Abstract
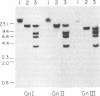
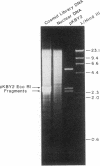
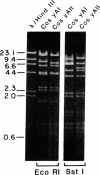
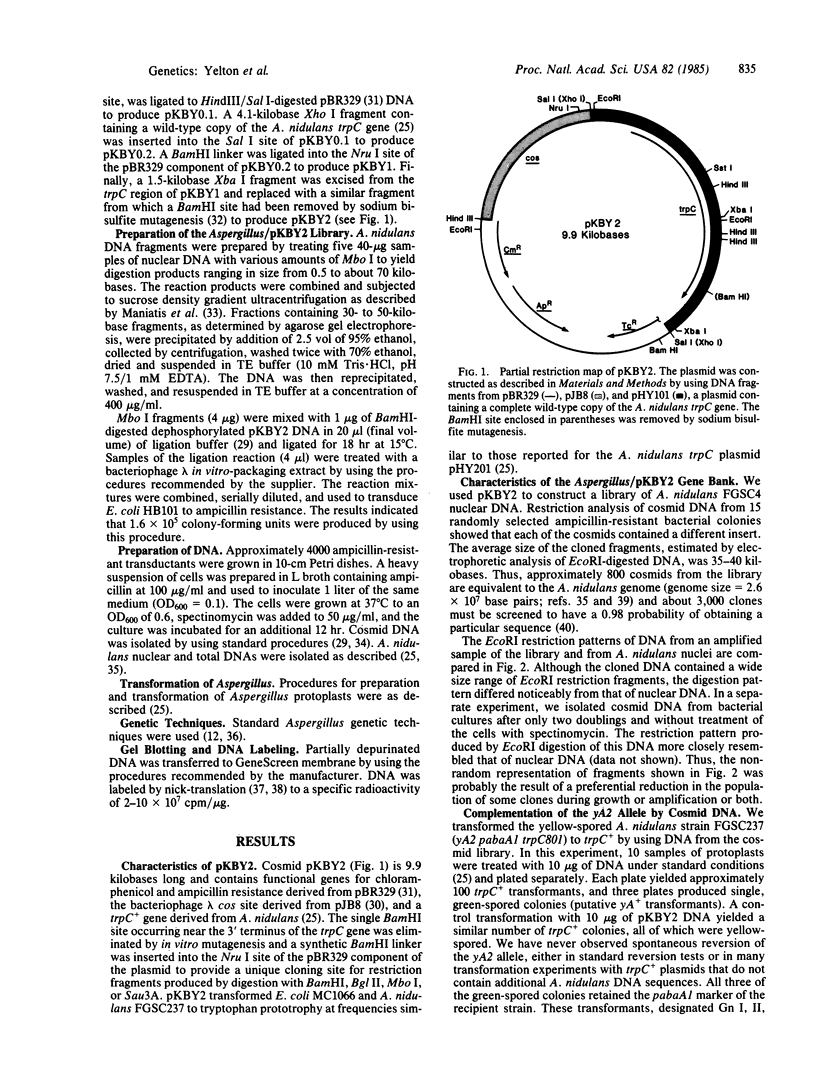
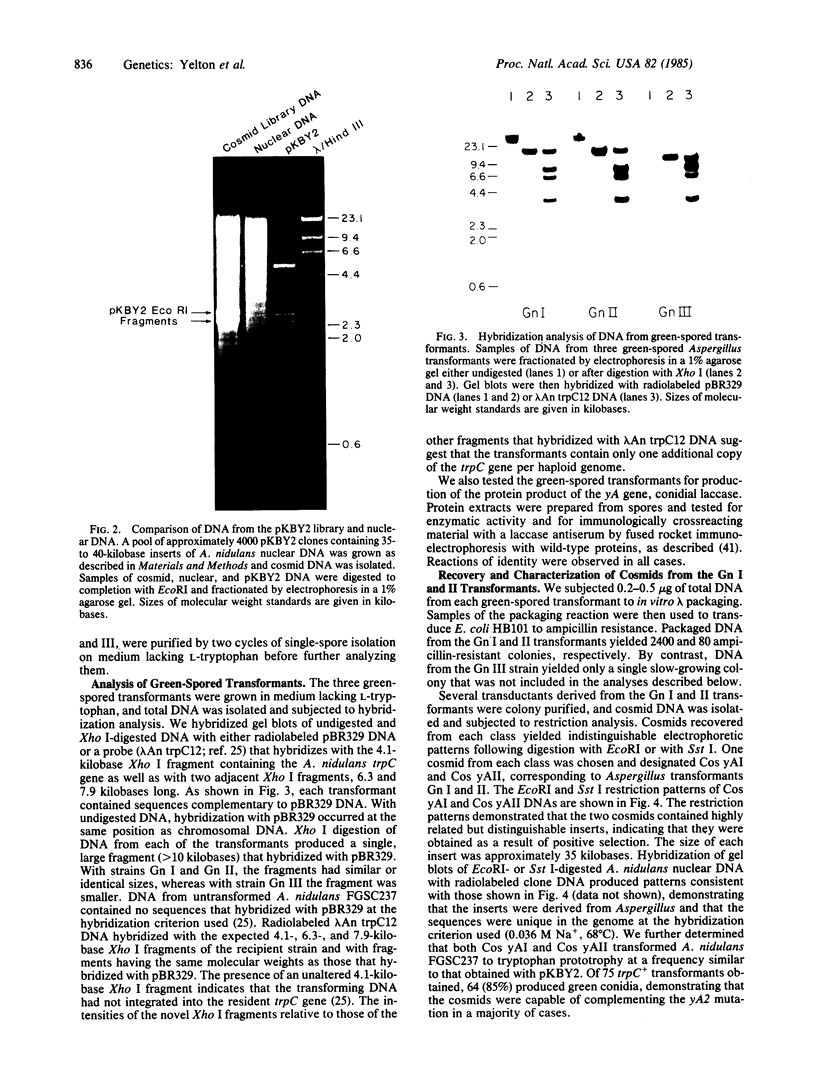
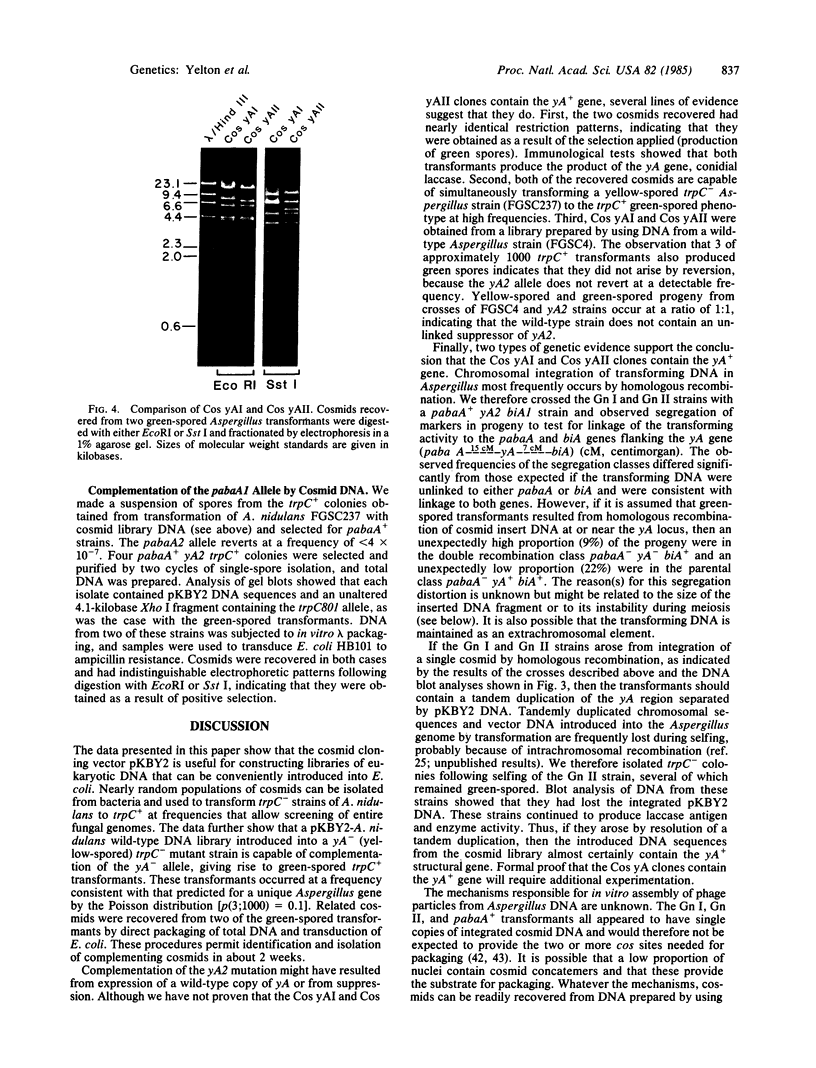
We constructed a 9.9-kilobase cloning vector, designated pKBY2, for isolating genes by complementation of mutations in Aspergillus nidulans. pKBY2 contains the bacteriophage λ cos site, to permit in vitro assembly of phage particles; a bacterial origin of replication and genes for resistance to ampicillin and chloramphenicol, to permit propagation in Escherichia coli; the A. nidulans trpC+ gene, to permit selection in Aspergillus; and a unique BamHI restriction site, to permit insertion of DNA fragments produced by digestion with restriction endonucleases BamHI, BglII, Mbo I, or Sau3A. We used this cosmid to form a quasirandom recombinant DNA library containing 35- to 40-kilobase DNA fragments from a wild-type strain of A. nidulans. DNA from this library transformed a yellow-spored (yA-) pabaA-trpC-Aspergillus strain (FGSC237) to trpC+ at frequencies of approximately 10 transformants per μg of DNA. Three of approximately 1000 trpC+pabaA- colonies obtained were putative yA+ transformants, because they produced wild-type (green) spores. DNA from each of the green-spored transformants contained pKBY2 sequences and DNA from two transformants transduced E. coli to ampicillin resistance following treatment in vitro with a λ packaging extract. The cosmids recovered in E. coli had similar restriction patterns and both yielded trpC+ transformants of A. nidulans FGSC237, 85% of which produced green spores. Several lines of evidence indicate that the recovered cosmids contain a wild-type copy of the yA gene.
Keywords: shuttle vector, gene library, gene transfer, fungi
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bainbridge B. W. Macromolecular composition and nuclear division during spore germination in Aspergillus nidulans. J Gen Microbiol. 1971 Jun;66(3):319–325. doi: 10.1099/00221287-66-3-319. [DOI] [PubMed] [Google Scholar]
- Ballance D. J., Buxton F. P., Turner G. Transformation of Aspergillus nidulans by the orotidine-5'-phosphate decarboxylase gene of Neurospora crassa. Biochem Biophys Res Commun. 1983 Apr 15;112(1):284–289. doi: 10.1016/0006-291x(83)91828-4. [DOI] [PubMed] [Google Scholar]
- Boyer H. W., Roulland-Dussoix D. A complementation analysis of the restriction and modification of DNA in Escherichia coli. J Mol Biol. 1969 May 14;41(3):459–472. doi: 10.1016/0022-2836(69)90288-5. [DOI] [PubMed] [Google Scholar]
- Casadaban M. J., Martinez-Arias A., Shapira S. K., Chou J. Beta-galactosidase gene fusions for analyzing gene expression in escherichia coli and yeast. Methods Enzymol. 1983;100:293–308. doi: 10.1016/0076-6879(83)00063-4. [DOI] [PubMed] [Google Scholar]
- Clarke L., Carbon J. A colony bank containing synthetic Col El hybrid plasmids representative of the entire E. coli genome. Cell. 1976 Sep;9(1):91–99. doi: 10.1016/0092-8674(76)90055-6. [DOI] [PubMed] [Google Scholar]
- Clewell D. B., Helinski D. R. Supercoiled circular DNA-protein complex in Escherichia coli: purification and induced conversion to an opern circular DNA form. Proc Natl Acad Sci U S A. 1969 Apr;62(4):1159–1166. doi: 10.1073/pnas.62.4.1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covarrubias L., Bolivar F. Construction and characterization of new cloning vehicles. VI. Plasmid pBR329, a new derivative of pBR328 lacking the 482-base-pair inverted duplication. Gene. 1982 Jan;17(1):79–89. doi: 10.1016/0378-1119(82)90103-2. [DOI] [PubMed] [Google Scholar]
- DiMaio D., Treisman R., Maniatis T. Bovine papillomavirus vector that propagates as a plasmid in both mouse and bacterial cells. Proc Natl Acad Sci U S A. 1982 Jul;79(13):4030–4034. doi: 10.1073/pnas.79.13.4030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hinnen A., Hicks J. B., Fink G. R. Transformation of yeast. Proc Natl Acad Sci U S A. 1978 Apr;75(4):1929–1933. doi: 10.1073/pnas.75.4.1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hohn B. DNA as substrate for packaging into bacteriophage lambda, in vitro. J Mol Biol. 1975 Oct 15;98(1):93–106. doi: 10.1016/s0022-2836(75)80103-3. [DOI] [PubMed] [Google Scholar]
- Hohn T., Katsura I. Structure and assembly of bacteriophage lambda. Curr Top Microbiol Immunol. 1977;78:69–110. doi: 10.1007/978-3-642-66800-5_3. [DOI] [PubMed] [Google Scholar]
- Hsiao C. L., Carbon J. High-frequency transformation of yeast by plasmids containing the cloned yeast ARG4 gene. Proc Natl Acad Sci U S A. 1979 Aug;76(8):3829–3833. doi: 10.1073/pnas.76.8.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ish-Horowicz D., Burke J. F. Rapid and efficient cosmid cloning. Nucleic Acids Res. 1981 Jul 10;9(13):2989–2998. doi: 10.1093/nar/9.13.2989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiessling U., Platzer M., Strauss M. Plasmid rescue - a tool for reproducible recovery of genes from transfected mammalian cells? Mol Gen Genet. 1984;193(3):513–519. doi: 10.1007/BF00382092. [DOI] [PubMed] [Google Scholar]
- Kushner P. J., Levinson B. B., Goodman H. M. A plasmid that replicates in both mouse and E. coli cells. J Mol Appl Genet. 1982;1(6):527–538. [PubMed] [Google Scholar]
- Lau Y. F., Kan Y. W. Direct isolation of the functional human thymidine kinase gene with a cosmid shuttle vector. Proc Natl Acad Sci U S A. 1984 Jan;81(2):414–418. doi: 10.1073/pnas.81.2.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law D. J., Timberlake W. E. Developmental regulation of laccase levels in Aspergillus nidulans. J Bacteriol. 1980 Nov;144(2):509–517. doi: 10.1128/jb.144.2.509-517.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maniatis T., Hardison R. C., Lacy E., Lauer J., O'Connell C., Quon D., Sim G. K., Efstratiadis A. The isolation of structural genes from libraries of eucaryotic DNA. Cell. 1978 Oct;15(2):687–701. doi: 10.1016/0092-8674(78)90036-3. [DOI] [PubMed] [Google Scholar]
- Maniatis T., Jeffrey A., Kleid D. G. Nucleotide sequence of the rightward operator of phage lambda. Proc Natl Acad Sci U S A. 1975 Mar;72(3):1184–1188. doi: 10.1073/pnas.72.3.1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasmyth K. A., Reed S. I. Isolation of genes by complementation in yeast: molecular cloning of a cell-cycle gene. Proc Natl Acad Sci U S A. 1980 Apr;77(4):2119–2123. doi: 10.1073/pnas.77.4.2119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliver P. T. Conidiophore and spore development in Aspergillus nidulans. J Gen Microbiol. 1972 Nov;73(1):45–54. doi: 10.1099/00221287-73-1-45. [DOI] [PubMed] [Google Scholar]
- PONTECORVO G., ROPER J. A., HEMMONS L. M., MACDONALD K. D., BUFTON A. W. J. The genetics of Aspergillus nidulans. Adv Genet. 1953;5:141–238. doi: 10.1016/s0065-2660(08)60408-3. [DOI] [PubMed] [Google Scholar]
- Rigby P. W., Dieckmann M., Rhodes C., Berg P. Labeling deoxyribonucleic acid to high specific activity in vitro by nick translation with DNA polymerase I. J Mol Biol. 1977 Jun 15;113(1):237–251. doi: 10.1016/0022-2836(77)90052-3. [DOI] [PubMed] [Google Scholar]
- Shortle D., Nathans D. Local mutagenesis: a method for generating viral mutants with base substitutions in preselected regions of the viral genome. Proc Natl Acad Sci U S A. 1978 May;75(5):2170–2174. doi: 10.1073/pnas.75.5.2170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinchcomb D. T., Struhl K., Davis R. W. Isolation and characterisation of a yeast chromosomal replicator. Nature. 1979 Nov 1;282(5734):39–43. doi: 10.1038/282039a0. [DOI] [PubMed] [Google Scholar]
- Stinchcomb D. T., Thomas M., Kelly J., Selker E., Davis R. W. Eukaryotic DNA segments capable of autonomous replication in yeast. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4559–4563. doi: 10.1073/pnas.77.8.4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K., Stinchcomb D. T., Davis R. W. A physiological study of functional expression in Escherichia coli of the cloned yeast imidazoleglycerolphosphate dehydratase gene. J Mol Biol. 1980 Jan 25;136(3):291–307. doi: 10.1016/0022-2836(80)90375-7. [DOI] [PubMed] [Google Scholar]
- Struhl K. The new yeast genetics. 1983 Sep 29-Oct 5Nature. 305(5933):391–397. doi: 10.1038/305391a0. [DOI] [PubMed] [Google Scholar]
- Tilburn J., Scazzocchio C., Taylor G. G., Zabicky-Zissman J. H., Lockington R. A., Davies R. W. Transformation by integration in Aspergillus nidulans. Gene. 1983 Dec;26(2-3):205–221. doi: 10.1016/0378-1119(83)90191-9. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E., Barnard E. C. Organization of a gene cluster expressed specifically in the asexual spores of A. nidulans. Cell. 1981 Oct;26(1 Pt 1):29–37. doi: 10.1016/0092-8674(81)90030-1. [DOI] [PubMed] [Google Scholar]
- Timberlake W. E. Developmental gene regulation in Aspergillus nidulans. Dev Biol. 1980 Aug;78(2):497–510. doi: 10.1016/0012-1606(80)90349-8. [DOI] [PubMed] [Google Scholar]
- Yager L. N., Kurtz M. B., Champe S. P. Temperature-shift analysis of conidial development in Aspergillus nidulans. Dev Biol. 1982 Sep;93(1):92–103. doi: 10.1016/0012-1606(82)90242-1. [DOI] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., Timberlake W. E. Transformation of Aspergillus nidulans by using a trpC plasmid. Proc Natl Acad Sci U S A. 1984 Mar;81(5):1470–1474. doi: 10.1073/pnas.81.5.1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yelton M. M., Hamer J. E., de Souza E. R., Mullaney E. J., Timberlake W. E. Developmental regulation of the Aspergillus nidulans trpC gene. Proc Natl Acad Sci U S A. 1983 Dec;80(24):7576–7580. doi: 10.1073/pnas.80.24.7576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann C. R., Orr W. C., Leclerc R. F., Barnard E. C., Timberlake W. E. Molecular cloning and selection of genes regulated in Aspergillus development. Cell. 1980 Oct;21(3):709–715. doi: 10.1016/0092-8674(80)90434-1. [DOI] [PubMed] [Google Scholar]