Abstract
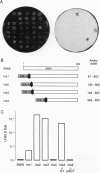
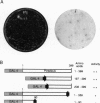
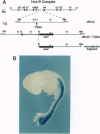
Transposon Tn1000 has been adapted to deliver novel DNA sequences for manipulating recombinant DNA. The transposition procedure for these "tagged" Tn1000s is simple and applicable to most plasmids in current use. For yeast molecular biology, tagged Tn1000s introduce a variety of yeast selective markers and replication origins into plasmids and cosmids. In addition, the beta-globin minimal promoter and lacZ gene of Tn(beta)lac serve as a mobile reporter of eukaryotic enhancer activity. In this paper, Tn(beta)lac was used to localize a mouse HoxB-complex enhancer in transgenic mice. Other tagged transposons create Gal4 DNA-binding-domain fusions, in either Escherichia coli or yeast plasmids, for use in one- and two-hybrid tests of transcriptional activation and protein-protein interaction, respectively. With such fusions, the Saccharomyces cerevisiae Swi6 G1/S-phase transcription factor and the Xenopus laevis Pintallavis developmental regulator are shown to activate transcription. Furthermore, the same transposon insertions also facilitated mapping of the Swi6 and Pintallavis domains responsible for transcriptional activation. Thus, as well as introducing novel sequences, tagged transposons share the numerous other applications of transposition such as producing insertional mutations, creating deletion series, or serving as mobile primer sites for DNA sequencing.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andrews B. J., Mason S. W. Gene expression and the cell cycle: a family affair. Science. 1993 Sep 17;261(5128):1543–1544. doi: 10.1126/science.8372349. [DOI] [PubMed] [Google Scholar]
- Apolinario E., Nocero M., Jin M., Hoffman C. S. Cloning and manipulation of the Schizosaccharomyces pombe his7+ gene as a new selectable marker for molecular genetic studies. Curr Genet. 1993 Dec;24(6):491–495. doi: 10.1007/BF00351711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berben G., Dumont J., Gilliquet V., Bolle P. A., Hilger F. The YDp plasmids: a uniform set of vectors bearing versatile gene disruption cassettes for Saccharomyces cerevisiae. Yeast. 1991 Jul;7(5):475–477. doi: 10.1002/yea.320070506. [DOI] [PubMed] [Google Scholar]
- Berg C. M., Vartak N. B., Wang G., Xu X., Liu L., MacNeil D. J., Gewain K. M., Wiater L. A., Berg D. E. The m gamma delta-1 element, a small gamma delta (Tn1000) derivative useful for plasmid mutagenesis, allele replacement and DNA sequencing. Gene. 1992 Apr 1;113(1):9–16. doi: 10.1016/0378-1119(92)90664-b. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Brent R., Ptashne M. A eukaryotic transcriptional activator bearing the DNA specificity of a prokaryotic repressor. Cell. 1985 Dec;43(3 Pt 2):729–736. doi: 10.1016/0092-8674(85)90246-6. [DOI] [PubMed] [Google Scholar]
- Broach J. R., Strathern J. N., Hicks J. B. Transformation in yeast: development of a hybrid cloning vector and isolation of the CAN1 gene. Gene. 1979 Dec;8(1):121–133. doi: 10.1016/0378-1119(79)90012-x. [DOI] [PubMed] [Google Scholar]
- Chevray P. M., Nathans D. Protein interaction cloning in yeast: identification of mammalian proteins that react with the leucine zipper of Jun. Proc Natl Acad Sci U S A. 1992 Jul 1;89(13):5789–5793. doi: 10.1073/pnas.89.13.5789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chien C. T., Bartel P. L., Sternglanz R., Fields S. The two-hybrid system: a method to identify and clone genes for proteins that interact with a protein of interest. Proc Natl Acad Sci U S A. 1991 Nov 1;88(21):9578–9582. doi: 10.1073/pnas.88.21.9578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Datta N., Hedges R. W. Trimethoprim resistance conferred by W plasmids in Enterobacteriaceae. J Gen Microbiol. 1972 Sep;72(2):349–355. doi: 10.1099/00221287-72-2-349. [DOI] [PubMed] [Google Scholar]
- Durfee T., Becherer K., Chen P. L., Yeh S. H., Yang Y., Kilburn A. E., Lee W. H., Elledge S. J. The retinoblastoma protein associates with the protein phosphatase type 1 catalytic subunit. Genes Dev. 1993 Apr;7(4):555–569. doi: 10.1101/gad.7.4.555. [DOI] [PubMed] [Google Scholar]
- Fields S., Song O. A novel genetic system to detect protein-protein interactions. Nature. 1989 Jul 20;340(6230):245–246. doi: 10.1038/340245a0. [DOI] [PubMed] [Google Scholar]
- Frasch M., Chen X., Lufkin T. Evolutionary-conserved enhancers direct region-specific expression of the murine Hoxa-1 and Hoxa-2 loci in both mice and Drosophila. Development. 1995 Apr;121(4):957–974. doi: 10.1242/dev.121.4.957. [DOI] [PubMed] [Google Scholar]
- Guyer M. S. The gamma delta sequence of F is an insertion sequence. J Mol Biol. 1978 Dec 15;126(3):347–365. doi: 10.1016/0022-2836(78)90045-1. [DOI] [PubMed] [Google Scholar]
- Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983 Jun 5;166(4):557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- Knöchel S., Lef J., Clement J., Klocke B., Hille S., Köster M., Knöchel W. Activin A induced expression of a fork head related gene in posterior chordamesoderm (notochord) of Xenopus laevis embryos. Mech Dev. 1992 Aug;38(2):157–165. doi: 10.1016/0925-4773(92)90007-7. [DOI] [PubMed] [Google Scholar]
- Krumlauf R. Hox genes in vertebrate development. Cell. 1994 Jul 29;78(2):191–201. doi: 10.1016/0092-8674(94)90290-9. [DOI] [PubMed] [Google Scholar]
- Lathe R., Vilotte J. L., Clark A. J. Plasmid and bacteriophage vectors for excision of intact inserts. Gene. 1987;57(2-3):193–201. doi: 10.1016/0378-1119(87)90122-3. [DOI] [PubMed] [Google Scholar]
- Liu L., Whalen W., Das A., Berg C. M. Rapid sequencing of cloned DNA using a transposon for bidirectional priming: sequence of the Escherichia coli K-12 avtA gene. Nucleic Acids Res. 1987 Nov 25;15(22):9461–9469. doi: 10.1093/nar/15.22.9461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall H., Studer M., Pöpperl H., Aparicio S., Kuroiwa A., Brenner S., Krumlauf R. A conserved retinoic acid response element required for early expression of the homeobox gene Hoxb-1. Nature. 1994 Aug 18;370(6490):567–571. doi: 10.1038/370567a0. [DOI] [PubMed] [Google Scholar]
- O'Kane C. J., Gehring W. J. Detection in situ of genomic regulatory elements in Drosophila. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9123–9127. doi: 10.1073/pnas.84.24.9123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pani L., Overdier D. G., Porcella A., Qian X., Lai E., Costa R. H. Hepatocyte nuclear factor 3 beta contains two transcriptional activation domains, one of which is novel and conserved with the Drosophila fork head protein. Mol Cell Biol. 1992 Sep;12(9):3723–3732. doi: 10.1128/mcb.12.9.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruiz i Altaba A., Jessell T. M. Pintallavis, a gene expressed in the organizer and midline cells of frog embryos: involvement in the development of the neural axis. Development. 1992 Sep;116(1):81–93. doi: 10.1242/dev.116.Supplement.81. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scherer S., Davis R. W. Replacement of chromosome segments with altered DNA sequences constructed in vitro. Proc Natl Acad Sci U S A. 1979 Oct;76(10):4951–4955. doi: 10.1073/pnas.76.10.4951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sedgwick S. G., Nguyen T. M., Ellis J. M., Crowne H., Morris G. E. Rapid mapping by transposon mutagenesis of epitopes on the muscular dystrophy protein, dystrophin. Nucleic Acids Res. 1991 Nov 11;19(21):5889–5894. doi: 10.1093/nar/19.21.5889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham M. H., Hunt P., Nonchev S., Papalopulu N., Graham A., Boncinelli E., Krumlauf R. Analysis of the murine Hox-2.7 gene: conserved alternative transcripts with differential distributions in the nervous system and the potential for shared regulatory regions. EMBO J. 1992 May;11(5):1825–1836. doi: 10.1002/j.1460-2075.1992.tb05234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sham M. H., Vesque C., Nonchev S., Marshall H., Frain M., Gupta R. D., Whiting J., Wilkinson D., Charnay P., Krumlauf R. The zinc finger gene Krox20 regulates HoxB2 (Hox2.8) during hindbrain segmentation. Cell. 1993 Jan 29;72(2):183–196. doi: 10.1016/0092-8674(93)90659-e. [DOI] [PubMed] [Google Scholar]
- Strausbaugh L. D., Bourke M. T., Sommer M. T., Coon M. E., Berg C. M. Probe mapping to facilitate transposon-based DNA sequencing. Proc Natl Acad Sci U S A. 1990 Aug;87(16):6213–6217. doi: 10.1073/pnas.87.16.6213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer M., Pöpperl H., Marshall H., Kuroiwa A., Krumlauf R. Role of a conserved retinoic acid response element in rhombomere restriction of Hoxb-1. Science. 1994 Sep 16;265(5179):1728–1732. doi: 10.1126/science.7916164. [DOI] [PubMed] [Google Scholar]
- Sundaresan V., Springer P., Volpe T., Haward S., Jones J. D., Dean C., Ma H., Martienssen R. Patterns of gene action in plant development revealed by enhancer trap and gene trap transposable elements. Genes Dev. 1995 Jul 15;9(14):1797–1810. doi: 10.1101/gad.9.14.1797. [DOI] [PubMed] [Google Scholar]
- Thomas S. M., Crowne H. M., Pidsley S. C., Sedgwick S. G. Structural characterization of the Salmonella typhimurium LT2 umu operon. J Bacteriol. 1990 Sep;172(9):4979–4987. doi: 10.1128/jb.172.9.4979-4987.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiting J., Marshall H., Cook M., Krumlauf R., Rigby P. W., Stott D., Allemann R. K. Multiple spatially specific enhancers are required to reconstruct the pattern of Hox-2.6 gene expression. Genes Dev. 1991 Nov;5(11):2048–2059. doi: 10.1101/gad.5.11.2048. [DOI] [PubMed] [Google Scholar]
- Yee S. P., Rigby P. W. The regulation of myogenin gene expression during the embryonic development of the mouse. Genes Dev. 1993 Jul;7(7A):1277–1289. doi: 10.1101/gad.7.7a.1277. [DOI] [PubMed] [Google Scholar]