In this issue of the JGP, Vedovato and Gadsby show us that the native Na/K pumps of Xenopus laevis oocytes are even more complex machines than we realized. Readers of the JGP will be aware that Na/K pumps extrude three Na ions and import two K ions into cells with each pump cycle at the expense of one ATP being hydrolyzed to ADP. It now turns out that Na/K pumps develop a selective passageway for protons to cross the membrane during this normal cycle, even in the presence of physiological ion concentrations on both sides of the membrane. The proton-conducting pathway develops transiently when the pump enters into the specific state that releases the first of the three Na ions into the extracellular space and thereby generates electrical current. This Na-binding site, which is special because it cannot be occupied by a K ion, is called the “Na-selective binding site III.” When site III is not occupied by Na, protons can enter the pump’s binding sites from the extracellular space and traverse site III to the cytoplasm. When the extracellular pH is lowered and membrane potential is negative, the proton flux through site III can be about as large as the Na and K fluxes generated by pump activity in oocytes. Therewith, the Na/K pump joins a list of hybrid membrane proteins that, on the one hand, engage in coupled transport via an alternating access mechanism, and, on the other hand, develop channel-like ion conductances in a state-dependent manner. This unexpected development raises new questions about the cellular function of Na/K pumps, especially in settings in which the extracellular or cytoplasmic space, or both, become acidified.
Background
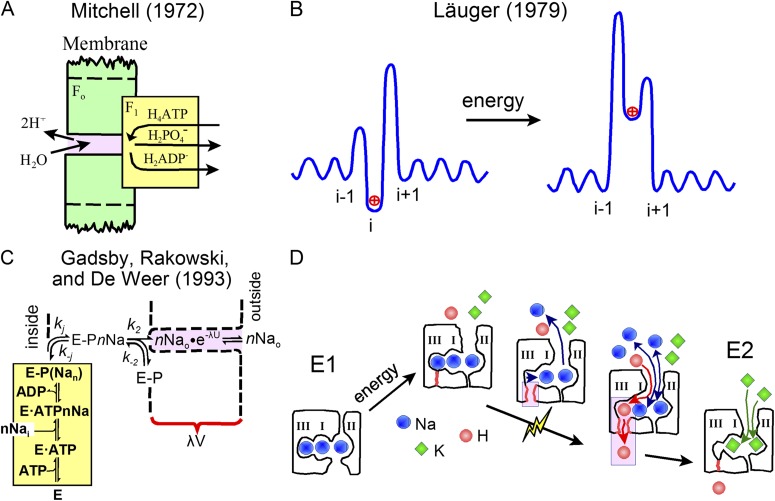
The idea that ion pumps are modified ion channels was obvious to Peter Mitchell (1972). Mitchell drew the F-ATPase as if the Fo subunits constitute a long aqueous channel leading up to the F1 sites of ATP synthesis (or hydrolysis; see Fig. 1 A). Equivalence of membrane potential and proton concentration in driving F-ATPase function could then be neatly explained if membrane potential would fall off along this narrow “access channel.” Mitchell’s thinking was extended and refined by Peter Läuger (1979): Access channels will exist on both sides of an ion pump. On the cis side, the access channel ends at a binding site, represented by an energy minimum at the base of a large energy barrier. When ions passively occupy the binding site, investment of energy (light, in the case of bacteriorhodopsin) then shifts the energy profiles so that the ions are forced to exit the pump through the access channel on the trans side (see Fig. 1 B). These ideas were subsequently put to use in the study of many transporters (Apell, 2004), but they found particular resonance with investigators of mammalian Na transporters.
Figure 1.
A progression of thoughts about ion transport and ion release from ion pumps. Panels A–C were reproduced from the original figures with permission from The Journal of Bioenergetics and Biomembranes, Quarterly Reviews of Biophysics, and Science, respectively. (A) Peter Mitchell’s diagram of an F-type ATPase suggesting that protons diffuse through a long “access channel” along which membrane potential falls off, namely between ATP synthesis/hydrolysis sites and the bulk medium (Mitchell, 1972). For a recent discussion and proposal of an alternative view, see Mulkidjanian (2006). (B) Peter Läuger’s energy diagram of proton translocation by a pump (Läuger, 1979). Microsecond charge–pulse relaxation studies subsequently were used to resolve multiple electrogenic reactions of bacteriorhodopsin that presumably reflect proton association with and dissociation from the carrier (Läuger et al., 1981). For a recent discussion of these principles, see Apell (2004). (C) Reaction scheme suggested by Gadsby et al. (1993) to explain why the extracellular Na concentration and membrane voltage act equivalently on ion flux during pump-mediated Na/Na exchange in squid giant axons. Voltage dependence of the pump was visualized as arising from an access channel on the extracellular side, similar to Peter Mitchell’s original diagram for a proton pump. Nevertheless, analysis of the Na release reactions at higher temporal resolution indicated that more complex interpretations would be required (Hilgemann, 1994; Wuddel and Apell, 1995). (D) A working model of Na release from the Na/K pump based on the study by Vedovato and Gadsby (2014) and recent structural advances (Kanai et al., 2013). From left to right: three Na ions bind with high affinity from the cytoplasmic side, with the binding sites oriented parallel to the membrane surface. Energy of phosphorylation by ATP promotes closure of the cytoplasmic “gate” of the pump and opening of the “gate” to the extracellular side. Release of the first Na ion to the outside involves a complex series of reactions, whereby one Na ion is released from site II and Na ions shift laterally from sites III and I, leaving site III vacant. These events generate the major charge movement of the pump cycle, perhaps involving both the movement of Na ions through electrical field and conformational changes of site III that open a proton pathway to the cytoplasmic side. After site III is vacated, proton passage is enabled from the extracellular space to site III and the cytoplasmic medium, presumably at instants when sites I and II are not occupied by Na. Finally, two K ions replace the two Na ions bound in sites I and II, thereby triggering dephosphorylation and closure of the proton pathway from the extracellular space.
In a seminal study that sets the background for the present study, Gadsby et al. (1993) determined that Na/K pumps function as if extracellular Na concentration and membrane potential were functionally equivalent, as suggested by Mitchell for protons in proton pumps. Specifically, changes of membrane potential act as if they selectively change the Na concentration that the pump experiences on the extracellular side. Accordingly, the stimulation of Na pump activity that occurs with depolarization from resting potential is caused mostly by Na being driven out of binding sites when they are open to the extracellular side (see Fig. 1 C). Nevertheless, Na binding from the cytoplasmic side has also been shown by optical methods to be voltage dependent. Notably, the voltage dependence occurs at a site that is not accessible to K (Domaszewicz and Apell, 1999), namely “site III,” through which protons evidently permeate when it is open to the outside. This electrogenic nature of Na binding and dissociation reactions is not unique to Na/K pumps. Extracellular Na binding has been found to be the major electrogenic transport step in many other Na-coupled transporters, including Na/glucose transporters (Hazama et al., 1997), GABA transporters (Mager et al., 1993), Na/phosphate transporters (Virkki et al., 2005), and to a lesser extent in Na/Ca exchangers (Hilgemann, 1996).
The fact that ion binding by Na/K pumps, as well as by other P-type ATPases, induces large fluorescence changes of electrochromic dyes in the adjacent membrane bilayer (Apell, 2004) provides an independent proof that ion binding is electrogenic. However, it can still be debated for each transporter whether the physical movement of Na ions or conformational changes of binding sites (that change field profile across binding sites) are the ultimate cause of charge movements. In any case, independent electrical methods with microsecond resolution revealed that Na release from Na/K pumps is in fact a very complex reaction with multiple kinetic components (Hilgemann, 1994; Wuddel and Apell, 1995).
More recently, Gadsby and colleagues were able to dissect the Na release mechanism into at least three partial reactions (Holmgren et al., 2000; Gadsby et al., 2012). This feat was accomplished using a microsecond voltage-clamp method in squid giant axons. In addition to the major reaction, associated with the initial release of one Na ion to the extracellular space, at least two additional partial reactions occur with release of the other two Na ions. Furthermore, the three reactions appear to occur in a sequential fashion (Holmgren et al., 2000; Gadsby et al., 2012). The sequential nature of these reactions provides one possible argument that these reactions take place in a structure similar to that of an ion channel. A more persuasive argument stems from the fact that toxins, known as palytoxins (from sea anemones), perturb the ion-translocating pump domains so as to generate a bona fide ion channel with a nonselective cation conductance approximately five times smaller than that of an acetylcholine-gated nicotinic receptor (Reyes and Gadsby, 2006).
That the pump is physiologically poised to undergo a more minute structural breach, specifically in the region of the third Na site, is now supported by the new experiments of Vedovato and Gadsby (2014). To make this case, the authors used incisive mutational analysis and clever manipulation of the pump to prevent it from completing a cycle, as well as by forcing it to carry out partial reactions that involve the proton-conducting state. The proton-conducting pathway evidently forms between Na site III and the cytoplasm in circumstances that match those in which the major charge movement of the pump occurs during normal pump operation.
It’s a very nice catch! But at the end of the day the authors effectively tell us not to be surprised by their surprising findings. Strong precedents for a hybrid function of membrane proteins as both transporters and channels already exist. Glutamate transporters of the EAAT type are also conformation-dependent Cl channels (Koch et al., 2007), and other neurotransmitter transporters generate state-dependent cation currents (Galli et al., 1996). In the larger picture, we are reminded that ABC transporters associated with cystic fibrosis turned out to be Cl channels (Quinton, 1990) and that, conversely, members of the CLC class of Cl channels were revealed to be Cl− proton exchangers (Accardi and Miller, 2004; Picollo and Pusch, 2005; Scheel et al., 2005).
Why are we still surprised?
If it’s possible, it will happen in biology. Darwin knew that well, and transport biophysicists have taken some time to catch up. Gadsby, who has straddled the worlds of transporters (i.e., Na/K pumps) and channels (i.e., CFTR channels) for decades, expects the list of hybrid membrane proteins to expand further as more high resolution measurements of transporter structure and function are achieved. He argues that “the required switching of cargo site access from one membrane side to the other often involves major changes in protein conformation such as rotation, twisting, rocking, and translation of transmembrane helices, or wholesale motion of protein domains. Unsurprisingly, these motions cause crevices to form between helices, helix bundles, or domains, not unlike the gaps between subway trains and curved station platforms. Propagation of those crevices, from one conformation to another, can allow penetration of solute ions like Cl, or of water, into, and even right through, the protein” (Gadsby, D.C., personal communication).
At least for the trimeric EAAT Na-coupled glutamate transporter, conformational changes of each of its individual transporter subunits can generate a transient Cl conductance (Leary et al., 2007); thus, three pores can form as the three subunits independently swing large distances with respect to the trimerization domain (Stolzenberg et al., 2012). The question for physiology is whether the protein flexibility needed for robust glutamate transport is the major determinant of Cl conductance or whether Cl conductance might be tuned by evolution to serve electrical signaling functions. What is established at this time is that transport rates of different EAATs can be very different, whereas the Cl conductances of different EAATs appear to be very similar (Torres-Salazar and Fahlke, 2007). This suggests, but certainly does not prove, that EAAT isoforms with low transport rates might primarily regulate excitability (e.g., in dendritic spines of Purkinje neurons). As for other excitatory neurotransmitter transporters, evidence continues to grow that currents generated by dopamine transporters underlie some of the important effects of both illicit and therapeutic drugs. Amphetamines induce dopamine transporters to generate long-lasting cation currents (Rodriguez-Menchaca et al., 2012) that are probably not strictly coupled to dopamine transport (Rodriguez-Menchaca et al., 2012). Nonselective cation current generated by catecholamine transporters may also be relevant to the ability of psychiatric drugs to act from inside of neurons by modulating the function of the secretory pathway (Lester et al., 2012). Clearly, all these recent data challenge the field to address more seriously the physiological roles of transporter conductances, now including a proton conductance of the Na/K pump. Along the way, it should become apparent how (and whether) the conducting mechanisms of transporters have been tuned by evolution to play specific roles, and to eventually be modulated by posttranslational signaling processes.
The details
Na/K pumps are the workhorses that drive ion homeostasis in animal cells, hydrolyzing the majority of all ATP produced in the brain (Hevner et al., 1992) and the kidney (Soltoff, 1986). The staggering complexity of their molecular function became apparent in the late 1960s and early 1970s as biochemists exploited isotopes, both nucleotides and ions, to study the pump’s enzymatic activity. Two pioneers of the day were R. Wayne Albers (1967) and Robert L. Post (Post et al., 1972). Although their work was not rewarded with a Nobel Prize, the “Post–Albers” scheme is core curriculum for any student of physiology and applies just as well to other P-type ATPases (see Fig. 1 of Vedovato and Gadsby, 2014): High affinity binding of three Na ions on the cytoplasmic side of the pump triggers autophosphorylation of the pump at an aspartate residue, coupled to a conformational change that occludes the Na ions into the pump, followed by their release to the outside; next, extracellular K ions bind with high affinity, and they become occluded in the pump as the pump dephosphorylates itself. Finally, the K ions are released into the cytoplasm, ATP binds, and the cycle repeats itself. This highly orchestrated pump cycle must occur in strict dependence on the occupation of binding sites and on the phosphorylation states of the pump with no ion slippage; otherwise, the work of the pump would be undermined. That the Na/K pump can nevertheless form a proton channel in the absence of Na and K was documented almost 20 years ago (Wang and Horisberger, 1995). Furthermore, the proton permeation pathway was clearly associated with the third Na site, linked to the major charge-moving step of the pump cycle (Li et al., 2006). Now we must ask how the pump forms this proton pathway during its normal transport cycle and still maintains adequately coupled Na and K transport function.
The cartoon in Fig. 1 D presents in rough form the interpretations of Vedovato and Gadsby (2014) in relation to recent advances in Na/K pump structure (Kanai et al., 2013; Nyblom et al., 2013). As in SERCA Ca pumps (Toyoshima and Inesi, 2004), which transport two Ca ions, the ion-binding sites of the Na/K pump are evidently oriented side by side (Kanai et al., 2013; Nyblom et al., 2013), as opposed to the perpendicular single file seen in K channels (Doyle et al., 1998). In the so-called E1 state of the pump, three Na ions bind with high affinity from the cytoplasmic side, and phosphorylation then results in a reaction sequence that closes access to the cytoplasm and opens access to the outside, thereby generating the E2 state. The initial release of one Na ion to the outside is in fact a complex series of coupled events. The immediate source of the Na ion released is suggested to be site II. There is, however, a coupled transfer of Na ions between sites: one Na ion shifts from site I to site II, and one shifts from site III to site I. As site III is vacated, the movement of Na and/or structural changes of site III generate the major pump charge movement. Coupled to these events, a (small) passageway through which protons can permeate opens from site III to the cytoplasm. With Na dissociation constants at sites I and II in the range of 20 mM (Lu et al., 1995), all Na sites may be empty a few percent of the time. That may be just time enough for protons to significantly breach the pump’s security barriers, or alternatively, protons may be able to occasionally sneak past sites I and II even when they are occupied by Na. Eventually, two K ions compete Na ions out of sites I and II, and the pump dephosphorylates itself in concert with the occlusion of K ions into the pump. Therewith, the proton permeation pathway becomes blocked and finally closed at both ends.
What is perhaps most impressive about this proposal is the extent to which the analysis of proton permeation through the pump has forced the authors to consolidate their thinking about ion translocation and charge movements in relation to pump structure. Clearly, the new proposal will catalyze a wide range of new structure–function studies to address these extraordinarily complex but fundamental reactions. Among the many remaining questions, it will be fascinating to learn how the proton pathway defined here for Na/K pumps relates to proton transport pathways that occur in other P-type pumps that actively pump protons.
Potential implications
The outstanding cellular question raised by this study is whether proton permeation through Na/K pumps is physiologically or pathologically important. It will clearly take time to provide a definitive answer, and the authors point the reader to the most relevant situations and literature. Ischemia and strenuous exercise are two obvious settings of interest. Suffice it here to say that acid flux across surface membranes is well ploughed turf. Therefore, an educated but very equivocal guess would be that Na/K pumps do not normally contribute substantial proton flux across surface membranes of most cells. Should that guess prove correct, the question will shift to whether the Na/K pump proton permeation pathway is subject to regulation and whether it is dependent on pump isoforms and possibly isoform combinations. In pursuing this second question, one must bear in mind that protons may act as congeners for both Na and K in Na/K pumps (Apell et al., 2011); certainly, that appears to be the case for Na/K pumps of red blood cells (Polvani and Blostein, 1988). In summary, careful and thoughtful experimentation has once again demonstrated new biological and physical possibilities that will take time and serious new work to be seen in their proper perspective.
Acknowledgments
I thank Hans-Jürgen Apell (Konstanz), Paul De Weer (Woods Hole), and David Gadsby (New York) for critical suggestions and patient tutelage.
This work was supported by National Institutes of Health grant R01HL067942.
The author declares no competing financial interests.
Sharona E. Gordon served as editor.
References
- Accardi A., Miller C. 2004. Secondary active transport mediated by a prokaryotic homologue of ClC Cl− channels. Nature. 427:803–807 10.1038/nature02314 [DOI] [PubMed] [Google Scholar]
- Albers R.W. 1967. Biochemical aspects of active transport. Annu. Rev. Biochem. 36:727–756 10.1146/annurev.bi.36.070167.003455 [DOI] [PubMed] [Google Scholar]
- Apell H.J. 2004. How do P-type ATPases transport ions? Bioelectrochemistry. 63:149–156 10.1016/j.bioelechem.2003.09.021 [DOI] [PubMed] [Google Scholar]
- Apell H.J., Benz G., Sauerbrunn D. 2011. Proton diet for the sodium pump. Biochemistry. 50:409–418 10.1021/bi101576s [DOI] [PubMed] [Google Scholar]
- Domaszewicz W., Apell H. 1999. Binding of the third Na+ ion to the cytoplasmic side of the Na,K-ATPase is electrogenic. FEBS Lett. 458:241–246 10.1016/S0014-5793(99)01162-X [DOI] [PubMed] [Google Scholar]
- Doyle D.A., Morais Cabral J., Pfuetzner R.A., Kuo A., Gulbis J.M., Cohen S.L., Chait B.T., MacKinnon R. 1998. The structure of the potassium channel: Molecular basis of K+ conduction and selectivity. Science. 280:69–77 10.1126/science.280.5360.69 [DOI] [PubMed] [Google Scholar]
- Gadsby D.C., Rakowski R.F., De Weer P. 1993. Extracellular access to the Na,K pump: Pathway similar to ion channel. Science. 260:100–103 10.1126/science.7682009 [DOI] [PubMed] [Google Scholar]
- Gadsby D.C., Bezanilla F., Rakowski R.F., De Weer P., Holmgren M. 2012. The dynamic relationships between the three events that release individual Na+ ions from the Na+/K+-ATPase. Nat Commun. 3:669 10.1038/ncomms1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli A., Blakely R.D., DeFelice L.J. 1996. Norepinephrine transporters have channel modes of conduction. Proc. Natl. Acad. Sci. USA. 93:8671–8676 10.1073/pnas.93.16.8671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazama A., Loo D.D., Wright E.M. 1997. Presteady-state currents of the rabbit Na+/glucose cotransporter (SGLT1). J. Membr. Biol. 155:175–186 10.1007/s002329900169 [DOI] [PubMed] [Google Scholar]
- Hevner R.F., Duff R.S., Wong-Riley M.T. 1992. Coordination of ATP production and consumption in brain: parallel regulation of cytochrome oxidase and Na+, K+-ATPase. Neurosci. Lett. 138:188–192 10.1016/0304-3940(92)90502-X [DOI] [PubMed] [Google Scholar]
- Hilgemann D.W. 1994. Channel-like function of the Na,K pump probed at microsecond resolution in giant membrane patches. Science. 263:1429–1432 10.1126/science.8128223 [DOI] [PubMed] [Google Scholar]
- Hilgemann D.W. 1996. Unitary cardiac Na+,Ca2+ exchange current magnitudes determined from channel-like noise and charge movements of ion transport. Biophys. J. 71:759–768 10.1016/S0006-3495(96)79275-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren M., Wagg J., Bezanilla F., Rakowski R.F., De Weer P., Gadsby D.C. 2000. Three distinct and sequential steps in the release of sodium ions by the Na+/K+-ATPase. Nature. 403:898–901 10.1038/35002599 [DOI] [PubMed] [Google Scholar]
- Kanai R., Ogawa H., Vilsen B., Cornelius F., Toyoshima C. 2013. Crystal structure of a Na+-bound Na+,K+-ATPase preceding the E1P state. Nature. 502:201–206 10.1038/nature12578 [DOI] [PubMed] [Google Scholar]
- Koch H.P., Brown R.L., Larsson H.P. 2007. The glutamate-activated anion conductance in excitatory amino acid transporters is gated independently by the individual subunits. J. Neurosci. 27:2943–2947 10.1523/JNEUROSCI.0118-07.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Läuger P. 1979. A channel mechanism for electrogenic ion pumps. Biochim. Biophys. Acta. 552:143–161 10.1016/0005-2736(79)90253-0 [DOI] [PubMed] [Google Scholar]
- Läuger P., Benz R., Stark G.L., Bamberg E., Jordan P.C., Fahr A., Brock W. 1981. Relaxation studies of ion transport systems in lipid bilayer membranes. Q. Rev. Biophys. 14:513–598 10.1017/S003358350000247X [DOI] [PubMed] [Google Scholar]
- Leary G.P., Stone E.F., Holley D.C., Kavanaugh M.P. 2007. The glutamate and chloride permeation pathways are colocalized in individual neuronal glutamate transporter subunits. J. Neurosci. 27:2938–2942 10.1523/JNEUROSCI.4851-06.2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester H.A., Miwa J.M., Srinivasan R. 2012. Psychiatric drugs bind to classical targets within early exocytotic pathways: Therapeutic effects. Biol. Psychiatry. 72:907–915 10.1016/j.biopsych.2012.05.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C., Geering K., Horisberger J.D. 2006. The third sodium binding site of Na,K-ATPase is functionally linked to acidic pH-activated inward current. J. Membr. Biol. 213:1–9 10.1007/s00232-006-0035-0 [DOI] [PubMed] [Google Scholar]
- Lu C.C., Kabakov A., Markin V.S., Mager S., Frazier G.A., Hilgemann D.W. 1995. Membrane transport mechanisms probed by capacitance measurements with megahertz voltage clamp. Proc. Natl. Acad. Sci. USA. 92:11220–11224 10.1073/pnas.92.24.11220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mager S., Naeve J., Quick M., Labarca C., Davidson N., Lester H.A. 1993. Steady states, charge movements, and rates for a cloned GABA transporter expressed in Xenopus oocytes. Neuron. 10:177–188 10.1016/0896-6273(93)90309-F [DOI] [PubMed] [Google Scholar]
- Mitchell P. 1972. Chemiosmotic coupling in energy transduction: A logical development of biochemical knowledge. J. Bioenerg. 3:5–24 10.1007/BF01515993 [DOI] [PubMed] [Google Scholar]
- Mulkidjanian A.Y. 2006. Proton in the well and through the desolvation barrier. Biochim. Biophys. Acta. 1757:415–427 10.1016/j.bbabio.2006.04.023 [DOI] [PubMed] [Google Scholar]
- Nyblom M., Poulsen H., Gourdon P., Reinhard L., Andersson M., Lindahl E., Fedosova N., Nissen P. 2013. Crystal structure of Na+, K+-ATPase in the Na+-bound state. Science. 342:123–127 10.1126/science.1243352 [DOI] [PubMed] [Google Scholar]
- Picollo A., Pusch M. 2005. Chloride/proton antiporter activity of mammalian CLC proteins ClC-4 and ClC-5. Nature. 436:420–423 10.1038/nature03720 [DOI] [PubMed] [Google Scholar]
- Polvani C., Blostein R. 1988. Protons as substitutes for sodium and potassium in the sodium pump reaction. J. Biol. Chem. 263:16757–16763 [PubMed] [Google Scholar]
- Post R.L., Hegyvary C., Kume S. 1972. Activation by adenosine triphosphate in the phosphorylation kinetics of sodium and potassium ion transport adenosine triphosphatase. J. Biol. Chem. 247:6530–6540 [PubMed] [Google Scholar]
- Quinton P.M. 1990. Cystic fibrosis. Righting the wrong protein. Nature. 347:226 10.1038/347226a0 [DOI] [PubMed] [Google Scholar]
- Reyes N., Gadsby D.C. 2006. Ion permeation through the Na+,K+-ATPase. Nature. 443:470–474 10.1038/nature05129 [DOI] [PubMed] [Google Scholar]
- Rodriguez-Menchaca A.A., Solis E., Jr, Cameron K., De Felice L.J. 2012. S(+)amphetamine induces a persistent leak in the human dopamine transporter: molecular stent hypothesis. Br. J. Pharmacol. 165:2749–2757 10.1111/j.1476-5381.2011.01728.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheel O., Zdebik A.A., Lourdel S., Jentsch T.J. 2005. Voltage-dependent electrogenic chloride/proton exchange by endosomal CLC proteins. Nature. 436:424–427 10.1038/nature03860 [DOI] [PubMed] [Google Scholar]
- Soltoff S.P. 1986. ATP and the regulation of renal cell function. Annu. Rev. Physiol. 48:9–31 10.1146/annurev.ph.48.030186.000301 [DOI] [PubMed] [Google Scholar]
- Stolzenberg S., Khelashvili G., Weinstein H. 2012. Structural intermediates in a model of the substrate translocation path of the bacterial glutamate transporter homologue GltPh. J. Phys. Chem. B. 116:5372–5383 10.1021/jp301726s [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torres-Salazar D., Fahlke C. 2007. Neuronal glutamate transporters vary in substrate transport rate but not in unitary anion channel conductance. J. Biol. Chem. 282:34719–34726 10.1074/jbc.M704118200 [DOI] [PubMed] [Google Scholar]
- Toyoshima C., Inesi G. 2004. Structural basis of ion pumping by Ca2+-ATPase of the sarcoplasmic reticulum. Annu. Rev. Biochem. 73:269–292 10.1146/annurev.biochem.73.011303.073700 [DOI] [PubMed] [Google Scholar]
- Vedovato N., Gadsby D.C. 2014. Route, mechanism, and implications of proton import during Na+/K+ exchange by native Na+/K+-ATPase pumps. J. Gen. Physiol. 143:449–464 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Virkki L.V., Forster I.C., Biber J., Murer H. 2005. Substrate interactions in the human type IIa sodium-phosphate cotransporter (NaPi-IIa). Am. J. Physiol. Renal Physiol. 288:F969–F981 10.1152/ajprenal.00293.2004 [DOI] [PubMed] [Google Scholar]
- Wang X., Horisberger J.D. 1995. A conformation of Na+-K+ pump is permeable to proton. Am. J. Physiol. 268:C590–C595 [DOI] [PubMed] [Google Scholar]
- Wuddel I., Apell H.J. 1995. Electrogenicity of the sodium transport pathway in the Na,K-ATPase probed by charge-pulse experiments. Biophys. J. 69:909–921 10.1016/S0006-3495(95)79965-9 [DOI] [PMC free article] [PubMed] [Google Scholar]