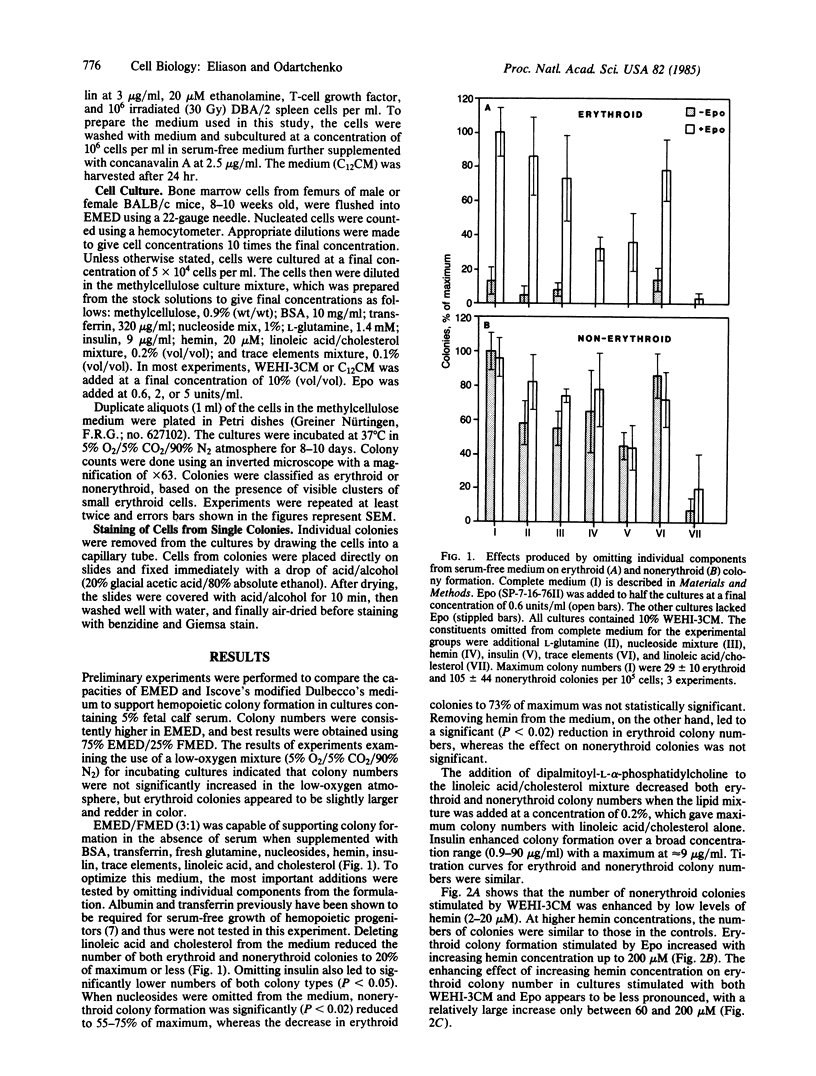
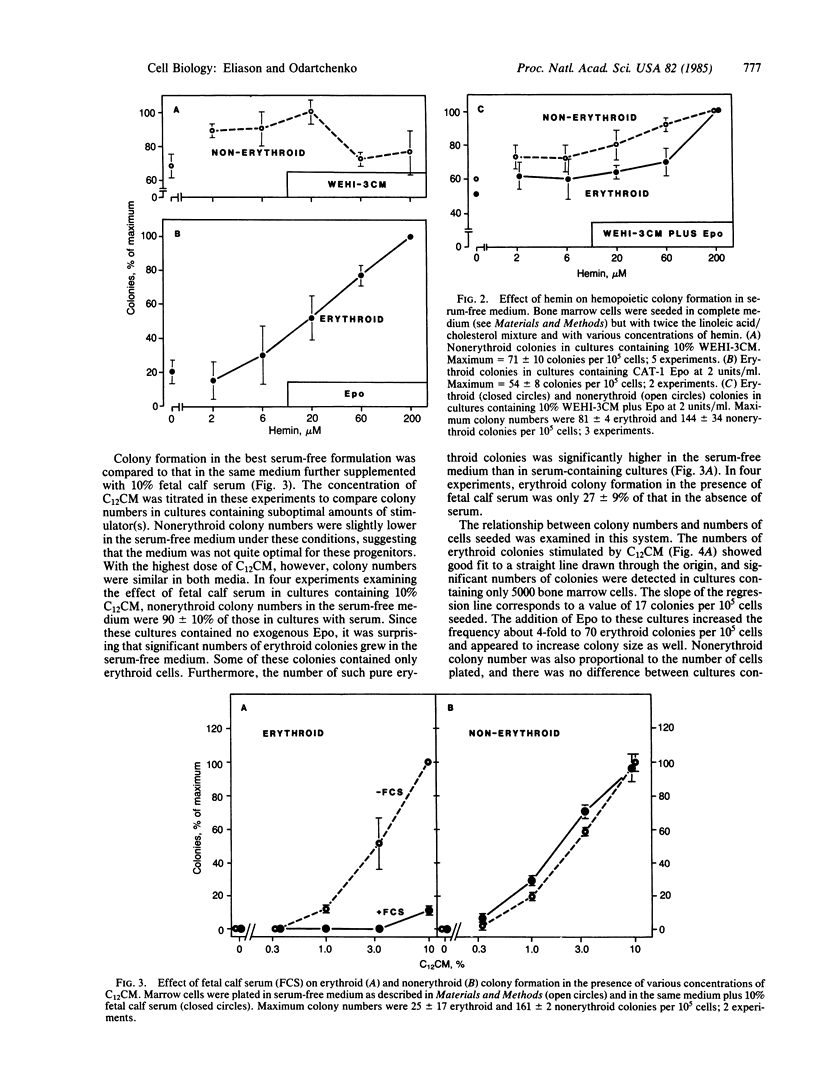
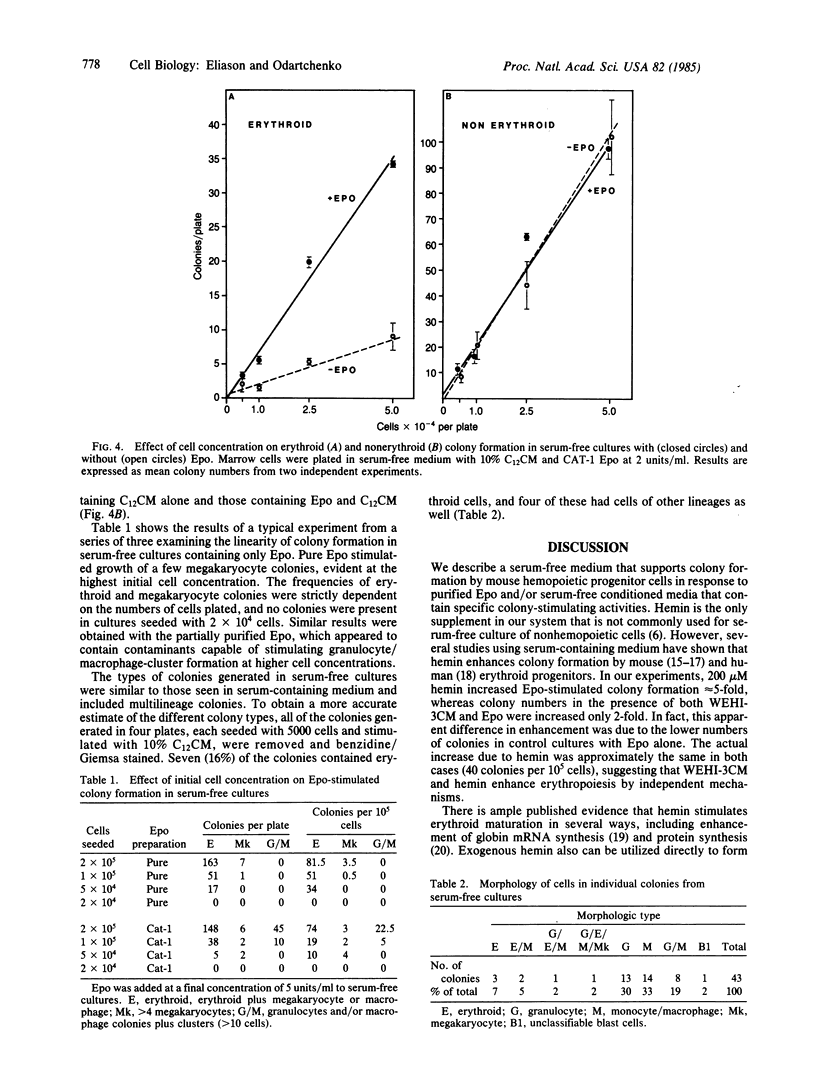
Abstract
A serum-free medium has been developed for clonal growth of murine hemopoietic progenitor cells. In this medium, the number of nonerythroid colonies induced by factors produced by cloned T lymphocytes was 90 +/- 10% of the number generated in serum-containing medium. Erythroid colony number in serum-free cultures containing the T-cell factors but no exogenous erythropoietin was significantly higher than that in cultures with serum, and the cloning efficiency was independent of cell concentration. Further addition of erythropoietin increased erythroid colony number approximately equal to 4-fold. Pure erythropoietin alone stimulated erythroid colony formation, but the cloning efficiency was highly dependent on cell concentration. Analysis of individual colonies generated in serum-free cultures containing the T-cell factors indicated that some contained cells of several hemopoietic lineages, demonstrating that multipotential progenitors can give rise to colonies in this system.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barnes D., Sato G. Methods for growth of cultured cells in serum-free medium. Anal Biochem. 1980 Mar 1;102(2):255–270. doi: 10.1016/0003-2697(80)90151-7. [DOI] [PubMed] [Google Scholar]
- Burgess A. W., Camakaris J., Metcalf D. Purification and properties of colony-stimulating factor from mouse lung-conditioned medium. J Biol Chem. 1977 Mar 25;252(6):1998–2003. [PubMed] [Google Scholar]
- Eliason J. F., Fekete A., Odartchenko N. Improving techniques for clonogenic assays. Recent Results Cancer Res. 1984;94:267–275. doi: 10.1007/978-3-642-82295-7_29. [DOI] [PubMed] [Google Scholar]
- Eliason J. F. Long-term production of hemopoietic progenitors in cultures containing low levels of serum. Exp Hematol. 1984 Aug;12(7):559–567. [PubMed] [Google Scholar]
- Eliason J. F., Van Zant G. Erythropoietin-dependent and erythropoietin-independent enhancement of colony formation by immature erythroid progenitors (BFUe). Cell Tissue Kinet. 1983 Jan;16(1):65–76. [PubMed] [Google Scholar]
- Fagg B. Is erythropoietin the only factor which regulates late erythroid differentiation? Nature. 1981 Jan 15;289(5794):184–186. doi: 10.1038/289184a0. [DOI] [PubMed] [Google Scholar]
- Gross M. Control of protein synthesis by hemin. Evidence that the hemin-controlled translational repressor inhibits formation of 80 S initiation complexes from 48 S intermediate initiation complexes. J Biol Chem. 1979 Apr 10;254(7):2370–2377. [PubMed] [Google Scholar]
- Holden S. A., Steinberg H. N., Matzinger E. A., Monette F. C. Further characterization of the hemin-induced enhancement of primitive erythroid progenitor cell growth in vitro. Exp Hematol. 1983 Nov;11(10):953–960. [PubMed] [Google Scholar]
- Ihle J. N., Keller J., Oroszlan S., Henderson L. E., Copeland T. D., Fitch F., Prystowsky M. B., Goldwasser E., Schrader J. W., Palaszynski E. Biologic properties of homogeneous interleukin 3. I. Demonstration of WEHI-3 growth factor activity, mast cell growth factor activity, p cell-stimulating factor activity, colony-stimulating factor activity, and histamine-producing cell-stimulating factor activity. J Immunol. 1983 Jul;131(1):282–287. [PubMed] [Google Scholar]
- Iscove N. N., Guilbert L. J., Weyman C. Complete replacement of serum in primary cultures of erythropoietin-dependent red cell precursors (CFU-E) by albumin, transferrin, iron, unsaturated fatty acid, lecithin and cholesterol. Exp Cell Res. 1980 Mar;126(1):121–126. doi: 10.1016/0014-4827(80)90476-0. [DOI] [PubMed] [Google Scholar]
- Kelso A., Glasebrook A. L., Kanagawa O., Brunner K. T. Production of macrophage-activating factor by T lymphocyte clones and correlation with other lymphokine activities. J Immunol. 1982 Aug;129(2):550–556. [PubMed] [Google Scholar]
- Kubota K., Motoyoshi K., Kajigaya S., Suda T., Saito M., Miura Y. Morphological examinations of murine granulocyte/macrophage colonies grown in serum-free cultures. Exp Hematol. 1983 May;11(5):364–370. [PubMed] [Google Scholar]
- Kurtz A., Jelkmann W., Bauer C. A new candidate for the regulation of erythropoiesis. Insulin-like growth factor I. FEBS Lett. 1982 Nov 22;149(1):105–108. doi: 10.1016/0014-5793(82)81081-8. [DOI] [PubMed] [Google Scholar]
- Kurtz A., Jelkmann W., Bauer C. Insulin stimulates erythroid colony formation independently of erythropoietin. Br J Haematol. 1983 Feb;53(2):311–316. doi: 10.1111/j.1365-2141.1983.tb02025.x. [DOI] [PubMed] [Google Scholar]
- Lu L., Broxmeyer H. E. The selective enhancing influence of hemin and products of human erythrocytes on colony formation by human multipotential (CFUGEMM) and erythroid (BFUE) progenitor cells in vitro. Exp Hematol. 1983 Sep;11(8):721–729. [PubMed] [Google Scholar]
- Malik Z., Bessler H., Djaldetti M. The role of hemin in the regulation of heme synthesis by fetal mouse liver erythroblasts in culture. Exp Hematol. 1979 Apr;7(4):183–188. [PubMed] [Google Scholar]
- Miyake T., Kung C. K., Goldwasser E. Purification of human erythropoietin. J Biol Chem. 1977 Aug 10;252(15):5558–5564. [PubMed] [Google Scholar]
- Nicola N. A., Metcalf D., Matsumoto M., Johnson G. R. Purification of a factor inducing differentiation in murine myelomonocytic leukemia cells. Identification as granulocyte colony-stimulating factor. J Biol Chem. 1983 Jul 25;258(14):9017–9023. [PubMed] [Google Scholar]
- Porter P. N., Meints R. H., Mesner K. Enhancement of erythroid colony growth in culture by hemin. Exp Hematol. 1979 Jan;7(1):11–16. [PubMed] [Google Scholar]
- Rich I. N., Heit W., Kubanek B. Extrarenal erythropoietin production by macrophages. Blood. 1982 Oct;60(4):1007–1018. [PubMed] [Google Scholar]
- Ross J., Sautner D. Induction of globin mRNA accumulation by hemin in cultured erythroleukemic cells. Cell. 1976 Aug;8(4):513–520. doi: 10.1016/0092-8674(76)90219-1. [DOI] [PubMed] [Google Scholar]
- Rothmann J., Hertogs C. F., Malik Z., Pluznik D. H. Hemin stimulating effect on colony formation of leukemic and bone marrow cells. Exp Hematol. 1983 Feb;11(2):147–153. [PubMed] [Google Scholar]
- Stanley E. R., Heard P. M. Factors regulating macrophage production and growth. Purification and some properties of the colony stimulating factor from medium conditioned by mouse L cells. J Biol Chem. 1977 Jun 25;252(12):4305–4312. [PubMed] [Google Scholar]
- Stewart S., Zhu B., Axelrad A. A "serum-free" medium for the production of erythropoietic bursts by murine bone marrow cells. Exp Hematol. 1984 Jun;12(5):309–318. [PubMed] [Google Scholar]
- Wagemaker G., Peters M. F., Bol S. J. Induction of erythropoietin responsiveness in vitro by a distinct population of bone marrow cells. Cell Tissue Kinet. 1979 Sep;12(5):521–537. doi: 10.1111/j.1365-2184.1979.tb00174.x. [DOI] [PubMed] [Google Scholar]
- Worton R. G., McCulloch E. A., Till J. E. Physical separation of hemopoietic stem cells from cells forming colonies in culture. J Cell Physiol. 1969 Oct;74(2):171–182. doi: 10.1002/jcp.1040740209. [DOI] [PubMed] [Google Scholar]


