Abstract
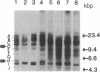
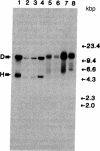
DNA of Marek disease virus (MDV) consists of two unique regions UL and US flanked by long inverted repeat regions TRL and IRL, and short inverted repeat regions TRS and IRS, respectively, similar to herpes simplex virus DNA. Comparison of restriction patterns between pathogenic and nonpathogenic MDV DNA was made to identify a region of viral DNA different between these two types of MDV, as it may be responsible for the tumorigenicity of MDV in chickens. The results indicated that BamHI-D and -H, located at the long inverted repeat regions TRL and IRL, were specifically expanded in nonpathogenic viral DNA. The location of the expanded region has been determined within 1.5 kilobase pairs of the Bgl I/Pst I fragment of BamHI-D and -H, close to the junction between the inverted repeat and the unique region. The possibility that a gene responsible for tumor induction may be disrupted by such expansion has been discussed.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bornkamm G. W., Delius H., Fleckenstein B., Werner F. J., Mulder C. Structure of Herpesvirus saimiri genomes: arrangement of heavy and light sequences in the M genome. J Virol. 1976 Jul;19(1):154–161. doi: 10.1128/jvi.19.1.154-161.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill A. E., Chubb R. C., Baxendale W. The attenuation, with loss of oncogenicity, of the herpes-type virus of Marek's disease (strain HPRS-16) on passage in cell culture. J Gen Virol. 1969 Jun;4(4):557–564. doi: 10.1099/0022-1317-4-4-557. [DOI] [PubMed] [Google Scholar]
- Churchill A. E., Payne L. N., Chubb R. C. Immunization against Marek's disease using a live attenuated virus. Nature. 1969 Feb 22;221(5182):744–747. doi: 10.1038/221744a0. [DOI] [PubMed] [Google Scholar]
- Coleman R. M., Schierman L. W. Transplantable Marek's disease lymphomas. I. Growth characteristics during development in two inbred lines of chickens. Avian Dis. 1982 Apr-Jun;26(2):245–256. [PubMed] [Google Scholar]
- Eidson C. S., Schmittle S. C. Studies on acute Marek's disease. I. Characteristics of isolate GA in chickens. Avian Dis. 1968 Aug;12(3):467–476. [PubMed] [Google Scholar]
- Frenkeĺ N., Locker H., Batterson W., Hayward G. S., Roizman B. Anatomy of herpes simplex virus DNA. VI. Defective DNA originates from the S component. J Virol. 1976 Nov;20(2):527–531. doi: 10.1128/jvi.20.2.527-531.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukuchi K., Sudo M., Lee Y. S., Tanaka A., Nonoyama M. Structure of Marek's disease virus DNA: detailed restriction enzyme map. J Virol. 1984 Jul;51(1):102–109. doi: 10.1128/jvi.51.1.102-109.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galloway D. A., McDougall J. K. The oncogenic potential of herpes simplex viruses: evidence for a 'hit-and-run' mechanism. Nature. 1983 Mar 3;302(5903):21–24. doi: 10.1038/302021a0. [DOI] [PubMed] [Google Scholar]
- Henry B. E., Newcomb W. W., O'Callaghan D. J. Biological and biochemical properties of defective interfering particles of equine herpesvirus type 1. Virology. 1979 Jan 30;92(2):495–506. doi: 10.1016/0042-6822(79)90152-1. [DOI] [PubMed] [Google Scholar]
- Hirai K., Honma H., Ikuta K., Kato S. Genetic relatedness of virulent and avirulent strains of Marek's disease virus. Arch Virol. 1984;79(3-4):293–298. doi: 10.1007/BF01310818. [DOI] [PubMed] [Google Scholar]
- Hirai K., Ikuta K., Kato S. Restriction endonuclease analysis of the genomes of virulent and avirulent Marek's disease viruses. Microbiol Immunol. 1981;25(7):671–681. doi: 10.1111/j.1348-0421.1981.tb00070.x. [DOI] [PubMed] [Google Scholar]
- Hirai K., Ikuta K., Kato S. Structural changes in the DNA of Marek's disease virus during serial passage in cultured cells. Virology. 1981 Dec;115(2):385–389. doi: 10.1016/0042-6822(81)90119-7. [DOI] [PubMed] [Google Scholar]
- Kaerner H. C., Maichle I. B., Ott A., Schröder C. H. Origin of two different classes of defective HSV-1 Angelotti DNA. Nucleic Acids Res. 1979 Apr;6(4):1467–1478. doi: 10.1093/nar/6.4.1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koomey J. M., Mulder C., Burghoff R. L., Fleckenstein B., Desrosiers R. C. Deletion of DNA sequence in a nononcogenic variant of Herpesvirus saimiri. J Virol. 1984 May;50(2):662–665. doi: 10.1128/jvi.50.2.662-665.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Rispens B. H., van Vloten H., Mastenbroek N., Maas H. J., Schat K. A. Control of Marek's disease in the Netherlands. I. Isolation of an avirulent Marek's disease virus (strain CVI 988) and its use in laboratory vaccination trials. Avian Dis. 1972 Apr;16(1):108–125. [PubMed] [Google Scholar]
- Rixon F. J., Ben-Porat T. Structural evolution of the DNA of pseudorabies-defective viral particles. Virology. 1979 Aug;97(1):151–163. doi: 10.1016/0042-6822(79)90381-7. [DOI] [PubMed] [Google Scholar]
- Roizman B. The structure and isomerization of herpes simplex virus genomes. Cell. 1979 Mar;16(3):481–494. doi: 10.1016/0092-8674(79)90023-0. [DOI] [PubMed] [Google Scholar]
- Schaffer P. A., Falk L. A., Deinhardt F. Attenuation of herpesvirus saimiri for marmosets after successive passage in cell culture at 39 degrees C. J Natl Cancer Inst. 1975 Nov;55(5):1243–1246. doi: 10.1093/jnci/55.5.1243. [DOI] [PubMed] [Google Scholar]
- Southern E. M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975 Nov 5;98(3):503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- Stinski M. F., Mocarski E. S., Thomsen D. R. DNA of human cytomegalovirus: size heterogeneity and defectiveness resulting from serial undiluted passage. J Virol. 1979 Jul;31(1):231–239. doi: 10.1128/jvi.31.1.231-239.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka A., Lee Y. S., Nonoyama M. Heterogeneous population of virus DNA in serially passaged Marek's disease virus preparation. Virology. 1980 Jun;103(2):510–513. doi: 10.1016/0042-6822(80)90209-3. [DOI] [PubMed] [Google Scholar]