Abstract
Amyotrophic lateral sclerosis (ALS) is a progressively paralytic neurodegenerative disease that can be caused by mutations in Cu,Zn-superoxide dismutase 1 (SOD1). Transgenic mice that over-express mutant SOD1 develop paralysis and accumulate aggregates of mutant protein in the brainstem and spinal cord. The present study uses a cell culture model to demonstrate αB-crystallin is capable of reducing aggregation of mutant SOD1. To test the role of αB-crystallin in modulating SOD1 aggregation in vivo, αB-crystallin deficient mice were bred to mice expressing two different SOD1 mutants (G37R and L126Z mutants). Although completely eliminating αB-crystallin reduced the interval to disease endstage by 20–30 days in mice expressing either mutant, there were no detectable changes in the levels of sedimentable, SOD1 aggregates in the spinal cord of symptomatic mice. Because αB-crystallin is most abundantly expressed in muscle, we expected that the loss of this chaperone would leave this tissue vulnerable to mutant SOD1 aggregation. However, there was no evidence of mutant SOD1 aggregation in the muscle of mice lacking αB-crystallin. Our findings indicate that a significant perturbation to the protein homeostasis network of muscle is not sufficient to induce the aggregation of misfolded mutant SOD1. These outcomes have implications regarding the role of chaperones in modulating the tissue specific accumulations of misfolded SOD1.
Keywords: Amyotrophic lateral sclerosis, Cu/Zn-superoxide dismutase, αB-crystallin, protein misfolding, heat shock proteins
Introduction
Dominantly inherited mutations in superoxide dismutase 1 (SOD1) have been linked to familial amyotrophic lateral sclerosis (fALS)(Rosen et al. 1993). More than 100 mutations in SOD1 are known to cause fALS (http://alsod.iop.kcl.ac.uk). Some mutations in SOD1 retain high activity (Ratovitski et al. 1999) and the targeted deletion of SOD1 in mice does not induce ALS-like symptoms (Reaume et al. 1996); thus, mutant SOD1 is thought to cause disease by the acquisition of toxic properties. A recent study from our laboratory has demonstrated that one property that may be shared by all fALS mutants of SOD1 is a higher inherent propensity to form detergent-insoluble aggregates (Prudencio et al. 2009). Moreover, computational modeling approaches (Wang et al. 2008b) and SOD1 aggregation models (Prudencio et al. 2009) have revealed an association between high aggregation propensity of mutant SOD1 and short disease duration in patients.
All mouse models of fALS that overexpress mutant human SOD1 share a similar phenotype of motor neuron loss, muscle wasting, and hindlimb paralysis (Turner and Talbot 2008). In these models of fALS, mutant SOD1 is ubiquitously expressed; however, cell death is largely restricted to motor neurons. In all SOD1 transgenic mice, the appearance of symptoms is associated with an accumulation of sedimentable structures that are detergent-insoluble, which is diagnostic for protein aggregation (Johnston et al. 2000;Shinder et al. 2001;Wang et al. 2003). SOD1 aggregates are selectively detected in the spinal cord, and these aberrant structures are absent in muscle and other tissue (Wang et al. 2002).
Recent studies in invertebrate models have suggested that the aggregation of mutant proteins may be modulated by constituents of the protein homeostasis network, which primarily includes the cellular chaperones and co-chaperones (for review see (Morimoto and Cuervo 2009)). Multiple laboratories have demonstrated that symptomatic fALS mice show evidence of upregulation of the small heat shock proteins termed Hsp25 (in human termed Hsp27) and αB-crystallin (Vleminckx et al. 2002;Wang et al. 2003;Wang et al. 2005a;Tummala et al. 2005). αB-Crystallin is of particular interest as previous studies have demonstrated, in in vitro aggregation assays, that the aggregation of mutant SOD1 (Wang et al. 2005a) and mutant α-synuclein (Wang et al. 2008a) can be inhibited by αB-crystallin.
αB-crystallin binds to exposed hydrophobic surfaces on denatured or misfolded proteins (Devlin et al. 2003;Sathish et al. 2003;Tanksale et al. 2002;Kumar et al. 2005), which allows it to inhibit protein aggregation (Horwitz 2003;Narberhaus 2002). In spinal cords of fALS mice expressing fALS mutants of SOD1 (G37R, G93A, G85R, L126Z), increased levels of oligomeric αB-crystallin, which are associated with its activation as a chaperone (Kumar and Rao 2000), are detected after the onset of symptoms (Wang et al. 2003). Moreover, in symptomatic L126Z mice, misfolded mutant SOD1 appears to specifically accumulate in motor neurons, which are not immunoreactive with αB-crystallin antibodies (Wang et al. 2005a). αB-crystallin is highly expressed in skeletal muscle (Dubin et al. 1989), which does not accumulate large, sedimentable SOD1 aggregates (Wang et al. 2002).
αB-crystallin knock-out mice have been developed in which αB-crystallin and a portion of HspB2 genes are deleted (the overlapping gene structure of HspB2 was unknown at the time the mice were generated) (Brady et al. 2001). HspB2 (also termed myotonic dystrophy kinase binding protein (Suzuki et al. 1998)) is highly abundant in the heart and muscle and is ubiquitously expressed at lower levels in most tissues (Suzuki et al. 1998;Shama et al. 1999). αB-crystallin knock-out mice have no overt pathology until 40 weeks of age, at which time they develop muscular degeneration, kyphosis, and osteoarthritis (Brady et al. 2001). Mice that retain one functional allele of αB-crystallin and HspB2 do not develop obvious phenotypes. Whether the muscle degeneration phenotypes are due to the loss of αB-crystallin, HspB2, or the combined loss of both is presently unknown.
In this study, we used three lines of SOD1 transgenic mice to study the effect of αB-crystallin on disease course, and on SOD1 aggregate accumulation and localization. Two of the models we used, Gn.G37R and Gn.L126Z mice, have mutations in human genomic SOD1 and are under the control of the human SOD1 promoter (Wong et al. 1995;Wang et al. 2005a), producing ubiquitous expression of human SOD1. Both strains of mice develop the characteristic ALS phenotype of hindlimb paralysis, motor neuron loss, and accumulation of detergent-insoluble mutant SOD1 in the spinal cord. In both lines of mice, misfolded forms of mutant SOD1 appear to accumulate selectively in motor neurons (Wong et al. 1995;Wang et al. 2005a;Watanabe et al. 2001). PrP.G37R mice have mutations in the human SOD1 cDNA with expression under the transcriptional control of the mouse prion promoter, which is predominantly expressed in neuronal and muscle tissue (Wang et al. 2005b). Mice that are heterozygous for PrP.G37R do not develop disease or pathology by two years of age and do not form SOD1 aggregates; however, mice that are homozygous for the PrP.G37R transgene develop hindlimb paralysis, motor neuron loss, and detergent-insoluble SOD1 species, indicating that threshold levels of mutant SOD1 expression are required to induce disease in this mouse model (Wang et al. 2005b). We report here that the complete elimination of αB-crystallin in these mouse models causes a detectable, but modest, reduction in the age at which mice reach clinical endstage (only for the Gn.G37R and Gn.L126Z mice) without changing the tissue distribution or abundance of detergent-insoluble SOD1 aggregates.
Materials and Methods
Transgenic mice
The SOD1 transgenic mice used in this study have been previously characterized. Three strains of mice that were used are transgenic for multiple copies of a 12 kb fragment of human genomic DNA that encompasses the entire human sod1 gene including the human SOD1 promoter: the G37R variant [line 29 (onset at 7–8 mo)] (Wong et al. 1995), the L126Z variant [line 45 (onset at 7–9 mo)](Wang et al. 2005a), and the wild type variant (line 76) (Wong et al. 1995). One SOD1 transgenic mouse strain was created using SOD1 cDNA under the control of the mouse prion promoter: G37R [line 39 (asymptomatic)] (Wang et al. 2005b). Mice homozygous null for αB-crystallin were obtained from Dr. Eric Wawrousek at the National Eye Institute and have been described previously (Brady et al. 2001). Mice were genotypes by PCR of DNA extracted from tail biopsy. All procedures involving mice were reviewed an approved by the University of Florida Institutional Animal Care and Use Committee. Methods used in the histologic analysis of tissues from these animals are described in Supplemental Information.
Analysis of SOD1 aggregation detergent extraction and centrifugation
The procedures used to separate aggregated forms of mutant SOD1 from soluble species, in spinal cord, muscle tissues, or transfected cell cultures, and to quantify the level of aggregated protein have been described previously (Wang et al. 2003;Karch and Borchelt 2008). Descriptions of the cell model, expression vectors, and methods of SOD1 analysis by immunoblotting are provided in Supplemental Information.
Results
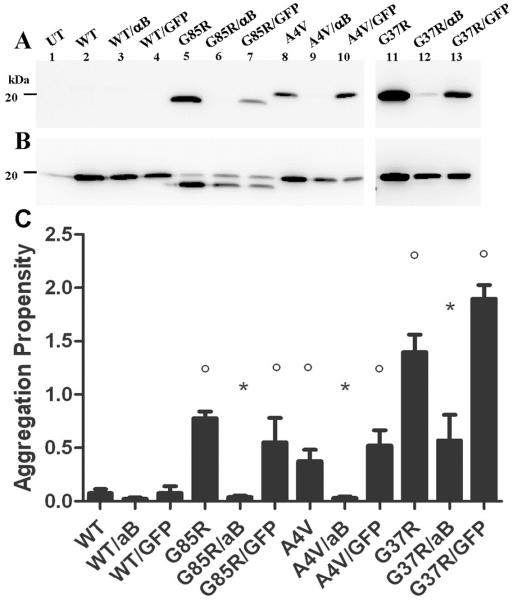
Previous studies have demonstrated that αB-crystallin prevents the in vitro aggregation of mutant SOD1-L126Z (Wang et al. 2005a). HEK293-FT cells do not constitutively express αB-crystallin nor can these cells be induced to express αB-crystallin in response to mutant SOD1 expression (Suppl. Fig. 1). These cells constitutively express Hsp70 with little or no further induction upon heat shock (Suppl. Fig. 2). Similarly, Hsp40 is constitutively induced, but at lower levels (Suppl. Fig. 2). These cells express low levels of Hsp27; just at the level of detection by immunoblot (data not shown). To assess the effect of αB-crystallin on mutant SOD1 aggregation in cell culture, vectors encoding fALS mutants (G85R, A4V, G37R) were transfected alone, with vectors encoding αB-crystallin, or with vectors encoding GFP in HEK293-FT cells. The cell lysates were fractionated into detergent-insoluble and detergent-soluble fractions by sonication and high-speed centrifugation in non-ionic detergent following previously described protocols (Karch and Borchelt 2008). In this assay, the amount of protein from the insoluble fraction that was analyzed is ~4 times that of the soluble fraction and this difference in protein loading should be kept in mind when viewing the data. In this study, SOD1 aggregates are defined as protein that is insoluble in non-ionic detergent and sediments upon centrifugation. All fALS-linked SOD1 mutants formed detergent-insoluble species (Fig. 1A, lanes 5, 8, 11) and had an aggregation potential significantly different from wild-type protein SOD1 (Fig. 1C). The co-expression of αB-crystallin with each SOD1 mutant (G85R, A4V, G37R) reduced aggregation (Fig. 1A, lanes 6, 9, 12) to levels that were not significantly different from wild-type (Fig. 1C). To control for non-specific effects on mutant SOD1 expression that could be caused by co-transfection of the αB-crystallin expression vectors, we compared the effect of αB-crystallin co-expression to co-expression of GFP In cells co-expressing GFP, there was a slight reduction in mutant SOD1 protein in the detergent-insoluble protein fraction due to an overall reduction in mutant SOD1 expression as a result of co-transfection (Fig. 1A, lanes 7, 10, 13); however, the aggregation propensity of mutant SOD1 (a ratio of soluble to insoluble protein) expressed with GFP was similar to mutant SOD1 expressed alone (Fig. 1C). Notably, the amount of mutant SOD1 detected in the soluble fraction of cells co-expressing mutant SOD1 with αB-crystallin and GFP were similar, indicating similar levels of expression of mutant SOD1 under both conditions (Fig. 1B). Immunoblots for αB-crystallin confirmed the high expression level of this chaperone in cells transfected with the appropriate vectors (Suppl. Fig. 1). In these cells both soluble and insoluble forms of αB-crystallin were detected (Suppl. Fig. 1). Thus, when co-expressed at high levels in cultured cells, αB-crystallin reduces mutant SOD1 aggregation in cell culture.
Figure 1.
αB-crystallin reduces mutant SOD1 aggregation in cell culture. HEK293FT cells were transfected with mutant SOD1 (4 μg) alone, with mutant SOD1 (2 μg) and αB-crystallin (2 μg), or with mutant SOD1 (2 μg) and GFP (2 μg). Cell lysates were extracted in non-ionic detergent and analyzed in 18% Tris-Glycine gels as described in Supplemental Information. Immunoblots were probed with m/hSOD1 antiserum. A. Detergent-insoluble (20 μg). B. Detergent-soluble (5 μg). C. Quantification of aggregation propensity measured as a ratio of detergent insoluble to soluble SOD1 and graphed with error bars representing the SEM. *, significantly different from mutant SOD1 (p<0.05). °, significantly different from WT SOD1 (p<0.05). The image shown is representative of 3 repetitions of the experiment. Data from at least 3 experiments were used to quantify aggregation propensities in panel C. Note, cells expressing G37R, G37R/αB, and G37R/GFP were harvested 48 hours after transfection; all others were harvested 24 hours post-transfection.
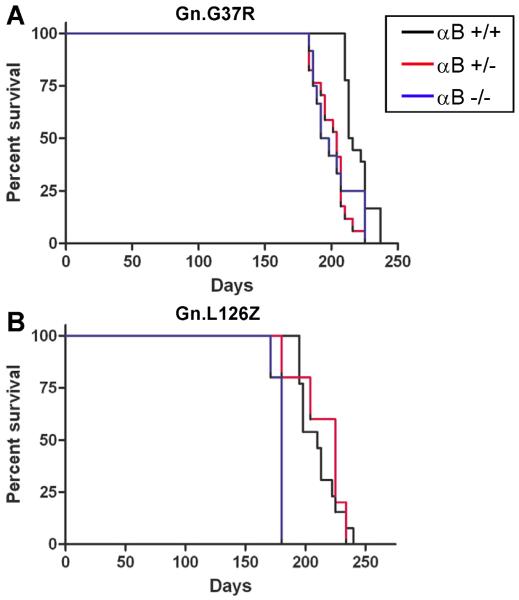
Mice lacking αB-crystallin have been previously described as developing late onset muscular degeneration (Brady et al. 2001). Because of overlapping gene structure, mice with the targeted deletion of αB-crystallin also lack HspB2, which is a specific chaperone for myotonic dystrophy kinase. Mice lacking αB-crystallin do not show evidence of motor neuron degeneration and live for 1 year before the loss of muscle mass requires euthanasia. Though imperfect, this model allows us to examine the impact of eliminating αB-crystallin upon the course of disease and the distribution of SOD1 aggregates in fALS mouse models. Moreover, because αB-crystallin null mice develop myopathy at 40 weeks (Brady et al. 2001), we indirectly asked whether concurrent muscle degeneration could compound the ALS phenotype in mice overexpressing mutant SOD1. Mutant SOD1 transgenic mice (Gn.G37R, Gn.L126Z, PrP.G37R) were crossed to mice that were homozygous null for αB-crystallin to produce mutant SOD1 mice in which αB-crystallin was reduced (heterozygous; +/−) or eliminated (KO; −/−). In the Gn.G37R and Gn.L126Z mice, the elimination of αB-crystallin resulted in a modest change in disease course (Fig. 2). Gn.G37R and Gn.L126Z mice lacking αB-crystallin developed characteristic hindlimb paralysis that appeared to progress at approximately the same rate, in regards to the interval between the first appearance of gait abnormalities and humane endpoint. Gn.G37R mice with reduced αB-crystallin (n=17, mean: 204 days) and no αB-crystallin (n=12, mean: 195 days) had lifespans significantly shorter than Gn.G37R mice with wild-type levels of αB-crystallin (mean: 214.5 days; p<0.0001, p=0.001, respectively) (Fig. 2A). The lifespans of Gn.G37R mice that were heterozygous for αB-crystallin deficiency were not different from Gn.G37R mice with no αB-crystallin (p=0.6919). Gn.L26Z mice that were heterozygous for αB-crystallin deficiency (n=8, mean: 225 days) had lifespans similar to Gn.L126Z mice expressing wild-type levels of αB-crystallin (mean: 210 days; p= 0.5552) (Fig. 2B). Gn.L126Z mice lacking αB-crystallin reached endstage earlier than Gn.L126Z mice expressing wild-type levels of αB-crystallin and Gn.L126Z mice expressing reduced levels of αB-crystallin (n=8, mean: 180 days; p<0.0001, p=0.0145, respectively) (Fig. 2B). In the study involving the L126Z mice, all of the knockout animals were generated as a single cohort and all reached endstage within a few days of each other, producing the unusual survival plot for this study. Thus, reduction or elimination of αB-crystallin is capable of altering disease course in SOD1 transgenic mice. However, the effect is not particularly robust and does not show a reproducible pattern of dose-dependency; Gn.L126Z mice heterozygous for αB-crystallin deletion are not significantly different from fALS mice wild-type for αB-crystallin.
Figure 2.
Reduction or elimination of αB-crystallin in mutant SOD1 transgenic mice modestly alters the age at which mice reach end-stage criteria. Kaplan-Meier survival curves of mutant SOD1 transgenic mice with WT (black), heterozygous (red), or homozygous null (blue) αB-crystallin. A. Gn.G37R mice. B. Gn.L126Z mice. Note: PrP.G37R mice did not develop symptoms (n=10 per condition).
To more stringently test the role of αB-crystallin in fALS disease course, we used mice that express G37R under the control of the mouse prion promoter (PrP.G37R). Mice that are heterozygous for the PrP.G37R transgene do not develop fALS symptoms, whereas a 2-fold increase in expression by generating homozygous animals produces disease (Wang et al. 2005b). This model allows us to determine whether eliminating αB-crystallin changes the threshold of mutant SOD1 expression that is required to induce disease. Eliminating αB-crystallin in heterozygous PrP.G37R mice did not induce fALS symptoms (mice were harvested at 40 weeks due to muscle atrophy). Thus, in PrP.G37R mice, the second insult provided by the elimination of αB-crystallin did not create enough of a burden to initiate disease in mice that are otherwise asymptomatic.
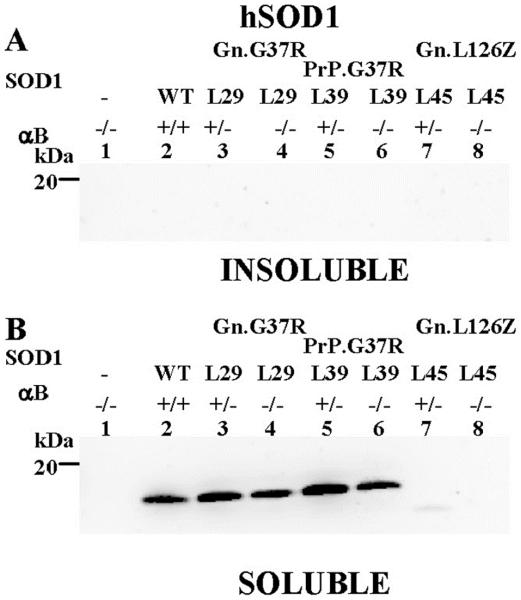
Because the elimination of αB-crystallin altered the disease course in Gn.G37R and Gn.L126Z mice, we were interested in the effects of αB-crystallin on mutant SOD1 aggregate accumulation and location. In mutant SOD1-L126Z mice, SOD1 aggregates are absent in muscle and are only detected in the spinal cord and brainstem (Suppl. Fig. 3). We hypothesized that the normally high level of expression of αB-crystallin in muscle could protect this tissue from the accumulation of SOD1 aggregates. To test this hypothesis, hindlimb muscle from Gn.G37R, Gn.L126Z, and PrP.G37R (αB +/+, αB +/−, αB −/−) was extracted in non-ionic detergent and fractionated into detergent insoluble and soluble fractions. In muscle from symptomatic mice (Gn.G37R and Gn.L126Z), SOD1 was not detected in the detergent-insoluble fraction when αB-crystallin was reduced (+/−) or eliminated (−/−) (Fig. 3A, lanes 3–4, 7–8). In these tissues, all mutant SOD1 was found in the detergent-soluble fraction (Fig. 3B, lanes 3–4), however in the L126Z mice only faint bands were apparent for SOD1-L126Z, due to the short half-life of this protein (Wang et al. 2005a) (Fig. 3B, lanes 7–8). In asymptomatic mice (PrP.G37R), SOD1 was not detected in the detergent-insoluble fraction when αB-crystallin was reduced (+/−) or eliminated (−/−) (Fig. 3A, lanes 5–6); however, detergent-soluble SOD1 was detected (Fig. 3B, lanes 5–6). Thus, the reduction or elimination of αB-crystallin in muscle of the SOD1 transgenic mice does not result in the accumulation of detergent-insoluble SOD1 in this tissue.
Figure 3.
SOD1 aggregation is absent in muscle tissue. Muscle tissues from hindlimbs (gastrocnemius and soleus) were isolated from mice and extracted in non-ionic detergent and analyzed by SDS-PAGE in 18% Tris-Glycine gels. Immunoblots were probed with hSOD1 antiserum. A. Detergent-insoluble (20 μg). B. Detergent-soluble (5 μg). The image shown is representative of 2 repetitions of the experiment.
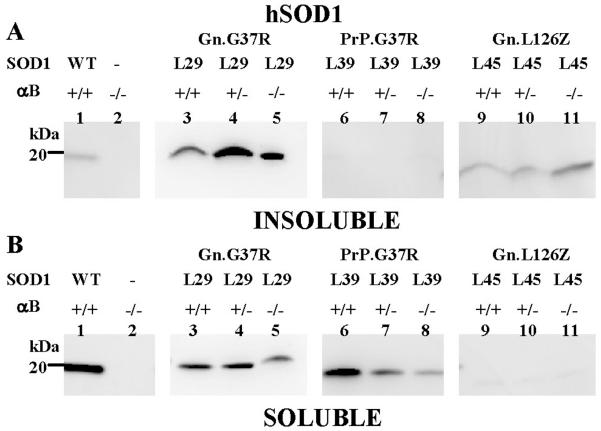
To determine whether the reduction or elimination of αB-crystallin could alter the abundance of SOD1 aggregates in the spinal cord tissue, we measured detergent-insoluble SOD1 using the detergent extraction assay described above. In symptomatic Gn.G37R mice, which normally produce detergent-insoluble SOD1, there was little change in the overall levels of detergent-insoluble SOD1 in spinal cord when αB-crystallin was reduced (+/−) or eliminated (−/−) (Fig. 4A, lanes 3–5). PrP.G37R mice, which do not produce SOD1 aggregates when heterozygous for the transgene, produced levels of detergent-insoluble SOD1 similar to or less than that of mice that overexpress WT SOD1 (Fig. 4A, compare lanes 1 and 6–8). In repeated measurements, we found no evidence of statistically significant differences in the ratio of detergent insoluble to soluble SOD1 among the αB-crystallin genotypes (Suppl. Fig. 4A; Suppl. Fig. 5). In the Gn.L126Z mice, the elimination of αB-crystallin resulted in a much larger variability in the ratio of soluble to insoluble SOD1 protein (Suppl. Fig. 4B). The low level of soluble L126Z protein in spinal cords (Fig. 4B, lanes 9–11) causes higher variability in values for aggregation propensity. It is possible that the spinal cords of these mice contain slightly higher levels of aggregated L126Z mutant protein (Fig. 4A, lanes 9–10), although we could not establish statistical significance (Suppl. Fig. 4B). Levels of detergent-soluble protein varied slightly in Gn.G37R and PrP.G37R spinal cords among the αB-crystallin genotypes; however, no consistent statistically validated difference was detected (Fig. 4B, lanes 3–8). Moreover, we were not able to detect any changes in the cellular distribution of mutant SOD1 immunoreactivity in spinal cords of mutant mice lacking αB-crystallin (Supplemental Information). We conclude that the reduction or elimination of αB-crystallin does not consistently alter the abundance of detergent-insoluble SOD1 in the spinal cords of mutant SOD1 transgenic mice.
Figure 4.
Reduction or elimination of αB-crystallin in SOD1 transgenic mice does not alter aggregation in spinal cord tissue. Spinal cords were extracted in non-ionic detergent and electrophoresed in 18% Tris-Glycine gels. Immunoblots were probed with hSOD1 antiserum. A. Detergent-insoluble (20 μg). B. Detergent-soluble (5 μg). The image shown is representative of 3 repetitions of the experiment.
Previous studies have independently demonstrated up-regulation of αB-crystallin in symptomatic fALS mice (Gn.G37R, Gn.L126Z and PrP.G37R mice) (Wang et al. 2003;Tummala et al. 2005;Wang et al. 2008a). To compare induction of αB-crystallin in these strains of mice directly, in side-by-side analyses, spinal cords from symptomatic mice were analyzed by the detergent extraction assay described above, producing detergent insoluble and soluble fractions that were immunoblotted to detect αB-crystallin. Although the levels of soluble αB-crystallin were similar among the three genotypes, the levels of insoluble αB-crystallin in spinal cords of symptomatic Gn.G37R mice were much higher than that of symptomatic Gn.L126Z mice (Suppl. Fig. 6A, lanes 3,4). In symptomatic Gn.L126Z mice, which seem to have a very weak upregulation of αB-crystallin, we noted a robust immunoreactivity for αB-crystallin in cells of astrocyte morphology (Suppl. Fig. 7G), whereas in the Gn.G37R mice which show a robust upregulation of αB-crystallin by immunoblot, the majority of the immunoreactivity appeared to be in cells exhibiting oligodendrocyte morphologies (Suppl. Fig. 7A). Moreover, as compared to nontransgenic animals (Suppl. Fig. 7J), it was difficult to demonstrate significant upregulation of αB-crystallin immunoreactivity in any cell type in the Gn.G37R mice (Suppl. Fig. 7). Thus, in this side-by-side comparison of different fALS mice, we find that Gn.L126Z mice differ from other mutants in regards to the magnitude of αB-crystallin induction and the types of cells that seem to produce the chaperone.
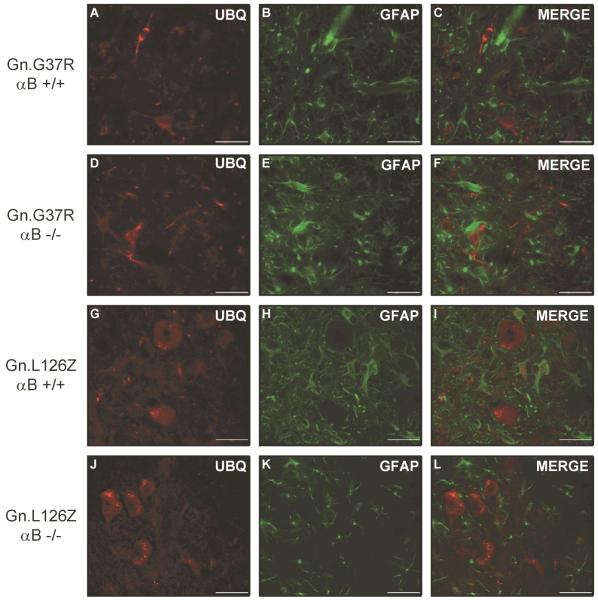
Accumulation of ubiquitin-immunoreactivity is a common pathologic feature of the Gn.G37R and Gn.L126Z mice (Wong et al. 1995;Wang et al. 2005a). Thus, we asked whether reducing or eliminating αB-crystallin altered the accumulation of ubiquitin-positive structures in symptomatic transgenic mice (Gn.G37R and Gn.L126Z). In symptomatic Gn.G37R and Gn.L126Z mice accumulations of ubiquitin immunoreactivity were observed in cells that exhibit neuronal morphologies (Fig. 5). Astrocytes were identified by co-immunostaining with antibodies to glial fibrillary acidic protein (GFAP). There was no obvious difference in the level of ubiquitin immunoreactivity in comparing mutant mice with wild-type levels (+/+) or no (−/−) αB-crystallin (Fig. 5A–F). Notably, in the Gn.L126Z mice which show a robust induction of αB-crystallin in astrocytes (see Suppl. Fig. 7), there was no obvious accumulation of ubiquitin immunoreactivity in astrocytes in mutant mice lacking αB-crystallin. We conclude that eliminating the expression of αB-crystallin in cells of the spinal cord has no effect the severity or distribution of ubiquitin immunoreactive pathology in Gn.G37R or Gn.L126Z mice.
Figure 5.
Ubiquitin-positive structures do not change in location or abundance in the absence of αB-crystallin. Mice were perfused with 4% paraformaldehyde and spinal cord tissue was immersed in sucrose prior to cryostat sectioning. Sections were stained with ubiquitin (A, D, G, J) and GFAP (B, E, H, K), followed by staining with secondary fluorescent antibodies: anti-rabbit-AlexaFluor 568 (A, D, G, J) and anti-mouse-AlexaFluor 488 (B, E, H, K). Ventral horn of spinal cord is shown at an original magnification of 40x. The image shown is representative of at least 3 repetitions of the experiment. Scale bar – 40 microns.
Discussion
This study examines the role of αB-crystallin as a modifier of mutant SOD1 misfolding and toxicity. In cultured cells, co-expression of αB-crystallin dramatically reduced the formation of detergent-insoluble aggregates of mutant SOD1. Eliminating αB-crystallin in the Gn.G37R and Gn.L126Z mice modestly shortened the interval (statistically validated) to which these mice reach humane endpoints (obvious paralysis), however we did not observe a dose-dependent effect on survival. In the Gn.G37R mice that were heterozygous for the targeted αB-crystallin allele, life expectancy was similar to that of the mice that were null for αB-crystallin, whereas the Gn.L126Z mice that were heterozygous for αB-crystallin developed paralysis at the same age as mice with normal levels of αB-crystallin. The lack of a consistent dose-dependent effect could indicate that the change in age at which paralysis develops in mice lacking one or both functional alleles of αB-crystallin is not entirely due to an effect of the chaperone. Notably, the background strain of Gn.G37R and Gn.L126Z mice is the same but it is possible that some variation in the inheritance of some factor in the background strain of the αB-crystallin KO mice is also influencing the course of disease in these mice. Overall, only the complete elimination of αB-crystallin has an impact on the course of disease in both of the symptomatic mouse models.
Eliminating αB-crystallin in mice that express G37R SOD1 at levels below the threshold for inducing disease was not sufficient to produce fALS-like symptoms. Similarly, the absence of αB-crystallin did not alter the amount of detergent-insoluble, sedimentable SOD1 that accumulated in the spinal cord; or produce an obvious change in the distribution of SOD1 immunoreactivity that accumulated in spinal cords of mutant mice lacking αB-crystallin. Because of the high level of αB-crystallin expression in muscle (Dubin et al. 1989;Iwaki et al. 1990), we had anticipated that this tissue would be most affected by the loss of this chaperone. However, somewhat surprisingly, the muscle of mice expressing mutant SOD1 did not accumulate mutant SOD1 aggregates. Together, this study illustrates that eliminating a component of the protein homeostasis network with a demonstrated ability to reduce mutant SOD1 aggregation does not result in a robust change in the pathogenesis of disease in mutant SOD1 mouse models.
Myopathy associated with ALS disease course
Muscle pathology has been described early in the disease course in mutant SOD1 transgenic mice (Fischer et al. 2004). The αB-crystallin KO mice we used develop a profound myopathy (Brady et al. 2001), which may result in part, or whole, from the loss of overlapping gene for HspB2. We anticipated the myogenic muscle atrophy that occurs in these mice could synergize to hasten the onset of disease or otherwise change disease course. Dobrowolny and colleagues recently demonstrated that the specific expression of mutant SOD1 in muscle resulted in myopathy without motor neuron disease (Dobrowolny et al. 2008). Deletion of αB-crystallin in the Gn.G37R and Gn.L126Z mice resulted in mice reaching humane endpoints at significantly, albeit modestly, younger ages. Although we did not specifically assess the age at which symptoms first appeared, the antecedent appearance of disease in these animals did not appear to be obviously different from what is expected of these mouse models. The interval between the first noticeable abnormality in gait and complete hindlimb paralysis was not obviously different. Thus, we do not find evidence that an underlying myogenic degeneration of muscle dramatically alters the course of motor neuron degeneration caused by mutant SOD1.
The role of heat shock proteins in the ALS disease course
In ALS, motor neurons are less capable of inducing heat shock proteins (Batulan et al. 2003). In mouse models of fALS, Hsp40, Hsp60. Hsp70, and Hsp90 remain unchanged throughout the disease course (Wang et al. 2008a). Only the small heat shock proteins Hsp25 and αB-crystallin are upregulated in symptomatic SOD1 transgenic mice (Wang et al. 2005a;Wang et al. 2008a). Previous studies investigating the effects of heat shock proteins on ALS have shown little, if any, effect on disease course and progression when expression of Hsp27 or Hsp70 is artificially elevated in motor neurons (Batulan et al. 2003;Krishnan et al. 2008;Sharp et al. 2008). Hsp25, which is the murine homologue of human Hsp27, is normally relatively abundant in the motor neurons of mice (Wang et al. 2005a). By contrast, based on immunohistochemical data, it appears that αB-crystallin protein is not detected in motor neurons under normal conditions nor in symptomatic fALS mice (Wang et al. 2005a)(see Suppl. Fig. 7). However, in situ evidence from the Allen Brain Atlas indicates that αB-crystallin mRNA is expressed in the majority of α-motor neurons throughout the spinal cord, leaving open the possibility that some level of αB-crystallin is present in motor neurons.
In our cell culture model of mutant SOD1 aggregation, the co-expression of high levels of αB-crystallin is capable of slowing the formation of large, detergent-insoluble, sedimentable aggregates of the mutant protein. Interestingly, in the cell model we use, HEK293FT cells, it appears that Hsp70 and Hsp40 are constitutively induced (see Suppl. Fig. 2). We presume that the high levels of mutant protein overexpression that are achieved in this cell model overwhelm the activities of these chaperones. However, whether the effects of αB-crystallin over-expression that were observed in this model require the co-expression of these other chaperones is unknown. Because of the high levels of expression of both mutant SOD1 and αB-crystallin in our cell model, we think it likely that the effect of αB-crystallin on aggregation is a direct effect.
With one exception (Bruijn et al. 1997), SOD1 immunoreactive inclusion pathology, when detected, is largely confined to motor neurons (reviewed in (Turner and Talbot 2008)). A recent study of mice that express SOD1-G85R fused to YFP has provided compelling evidence that the majority of aggregates formed by G85R-SOD1-YFP occur in motor neurons (Wang et al. 2009). Thus, the fact that we do not observe a dose-dependent or robust change in disease course in fALS mice lacking αB-crystallin could be viewed as a predictable outcome if this chaperone is not expressed in the cells most affected by the disease. More surprising to us was the absence of an effect on SOD1 aggregation in muscle when αB-crystallin expression was eliminated. This finding indicates that a significant perturbation to the protein homeostasis network of muscle is not sufficient to induce the aggregation of misfolded mutant SOD1. These outcomes have implications regarding the role of αB-crystallin in modulating the tissue specific accumulations of misfolded SOD1.
Supplementary Material
Acknowledgements
We are very grateful to Dr. Eric F. Wawrousek of the National Eye Institute for providing the αB-crystallin knockout mice and for critique of the manuscript. We would also like to thank Duane Winkler and P. John Hart of the University of Texas Health Science Center at San Antonio for providing the purified SOD1 wild-type protein. We would like to thank Mercedes Prudencio and Guilian Xu for thoughtful advice and discussion in the preparation of this manuscript. We thank Hilda Slunt Brown and Susan Fromholt for technical assistance. This work was supported by National Institute of Neurological Disease and Stroke [NS044278 to DRB]
References
- Batulan Z, Shinder GA, Minotti S, He BP, Doroudchi MM, Nalbantoglu J, Strong MJ, Durham HD. High threshold for induction of the stress response in motor neurons is associated with failure to activate HSF1. J Neurosci. 2003;23:5789–5798. doi: 10.1523/JNEUROSCI.23-13-05789.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady JP, Garland DL, Green DE, Tamm ER, Giblin FJ, Wawrousek EF. AlphaB-crystallin in lens development and muscle integrity: a gene knockout approach. Invest Ophthalmol Vis Sci. 2001;42:2924–2934. [PubMed] [Google Scholar]
- Bruijn LI, Becher MW, Lee MK, Anderson KL, Jenkins NA, Copeland NG, Sisodia SS, Rothstein JD, Borchelt DR, Price DL, Cleveland DW. ALS-linked SOD1 mutant G85R mediates damage to astrocytes and promotes rapidly progressive disease with SOD1-containing inclusions. Neuron. 1997;18:327–338. doi: 10.1016/s0896-6273(00)80272-x. [DOI] [PubMed] [Google Scholar]
- Devlin GL, Carver JA, Bottomley SP. The selective inhibition of serpin aggregation by the molecular chaperone, alpha-crystallin, indicates a nucleation-dependent specificity. J Biol Chem. 2003;278: 48644–48650. doi: 10.1074/jbc.M308376200. [DOI] [PubMed] [Google Scholar]
- Dobrowolny G, Aucello M, Rizzuto E, Beccafico S, Mammucari C, Boncompagni S, Belia S, Wannenes F, Nicoletti C, Del Prete Z, Rosenthal N, Molinaro M, Protasi F, Fano G, Sandri M, Musaro A. Skeletal muscle is a primary target of SOD1G93A-mediated toxicity. Cell Metab. 2008;8:425–436. doi: 10.1016/j.cmet.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Dubin RA, Wawrousek EF, Piatigorsky J. Expression of the murine alpha B-crystallin gene is not restricted to the lens. Mol Cell Biol. 1989;9:1083–1091. doi: 10.1128/mcb.9.3.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer LR, Culver DG, Tennant P, Davis AA, Wang M, Castellano-Sanchez A, Khan J, Polak MA, Glass JD. Amyotrophic lateral sclerosis is a distal axonopathy: evidence in mice and man. Exp Neurol. 2004;185:232–240. doi: 10.1016/j.expneurol.2003.10.004. [DOI] [PubMed] [Google Scholar]
- Horwitz J. Alpha-crystallin. Exp Eye Res. 2003;76:145–153. doi: 10.1016/s0014-4835(02)00278-6. [DOI] [PubMed] [Google Scholar]
- Iwaki T, Kume-Iwaki A, Goldman JE. Cellular distribution of alpha B-crystallin in non-lenticular tissues. J Histochem Cytochem. 1990;38:31–39. doi: 10.1177/38.1.2294148. [DOI] [PubMed] [Google Scholar]
- Johnston JA, Dalton MJ, Gurney ME, Kopito RR. Formation of high molecular weight complexes of mutant Cu, Zn-superoxide dismutase in a mouse model for familial amyotrophic lateral sclerosis. Proc Natl Acad Sci USA. 2000;97:12571–12576. doi: 10.1073/pnas.220417997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karch CM, Borchelt DR. A limited role for disulfide cross-linking in the aggregation of mutant SOD1 linked to familial amyotrophic lateral sclerosis. J Biol Chem. 2008;283:13528–13537. doi: 10.1074/jbc.M800564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan J, Vannuvel K, Andries M, Waelkens E, Robberecht W, Van Den BL. Over-expression of Hsp27 does not influence disease in the mutant SOD1(G93A) mouse model of amyotrophic lateral sclerosis. J Neurochem. 2008;106:2170–2183. doi: 10.1111/j.1471-4159.2008.05545.x. [DOI] [PubMed] [Google Scholar]
- Kumar LV, Rao CM. Domain swapping in human alpha A and alpha B crystallins affects oligomerization and enhances chaperone-like activity. J Biol Chem. 2000;275:22009–22013. doi: 10.1074/jbc.M003307200. [DOI] [PubMed] [Google Scholar]
- Kumar MS, Kapoor M, Sinha S, Reddy GB. Insights into hydrophobicity and the chaperone-like function of alpha A and alpha B-crystallins: An isothermal titration calorimetric study. J Biol Chem. 2005;280:21726–21730. doi: 10.1074/jbc.M500405200. [DOI] [PubMed] [Google Scholar]
- Morimoto RI, Cuervo AM. Protein homeostasis and aging: taking care of proteins from the cradle to the grave. J Gerontol A Biol Sci Med Sci. 2009;64:167–170. doi: 10.1093/gerona/gln071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narberhaus F. Alpha-crystallin-type heat shock proteins: socializing minichaperones in the context of a multichaperone network. Microbiol Mol Biol Rev. 2002;66:64–93. doi: 10.1128/MMBR.66.1.64-93.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prudencio M, Hart PJ, Borchelt DR, Andersen PM. Variation in aggregation propensities among ALS-associated variants of SOD1: correlation to human disease. Hum Mol Genet. 2009;18:3217–3226. doi: 10.1093/hmg/ddp260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratovitski T, Corson LB, Strain J, Wong P, Cleveland DW, Culotta VC, Borchelt DR. Variation in the biochemical/biophysical properties of mutant superoxide dismutase 1 enzymes and the rate of disease progression in familial amyotrophic lateral sclerosis kindreds. Hum Mol Genet. 1999;8:1451–1460. doi: 10.1093/hmg/8.8.1451. [DOI] [PubMed] [Google Scholar]
- Reaume AG, Elliott JL, Hoffman EK, Kowall NW, Ferrante RJ, Siwek DF, Wilcox HM, Flood DG, Beal MF, Brown RH, Jr., Scott RW, Snider WD. Motor neurons in Cu/Zn superoxide dismutase-deficient mice develop normally but exhibit enhanced cell death after axonal injury. Nature Genetics. 1996;13:43–47. doi: 10.1038/ng0596-43. [DOI] [PubMed] [Google Scholar]
- Rosen DR, Siddique T, Patterson D, Figlewicz DA, Sapp P, Hentati A, Donaldson D, Goto J, O'Regan JP, Deng H-X, Rahmani Z, Krizus A, McKenna-Yasek D, Cayabyab A, Gaston SM, Berger R, Tanzi RE, Halperin JJ, Herzfeldt B, Van den Bergh R, Hung W-Y, Bird T, Deng G, Mulder DW, Smyth C, Laing NG, Soriano E, Pericak-Vance MA, Haines J, Rouleau GA, Gusella JS, Horvitz HR, Brown RH., Jr. Mutations in Cu/Zn superoxide dismutase gene are associated with familial amyotrophic lateral sclerosis. Nature. 1993;362:59–62. doi: 10.1038/362059a0. [DOI] [PubMed] [Google Scholar]
- Sathish HA, Stein RA, Yang G, Mchaourab HS. Mechanism of chaperone function in small heat-shock proteins. Fluorescence studies of the conformations of T4 lysozyme bound to alphaB-crystallin. J Biol Chem. 2003;278:44214–44221. doi: 10.1074/jbc.M307578200. [DOI] [PubMed] [Google Scholar]
- Shama KM, Suzuki A, Harada K, Fujitani N, Kimura H, Ohno S, Yoshida K. Transient up-regulation of myotonic dystrophy protein kinase-binding protein, MKBP, and HSP27 in the neonatal myocardium. Cell Struct Funct. 1999;24:1–4. doi: 10.1247/csf.24.1. [DOI] [PubMed] [Google Scholar]
- Sharp PS, Akbar MT, Bouri S, Senda A, Joshi K, Chen HJ, Latchman DS, Wells DJ, de Belleroche J. Protective effects of heat shock protein 27 in a model of ALS occur in the early stages of disease progression. Neurobiol Dis. 2008;30:42–55. doi: 10.1016/j.nbd.2007.12.002. [DOI] [PubMed] [Google Scholar]
- Shinder GA, Lacourse MC, Minotti S, Durham HD. Mutant Cu/Zn-superoxide dismutase proteins have altered solubility and interact with heat shock/stress proteins in models of amyotrophic lateral sclerosis. J Biol Chem. 2001;276:12791–12796. doi: 10.1074/jbc.M010759200. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Sugiyama Y, Hayashi Y, Nyu-i N, Yoshida M, Nonaka I, Ishiura S, Arahata K, Ohno S. MKBP, a novel member of the small heat shock protein family, binds and activates the myotonic dystrophy protein kinase. J Cell Biol. 1998;140:1113–1124. doi: 10.1083/jcb.140.5.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanksale A, Ghatge M, Deshpande V. Alpha-crystallin binds to the aggregation-prone molten-globule state of alkaline protease: implications for preventing irreversible thermal denaturation. Protein Sci. 2002;11:1720–1728. doi: 10.1110/ps.0201802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tummala H, Jung C, Tiwari A, Higgins CM, Hayward LJ, Xu Z. Inhibition of chaperone activity is a shared property of several Cu,Zn-superoxide dismutase mutants that cause amyotrophic lateral sclerosis. J Biol Chem. 2005;280:17725–17731. doi: 10.1074/jbc.M501705200. [DOI] [PubMed] [Google Scholar]
- Turner BJ, Talbot K. Transgenics, toxicity and therapeutics in rodent models of mutant SOD1-mediated familial ALS. Prog Neurobiol. 2008;85:94–134. doi: 10.1016/j.pneurobio.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Vleminckx V, Van Damme P, Goffin K, Delye H, Van Den BL, Robberecht W. Upregulation of HSP27 in a transgenic model of ALS. J Neuropathol Exp Neurol. 2002;61:968–974. doi: 10.1093/jnen/61.11.968. [DOI] [PubMed] [Google Scholar]
- Wang J, Farr GW, Zeiss CJ, Rodriguez-Gil DJ, Wilson JH, Furtak K, Rutkowski DT, Kaufman RJ, Ruse CI, Yates JR, III, Perrin S, Feany MB, Horwich AL. Progressive aggregation despite chaperone associations of a mutant SOD1-YFP in transgenic mice that develop ALS. Proc Natl Acad Sci U S A. 2009;106:1392–1397. doi: 10.1073/pnas.0813045106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Martin E, Gonzales V, Borchelt DR, Lee MK. Differential regulation of small heat shock proteins in transgenic mouse models of neurodegenerative diseases. Neurobiol Aging. 2008a;29:586–597. doi: 10.1016/j.neurobiolaging.2006.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Slunt H, Gonzales V, Fromholt D, Coonfield M, Copeland NG, Jenkins NA, Borchelt DR. Copper-binding-site-null SOD1 causes ALS in transgenic mice: aggregates of non-native SOD1 delineate a common feature. Hum Mol Genet. 2003;12:2753–2764. doi: 10.1093/hmg/ddg312. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu G, Borchelt DR. High molecular weight complexes of mutant superoxide dismutase 1: age- dependent and tissue-specific accumulation. Neurobiol Dis. 2002;9:139–148. doi: 10.1006/nbdi.2001.0471. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu G, Li H, Gonzales V, Fromholt D, Karch C, Copeland NG, Jenkins NA, Borchelt DR. Somatodendritic accumulation of misfolded SOD1-L126Z in motor neurons mediates degeneration: {alpha}B-crystallin modulates aggregation. Hum Mol Genet. 2005a;14:2335–2347. doi: 10.1093/hmg/ddi236. [DOI] [PubMed] [Google Scholar]
- Wang J, Xu G, Slunt HH, Gonzales V, Coonfield M, Fromholt D, Copeland NG, Jenkins NA, Borchelt DR. Coincident thresholds of mutant protein for paralytic disease and protein aggregation caused by restrictively expressed superoxide dismutase cDNA. Neurobiol Dis. 2005b;20:943–952. doi: 10.1016/j.nbd.2005.06.005. [DOI] [PubMed] [Google Scholar]
- Wang Q, Johnson JL, Agar NY, Agar JN. Protein aggregation and protein instability govern familial amyotrophic lateral sclerosis patient survival. PLoS Biol. 2008b;6:e170. doi: 10.1371/journal.pbio.0060170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe M, Dykes-Hoberg M, Cizewski C,V, Price DL, Wong PC, Rothstein JD. Histological Evidence of Protein Aggregation in Mutant SOD1 Transgenic Mice and in Amyotrophic Lateral Sclerosis Neural Tissues. Neurobiol Dis. 2001;8:933–941. doi: 10.1006/nbdi.2001.0443. [DOI] [PubMed] [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL. An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron. 1995;14:1105–1116. doi: 10.1016/0896-6273(95)90259-7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.