Abstract
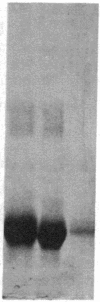
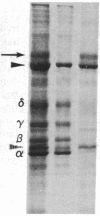
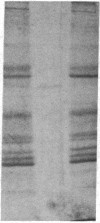
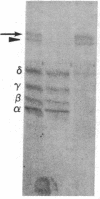
Integral membrane proteins that form water-filled channels through membranes often exist as aggregates of similar or identical subunits spanning the membrane. It has been suggested that the insertion into the membrane of the channel-forming domains of the subunits may impart unusual structural features to the membrane-intercalated portions of the protein. To test this proposal, we have investigated the interaction of a multisubunit channel-forming integral membrane protein, the acetylcholine receptor protein, with the nonionic detergent Triton X-114. Whereas non-channel-forming integral membrane proteins that have heretofore been studied form mixed micelles with the detergent, the acetylcholine receptor was excluded from the Triton X-114 micelles. The structural implications of this result are discussed.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson D. J., Blobel G. Molecular events in the synthesis and assembly of a nicotinic acetylcholine receptor. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):125–134. doi: 10.1101/sqb.1983.048.01.015. [DOI] [PubMed] [Google Scholar]
- Blobel G. Intracellular protein topogenesis. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1496–1500. doi: 10.1073/pnas.77.3.1496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bordier C. Phase separation of integral membrane proteins in Triton X-114 solution. J Biol Chem. 1981 Feb 25;256(4):1604–1607. [PubMed] [Google Scholar]
- Chang H. W., Bock E. Molecular forms of acetylcholine receptor. Effects of calcium ions and a sulfhydryl reagent on the occurrence of oligomers. Biochemistry. 1977 Oct 4;16(20):4513–4520. doi: 10.1021/bi00639a028. [DOI] [PubMed] [Google Scholar]
- Chen R., Krämer C., Schmidmayr W., Henning U. Primary structure of major outer membrane protein I of Escherichia coli B/r. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5014–5017. doi: 10.1073/pnas.76.10.5014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devillers-Thiery A., Giraudat J., Bentaboulet M., Changeux J. P. Complete mRNA coding sequence of the acetylcholine binding alpha-subunit of Torpedo marmorata acetylcholine receptor: a model for the transmembrane organization of the polypeptide chain. Proc Natl Acad Sci U S A. 1983 Apr;80(7):2067–2071. doi: 10.1073/pnas.80.7.2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finer-Moore J., Stroud R. M. Amphipathic analysis and possible formation of the ion channel in an acetylcholine receptor. Proc Natl Acad Sci U S A. 1984 Jan;81(1):155–159. doi: 10.1073/pnas.81.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon A. S., Milfay D., Diamond I. Identification of a molecular weight 43,000 protein kinase in acetylcholine receptor-enriched membranes. Proc Natl Acad Sci U S A. 1983 Oct;80(19):5862–5865. doi: 10.1073/pnas.80.19.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guy H. R. A structural model of the acetylcholine receptor channel based on partition energy and helix packing calculations. Biophys J. 1984 Jan;45(1):249–261. doi: 10.1016/S0006-3495(84)84152-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Charge shift electrophoresis: simple method for distinguishing between amphiphilic and hydrophilic proteins in detergent solution. Proc Natl Acad Sci U S A. 1977 Feb;74(2):529–532. doi: 10.1073/pnas.74.2.529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helenius A., Simons K. Solubilization of membranes by detergents. Biochim Biophys Acta. 1975 Mar 25;415(1):29–79. doi: 10.1016/0304-4157(75)90016-7. [DOI] [PubMed] [Google Scholar]
- Henderson R., Unwin P. N. Three-dimensional model of purple membrane obtained by electron microscopy. Nature. 1975 Sep 4;257(5521):28–32. doi: 10.1038/257028a0. [DOI] [PubMed] [Google Scholar]
- Heuser J. E., Salpeter S. R. Organization of acetylcholine receptors in quick-frozen, deep-etched, and rotary-replicated Torpedo postsynaptic membrane. J Cell Biol. 1979 Jul;82(1):150–173. doi: 10.1083/jcb.82.1.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong K., Hubbell W. L. Lipid requirements for Rhodopsin regenerability. Biochemistry. 1973 Oct 23;12(22):4517–4523. doi: 10.1021/bi00746a033. [DOI] [PubMed] [Google Scholar]
- Jardetzky O. Simple allosteric model for membrane pumps. Nature. 1966 Aug 27;211(5052):969–970. doi: 10.1038/211969a0. [DOI] [PubMed] [Google Scholar]
- Klymkowsky M. W., Stroud R. M. Immunospecific identification and three-dimensional structure of a membrane-bound acetylcholine receptor from Torpedo californica. J Mol Biol. 1979 Mar 5;128(3):319–334. doi: 10.1016/0022-2836(79)90091-3. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lenard J., Singer S. J. Protein conformation in cell membrane preparations as studied by optical rotatory dispersion and circular dichroism. Proc Natl Acad Sci U S A. 1966 Dec;56(6):1828–1835. doi: 10.1073/pnas.56.6.1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindstrom J., Anholt R., Einarson B., Engel A., Osame M., Montal M. Purification of acetylcholine receptors, reconstitution into lipid vesicles, and study of agonist-induced cation channel regulation. J Biol Chem. 1980 Sep 10;255(17):8340–8350. [PubMed] [Google Scholar]
- Merlie J. P., Sebbane R., Gardner S., Olson E., Lindstrom J. The regulation of acetylcholine receptor expression in mammalian muscle. Cold Spring Harb Symp Quant Biol. 1983;48(Pt 1):135–146. doi: 10.1101/sqb.1983.048.01.016. [DOI] [PubMed] [Google Scholar]
- Meunier J. C., Olsen R. W., Menez A., Fromageot P., Boquet P., Changeux J. P. Some physical properties of the cholinergic receptor protein from Electrophorus electricus revealed by a tritiated alpha-toxin from Naja nigricollis venom. Biochemistry. 1972 Mar 28;11(7):1200–1210. doi: 10.1021/bi00757a014. [DOI] [PubMed] [Google Scholar]
- Neubig R. R., Krodel E. K., Boyd N. D., Cohen J. B. Acetylcholine and local anesthetic binding to Torpedo nicotinic postsynaptic membranes after removal of nonreceptor peptides. Proc Natl Acad Sci U S A. 1979 Feb;76(2):690–694. doi: 10.1073/pnas.76.2.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noda M., Takahashi H., Tanabe T., Toyosato M., Kikyotani S., Furutani Y., Hirose T., Takashima H., Inayama S., Miyata T. Structural homology of Torpedo californica acetylcholine receptor subunits. Nature. 1983 Apr 7;302(5908):528–532. doi: 10.1038/302528a0. [DOI] [PubMed] [Google Scholar]
- Ratnam M., Lindstrom J. Structural features of the nicotinic acetylcholine receptor revealed by antibodies to synthetic peptides. Biochem Biophys Res Commun. 1984 Aug 16;122(3):1225–1233. doi: 10.1016/0006-291x(84)91223-3. [DOI] [PubMed] [Google Scholar]
- Schwartz A., Lindenmayer G. E., Allen J. C. The sodium-potassium adenosine triphosphatase: pharmacological, physiological and biochemical aspects. Pharmacol Rev. 1975 Mar;27(01):3–134. [PubMed] [Google Scholar]
- Singer S. J. Thermodynamics, the structure of integral membrane proteins, and transport. J Supramol Struct. 1977;6(3):313–323. doi: 10.1002/jss.400060304. [DOI] [PubMed] [Google Scholar]
- Tomita M., Furthmayr H., Marchesi V. T. Primary structure of human erythrocyte glycophorin A. Isolation and characterization of peptides and complete amino acid sequence. Biochemistry. 1978 Oct 31;17(22):4756–4770. doi: 10.1021/bi00615a025. [DOI] [PubMed] [Google Scholar]
- Wallach D. F., Zahler P. H. Protein conformations in cellular membranes. Proc Natl Acad Sci U S A. 1966 Nov;56(5):1552–1559. doi: 10.1073/pnas.56.5.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]