization of telomere-specific chromatin in rat. Cell. 1993 May 21;73(4):775–787. doi: 10.1016/0092-8674(93)90256-p. [DOI] [PubMed] [Google Scholar]
- Moens P. B., Moens T. Computer measurements and graphics of three-dimensional cellular ultrastructure. J Ultrastruct Res. 1981 May;75(2):131–141. doi: 10.1016/s0022-5320(81)80129-3. [DOI] [PubMed] [Google Scholar]
- Moens P. B., Pearlman R. E. Satellite DNA I in chromatin loops of rat pachytene chromosomes and in spermatids. Chromosoma. 1989 Oct;98(4):287–294. doi: 10.1007/BF00327315. [DOI] [PubMed] [Google Scholar]
- Moens P. B., Pearlman R. E. Telomere and centromere DNA are associated with the cores of meiotic prophase chromosomes. Chromosoma. 1990 Dec;100(1):8–14. doi: 10.1007/BF00337598. [DOI] [PubMed] [Google Scholar]
- Paulson J. R., Laemmli U. K. The structure of histone-depleted metaphase chromosomes. Cell. 1977 Nov;12(3):817–828. doi: 10.1016/0092-8674(77)90280-x. [DOI] [PubMed] [Google Scholar]
- Pearlman R. E., Tsao N., Moens P. B. Synaptonemal complexes from DNase-treated rat pachytene chromosomes contain (GT)n and LINE/SINE sequences. Genetics. 1992 Apr;130(4):865–872. doi: 10.1093/genetics/130.4.865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tommerup H., Dousmanis A., de Lange T. Unusual chromatin in human telomeres. Mol Cell Biol. 1994 Sep;14(9):5777–5785. doi: 10.1128/mcb.14.9.5777. [DOI] [PMC free article] [PubMed] [Google Scholar]