Abstract
Recombinant human erythropoietin (rhEPO) induces neurogenesis and angiogenesis. Using a coculture system of mouse brain endothelial cells (MBECs) and neural progenitor cells derived from the subventricular zone of adult mouse, we investigated the hypothesis that neural progenitor cells treated with rhEPO promote angiogenesis. Treatment of neural progenitor cells with rhEPO significantly increased their expression and secretion of vascular endothelial growth factor (VEGF) and activated phosphatidylinositol 3-kinase/Akt (PI3K/Akt) and extracellular signal-regulated kinase (ERK1/2). Selective inhibition of the Akt and ERK1/2 signaling pathways significantly attenuated the rhEPO-induced VEGF expression in neural progenitor cells. The supernatant harvested from neural progenitor cells treated with rhEPO significantly increased the capillary-like tube formation of MBECs. SU1498, a specific VEGF type-2 receptor (VEGFR2) antagonist, abolished the supernatant-enhanced angiogenesis. In addition, coculture of MBECs with neural progenitor cells treated with rhEPO substantially increased VEGFR2 mRNA and protein levels in MBECs. These in vitro results suggest that EPO enhances VEGF secretion in neural progenitor cells through activation of the PI3K/Akt and ERK1/2 signaling pathways and that neural progenitor cells treated with rhEPO upregulate VEGFR2 expression in cerebral endothelial cells, which along with VEGF secreted by neural progenitor cells promotes angiogenesis.
Keywords: angiogenesis, mouse brain endothelial cell, neural progenitor cell, rhEPO
Introduction
In the adult rodent brain, neural progenitor cells are localized adjacent to endothelial cells in the subventricular zone (SVZ) and the dentate gyrus (Palmer et al, 2000; Gotts and Chesselet, 2005). Cerebral ischemia induces neurogenesis and angiogenesis (Jin et al, 2001; Zhang et al, 2001; Arvidsson et al, 2002; Parent et al, 2002; Wang et al, 2004). Angiogenesis is coupled with neurogenesis (Palmer et al, 2000; Louissaint et al, 2002; Taguchi et al, 2004). Suppressing angiogenesis attenuates neuroblast migration toward the ischemic cortex (Ohab et al, 2006).
Erythropoietin (EPO), a hematopoietic cytokine, regulates neurogenesis and angiogenesis (Shingo et al, 2001; Wang et al, 2004). Treatment of stroke with recombinant human EPO (rhEPO) enhances neurogenesis and angiogenesis and improves neurologic outcome (Wang et al, 2004). Erythropoietin-enhanced angiogenesis promotes migration of new-born neurons toward the ischemic boundary region through MMP2 and MMP9 secreted by cerebral endothelial cells (Wang et al, 2006a, b), suggesting that EPO-induced angiogenesis is coupled with neurogenesis in the ischemic brain.
Adult SVZ neural progenitor cells express many genes involved in angiogenesis (Liu et al, 2007). Ischemic stroke upregulates angiogenic gene expression in SVZ neural progenitor cells (Liu et al, 2007). Using a coculture system, we recently showed that neural progenitor cells derived from the ischemic SVZ promote in vitro angiogenesis, indicating that neural progenitor cells modulate cerebral endothelial cell behavior (Teng et al, 2008). Neural progenitor cell proliferation and differentiation are regulated by EPO receptor (EPOR) (Studer et al, 2000; Shingo et al, 2001; Tsai et al, 2006). Erythropoietin also plays an important role in angiogenesis through upregulation of vascular endothelial growth factor (VEGF) in ischemic rats (Wang et al, 2004). Angiogenesis is a tightly controlled multistep process by which new blood vessels are formed by sprouting from the preexisting vasculature. The VEGF family involves at least VEGF A–D, which exert biologic functions through three related receptor tyrosine kinases, VEGF receptor (VEGFR)1, VEGFR2, and VEGFR3 (Matsumoto and Claesson-Welsh, 2001). Among them, VEGF A and its receptor VEGFR2 are required to initiate the formation of immature vessels by vasculogenesis or angiogenesis during embryonic development (Ferrara et al, 2003). In addition, phosphatidylinositol 3-kinase/Akt (PI3K/Akt) and extracellular signal-regulated kinase (ERK1/2) signaling mediates angiogenesis and VEGF expression in endothelial cells (Jiang et al, 2000). In this study, we investigated the effect of neural progenitor cells treated with rhEPO on cerebral endothelial cells and the induction of angiogenesis and the signaling pathways that mediate this process.
Materials and methods
All experimental procedures were approved by the Institutional Animal Care and Use Committee of Henry Ford Hospital. Wild-type male mice (C57BL6/J, 6 to 8 weeks) were purchased from The Jackson Laboratory (Bar Harbor, ME, USA). EPO receptor null male mice (ΔEPOR mice, C57BL6 background, 6 to 8 weeks) were provided by Dr Constance Tom Noguchi at NIDDK, NIH (Chen et al, 2007).
Neurosphere Culture
Subventricular zone neural progenitor cells were dissociated from wild-type male mice (n = 10) and ΔEPOR male mice (n = 10), as previously reported (Morshead et al, 1994; Chiasson et al, 1999). The cells were plated at a density of 2 × 104 cells/mL in growth medium. Growth medium contained DMEM-F-12 medium (Invitrogen Corporation, Carlsbad, CA, USA), 20 ng/mL of epidermal growth factor (EGF; R&D Systems, Minneapolis, MN, USA), and basic fibroblast growth factor (bFGF; R&D Systems). DMEM-F-12 medium contained l-glutamine (2 mmol/L), glucose (0.6%), putrescine (9.6 µg/mL), insulin (0.025 mg/mL), progesterone (6.3 ng/mL), apotransferrin (0.1 mg/mL), and sodium selenite (5.2 ng/mL). The generated neurospheres (primary sphere) were passaged by mechanical dissociation and reseeded as single cells at a density of 20 cells/µL in bFGF and EGF containing media (passage 1 cells). In this study, passage 3 cells were processed for the experiments.
Mouse Brain Endothelial Cell Culture
Mouse brain endothelial cells (MBECs; ATCC, Manassas, VA, USA) were incubated in Dulbecco’s modified Eagle’s medium and 10% fetal bovine serum (GIBCO, Grand Island, NY, USA) and maintained at 37°C in 5% CO2/95% ambient mixed air. The culture media were changed every 48 h. Passage 10 to 12 MBECs were used in the experiments.
Mouse VEGFR2 siRNA and Transfection
Mouse VEGFR2 short interfering RNA (siRNA) was purchased from Dharmacon Inc. (Chicago, IL, USA). Mouse brain endothelial cells were transfected using FuGENE 6 Transfection Reagent (Roche Applied Science, Indianapolis, IN, USA) as per the manufacturer’s instructions. The total amount of siRNA per transfection was kept constant to 1 µg/mL. The mRNA levels of VEGFR2 were measured 48 h after transfection.
Preparation of Conditioned Media
Neural progenitor cells (1 × 106) were plated on six-well tissue culture plates containing reduced growth media (5 ng/mL bFGF). The cells were treated with rhEPO (0, 1, 5, or 10 U/mL of epoietin α; Amgen, Thousand Oaks, CA, USA) in the presence or absence of the PI3K/Akt inhibitors LY294002 (10 µmol/L; Calbiochem, San Diego, CA, USA) and wortmannin (2 µmol/L; Sigma-Aldrich, St Louis, MO, USA) (Wang et al, 2005) or the ERK1/2 inhibitors U0126 (10 µmol/L; Calbiochem) and PD98059 (10 µmol/L; Calbiochem) (Sengupta et al, 1998) for 24 h. After washing the cells with phosphate-buffered saline, 2mL culture media (described above) was added to each well and the cells were incubated for another 48 h after which time the supernatant as conditioned medium was removed and immediately frozen at −80°C.
Enzyme-Linked Immunosorbent Assay
The concentration of VEGF in cultured neural progenitor cells supernatant was determined using a mouse VEGF ELISA kit according to the manufacturer’s instructions (R&D Systems). Using known concentrations of VEGF (0 to 500 pg/mL), a standard curve was calculated for each assay.
Capillary-Like Tube Formation Assay
Mouse brain-derived endothelial cells (2 × 104 cells) were incubated in Matrigel (BD Biosciences, San Jose, CA, USA) for 5 h in conditioned medium collected from neural progenitor cells treated with rhEPO (0, 1, 5, and 10 U/mL) in the presence or absence of the PI3K/Akt inhibitor LY294002 (10 µmol/L) or the ERK1/2 inhibitor U0126 (10 µmol/L) or a specific VEGFR2 antagonist (SU1498, 5 µmol/L; LC Laboratories, Woburn, MA, USA) (Haralabopoulos et al, 1994). All assays were performed in n = 6/group. For quantitative measurements of capillary tube formation, Matrigel wells were digitized under a × 10 objective (Olympus BX40) for measurement of total length of capillary tubes using a video camera (Sony DXC-970MD) interfaced with the MCID image analysis system (Imaging Research, St Catharines, ON, Canada) at 5 h. Tracks of endothelial cells organized into networks of cellular cords (tubes) were counted and averaged in randomly selected five microscopic fields (Rikitake et al, 2002).
Coculture of Neural Progenitor Cells with Mouse Brain Endothelial Cells
Twenty-four hours before coculture, MBECs (2 × 105) were plated into the lower chamber of the six-well plate in medium with 10% FBS. Neural progenitor cells (1 × 106) were plated on six-well plates containing reduced growth media (5 ng/mL bFGF). The cells were treated with rhEPO (0, 1, 5, and 10 U/mL) with or without the PI3K/Akt inhibitors LY294002 (10 µmol/L) and wortmannin (2 µmol/L) or the ERK1/2 inhibitors U0126 (10 µmol/L) or PD98059 (10 µmol/L). After washing both the cells with phosphate-buffered saline, neural progenitor cells were seeded in the upper chamber of a Falcon 0.4 µm cell culture insert, in a total of 3mL culture media (described above, n = 3/group). The cells were cocultured for an additional 24 or 48 h after which the MBECs were collected for mRNA or protein assay, respectively.
Immunocytochemistry and Quantification
Double immunofluorescent staining of cultured cells was performed, as described previously (Zhang et al, 2001, 2003). The following primary antibodies were used: rabbit anti-nestin (1:100; BD Biosciences) and rabbit anti-SOX2 (1:50; Santa Cruz Biotechnology Inc., Santa Cruz, CA, USA). Cultured cells were fixed in 4% paraformaldehyde for 15 to 20 mins at room temperature. Nonspecific binding sites were blocked with 1% bovine serum albumin for 60 mins at room temperature. The cells were then incubated with the primary antibodies listed above and with CY3-conjugated or fluorescein isothiocyanate-conjugated secondary antibodies. Nuclei were counterstained with 4′,6′-diamidino-2-phenylindole (Vector Laboratories, Burlingame, CA, USA).
Real-Time Reverse Transcription-PCR
Quantitative PCR was performed using SYBR Green real-time PCR method. Total RNA was isolated from neurosphere cultures using the Stratagene Absolutely RNA MicroRNA isolation kit (Stratagene, La Jolla, CA, USA). Quantitative reverse transcription-PCR (RT-PCR) was performed on an ABI 7000 PCR instrument (Applied Biosystems, Foster City, CA, USA) using three-stage program parameters provided by the manufacturer, as follows: 2 mins at 50°C, 10 mins at 95°C, and then 40 cycles of 15 secs at 95°C and 1 min at 60°C. Specificity of the amplification product produced was confirmed by examination of dissociation reaction plots. A distinct single peak indicated that a single DNA sequence was amplified during PCR. Each sample was tested in triplicate using quantitative RT-PCR, and samples obtained from three independent experiments were used for analysis of relative gene expression data using the 2−ΔΔCT method (Livak and Schmittgen, 2001). The following primers for real-time PCR were designed using Primer Express software (ABI): glyceraldehyde-3-phosphate dehydrogenase (FWD: AGAGAGAGGCCCTCAGTTGCT, REV: TTGTGAGGGAGATGCTCAGTGT), VEGF (FWD: GA AAATCACTGTGTGAGCCTTGTTC, REV: GAAAATCACT GTGAGCCTTGTTC), VEGFR2 (FWD: CGAAATTACTTTT TAGCCGAGGT, REV: TTAACATAAGCACACAGGCAGAA).
Western Blot Analysis
Western blots were performed according to published methods (Wang et al, 2006a, b). Briefly, lysates from neural progenitor cells and MBECs were sonicated for 10 secs and centrifuged at 10,000g for 10 mins. Protein concentration in the supernatants of cell extract was determined using a BCA protein assay kit (Pierce Biotechnology Inc., Rockford, IL, USA). Equal amounts of proteins were loaded on a 10% SDS-polyacrylamide gel. After electrophoresis, the proteins were transferred to nitrocellulose membranes and the blots were subsequently probed with the following antibodies: phosphospecific Akt (Ser473, 1:1,000), Akt (1:1,000; Cell Signaling Technology Inc., Beverly, MA, USA), phospho-glycogen synthase kinase 3 α/β (GSK3α/β) (1:1,000; Cell Signaling Technology Inc.), GSK3α/β (1:1,000; QED Bioscience Inc., San Diego, CA, USA), phosphospecific ERK1/2 (1:1,000), ERK1/2 (1:1,000; Santa Cruz Biotechnology Inc.), VEGFR2 (3 µg/mL, Abcam Inc., Cambridge, MA, USA), and β-actin (1:5,000; Abcam Inc.). For detection, horseradish peroxidase-conjugated secondary antibodies were used (1:2,000) followed by enhanced chemiluminescence development (Pierce Biotechnology Inc.). Normalization of results was ensured by running parallel western blots with β-actin antibody or total Akt and ERK1/2 used as an internal control. The optical density was quantified using an image processing and analysis program (Scion Image, Ederick, MA, USA).
Statistical Analysis
A one-way analysis of variance was performed and statistical significance was set at P < 0.05. All data are presented as mean ± s.e.
Results
Subventricular Zone Cells Exhibit Markers of Neural Progenitor Cells
Immunostaining analysis showed that the majority of SVZ cells cultured in the growth medium were nestin and SOX2 positive, markers of neural progenitor cells (Figure 1), which is consistent with published studies (Wang et al, 2007).
Figure 1.
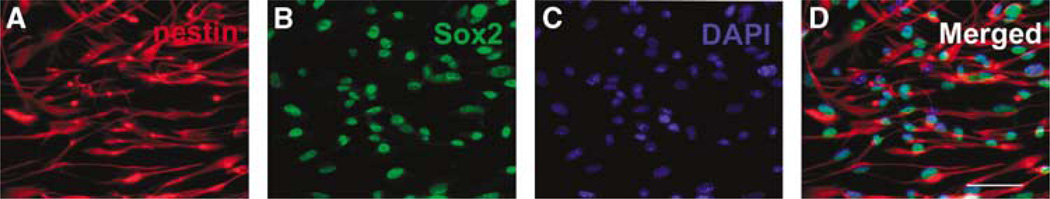
Adult SVZ cells are neural progenitor cells. Panels A to D show that the majority of SVZ cells are positive for markers of nestin (A, D) and SOX2 (B, D). Nuclei were stained with 4′,6′-diamidino-2-phenylindole (blue; C, D). Bar = 50 µm.
Recombinant Human Erythropoietin Stimulates Neural Progenitor Cells to Secrete Vascular Endothelial Growth Factor
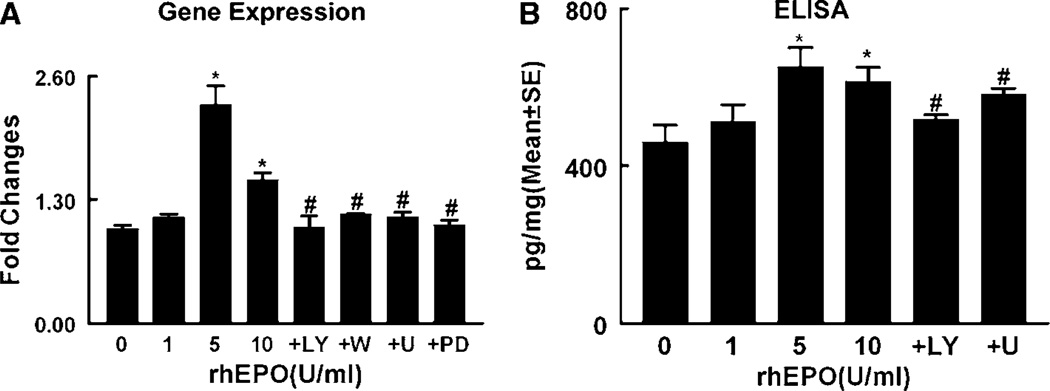
Adult neural progenitor cells express many angiogenic growth factors, including VEGF (Deleyrolle et al, 2006; Liu et al, 2007). To examine whether rhEPO upregulates VEGF expression, neural progenitor cells were incubated with rhEPO (0, 1, 5, and 10 U/mL) for 24 h. Real-time RT-PCR analysis showed that treatment with rhEPO dose-dependently upregulated VEGF expression in neural progenitor cells (Figure 2). To examine whether rhEPO increases VEGF proteins, VEGF levels were measured by means of an ELISA kit in the supernatant harvested from neural progenitor cells treated with rhEPO for 24 h. Incubation of neural progenitor cells in the presence of rhEPO (5 and 10 U/mL) significantly (P < 0.05) increased VEGF levels in the supernatant (Figure 2). The specific effect of rhEPO was confirmed because incubation of neural progenitor cells derived from ΔEPOR mice with rhEPO did not elevate VEGF levels in the supernatant (678.6 ± 78 pg/mg in the presence of 5 U/mL rhEPO versus 659.9 ± 58 pg/mg in control), although basal levels of VEGF were higher in the supernatant harvested from neural progenitor cells derived from ΔEPOR mice (659.9 ± 58 pg/mg) than that from wild-type neural progenitor cells (460.45 ± 43 pg/mg). These data indicate that exogenous EPO stimulates neural progenitor cells to secrete VEGF through endogenous EPOR.
Figure 2.
Effect of rhEPO on VEGF expression and secretion. Real-time RT-PCR (A) and enzyme-linked immunosorbent assay (B) show VEGF mRNA levels in neural progenitor cells (A) and VEGF protein levels in conditioned medium (B) after treatment with different concentrations of rhEPO (0, 1, 5, and 10 U/mL), rhEPO (5 U/mL) with LY294002 (+ LY) or wortmannin (+ W), and rhEPO (5 U/mL) with U0126 (+ U) or PD98059 (+ PD). *P < 0.05 versus the control (0) group and #P < 0.05 versus the rhEPO (5 U/mL) group. n = 3/group.
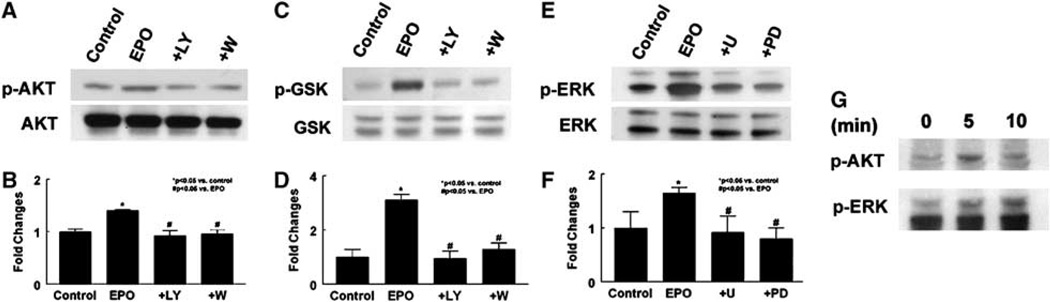
In addition, western blot analysis showed that treatment of neural progenitor cells with rhEPO (5 U/mL) for 30 mins increased phosphorylated Akt and ERK1/2 but did not alter the levels of total Akt and ERK1/2 (Figure 3). To determine the ability of activated Akt to phosphorylate its downstream targets, phosphorylation of GSK3α/β, a well-characterized Akt substrate, was measured. Erythropoietin-activated Akt significantly increased serine phosphorylation of GSK3α/β (Figure 3). To evaluate whether activation of Akt and ERK1/2 affects VEGF expression, levels of VEGF mRNA and proteins were determined using real-time RT-PCR and enzyme-linked immunosorbent assay, respectively. Incubation of neural progenitor cells with rhEPO (5 U/mL) in the presence of PI3K/Akt inhibitors (LY29402 and wortmannin) or ERK inhibitors (U0126 and PD98059) (Fukazawa et al, 2002) substantially attenuated activation of Akt or ERK1/2, respectively, and abolished VEGF upregulation in neural progenitor cells (Figure 2) and VEGF proteins in the supernatant (Figure 2). Western blots showed that rhEPO phosphorylated Akt as early as 5 mins after incubation, whereas phosphorylation of ERK1/2 occurred at 10 mins (Figure 3). These data suggest that the PI3K/Akt and ERK1/2 signaling pathways mediate exogenous EPO-augmented VEGF and that the PI3K/Akt pathway may activate ERK1/2.
Figure 3.
Effects of rhEPO on the PI3K/Akt and ERK1/2 signaling pathways. Western blot analysis shows that incubation of neural progenitor cells with rhEPO (EPO) significantly increased phosphorylated Akt (p-AKT; A, B), GSK3α/β (p-GSK; C, D), and ERK1/2 (p-ERK; E, F), but did not affect total Akt (Akt; A), GSK3α/β (GSK; C), and ERK1/2 (ERK; E) compared with the control group. Blockage of the PI3K/Akt pathway with LY294002 (+ LY; A–D) or wortmannin (+ W; A–D) suppressed EPO-activated Akt (A, B) and GSK3α/β (C, D). Inhibition of the ERK1/2 pathway with U0126 (+ U; E, F) or PD9321 (+ PD; E, F) blocked EPO-activated ERK1/2 (E, F). Panel G shows a time course of phosphorylation of Akt and ERK in neural progenitor cells treated with rhEPO. *P < 0.05 and #P < 0.05 versus the control and rhEPO groups, respectively. n = 3/group.
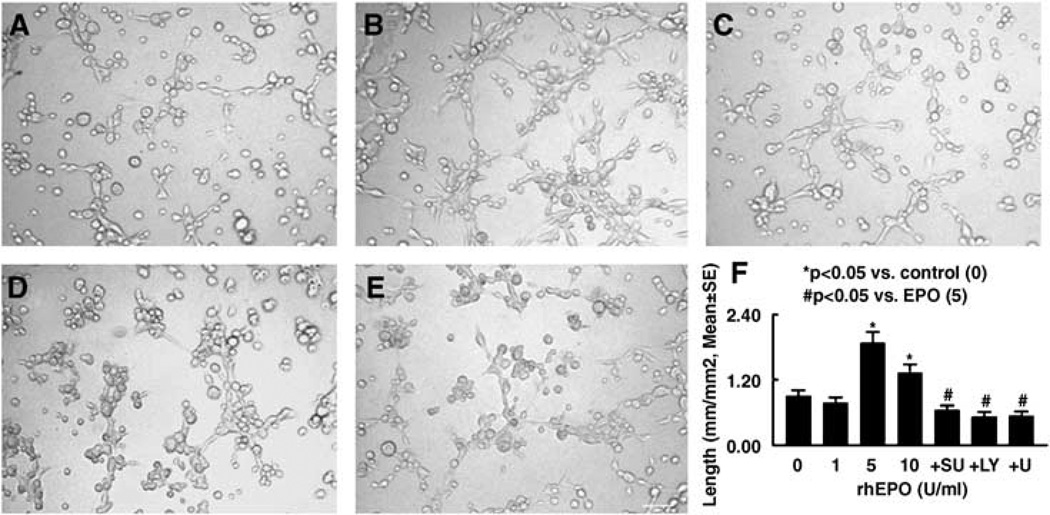
Supernatant Harvested from Neural Progenitor Cells Treated with Recombinant Human Erythropoietin Enhances In Vitro Angiogenesis
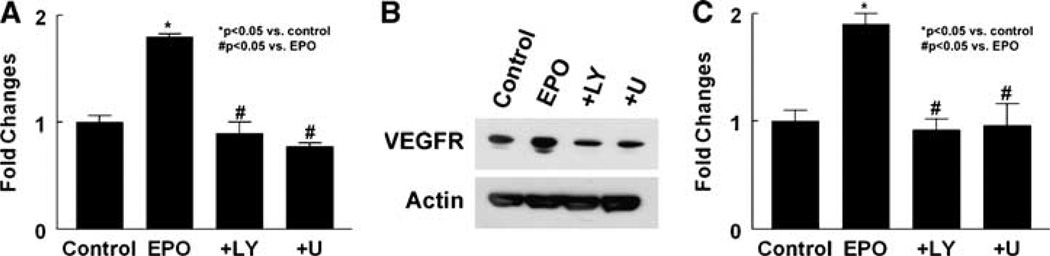
To examine whether VEGF secreted by neural progenitor cells treated with rhEPO promotes angiogenesis, we performed a capillary tube formation assay that has been widely used for in vitro angiogenesis (Haralabopoulos et al, 1994; Wang et al, 2004). Media for culturing neural progenitor cells contain bFGF (20 ng/mL), which stimulates angiogenesis (Schweigerer et al, 1987; Mu et al, 2006). To minimize the effect of bFGF on capillary tube formation, we cultured neural progenitor cells in reduced growth medium (5 ng/mL, bFGF). Incubation of MBECs with the supernatant harvested from neural progenitor cells treated with rhEPO (5 and 10 U/mL) significantly increased the number and length of capillary tubes compared with that in the control group (Figure 4). The supernatant also significantly increased mRNA and protein levels of VEGFR2 in MBECs, whereas the supernatant harvested from neural progenitor cells treated with rhEPO (5 U/mL) in the presence of PI3K/Akt inhibitors (LY29402) or ERK inhibitors (U0126) did not increase VEGFR2 levels in MBECs (Figure 5). Blockage of VEGFR2 with SU1498, a specific antagonist of VEGFR2, suppressed capillary tube formation induced by the supernatant (Figure 4). To further test the effect of VEGFR2 on angiogenesis, endogenous VEGFR2 in MBECs was attenuated using siRNA against mouse VEGFR2. Reverse transcription-PCR analysis showed that VEGFR2 siRNA significantly decreased VEGFR2 mRNA levels (0.47 ± 0.06, n = 3) in MBECs compared with levels (1.0 ± 0.01, n = 3) in the control group, indicating that the VEGFR2 siRNA is effective in attenuating endogenous VEGFR2 expression. When MBECs transfected with VEGR2 siRNA were cultured with the supernatant harvested from neural progenitor cells treated with rhEPO (5 U/mL), the supernatant did not significantly elevate VEGFR2 mRNA levels in these MEBCs (0.84 ± 0.01, n = 3) and did not significantly increase the number (1.16 ± 0.2 mm/mm2, n = 6) of capillary tubes compared with mRNA levels (1.0 ± 0.04, n = 3) and capillary tubes (0.75 ± 0.1 mm/mm2, n = 6) in the control group in which MBECs transfected with VEGR2 siRNA were cultured with the supernatant harvested from neural progenitor cells without treatment with rhEPO.
Figure 4.
Neural progenitor cells treated with rhEPO promote capillary tube formation. Panels A to E show capillary-like tube formation in conditioned media from control (A), neural progenitor cells treated with rhEPO (5 U/mL) alone (B), rhEPO and SU1498 (+ SU; C), rhEPO and LY294002 (+ LY; D), and rhEPO and U0126 (+ U; E). Panel F shows quantitative data of capillary tube formation. Bar = 100 µm. n = 6/group.
Figure 5.
Effect of neural progenitor cells treated with rhEPO on expression of VEGFR2 in MBECs. Real-time RT-PCR (A) shows VEGFR2 mRNA levels in MBECs cultured with the supernatant harvested from neural progenitor cells treated with rhEPO (EPO), rhEPO and LY294002 (+ LY), or rhEPO and U0126 (+ U). Western blot (B) analysis shows VEGFR2 protein levels in MBECs cocultured with neural progenitor cells treated with rhEPO (EPO), rhEPO and LY294002 (+ LY), or rhEPO and U0126 (+ U). Panel C is quantitative data of VEGFR2 protein levels. Glyceraldehyde-3-phosphate dehydrogenase and actin were used as internal controls for real-time RT-PCR and western blot analysis, respectively. *P < 0.05 and #P < 0.05 versus the control and rhEPO groups, respectively. n = 3/group.
To examine whether EPOR is required for upregulation of VEGFR2 in MBECs induced by the supernatant, rhEPO (5 U/mL) was incubated with neural progenitor cells derived from ΔEPOR mice. The supernatant harvested from this incubation did not elevate VEGFR2 mRNA levers in MBECs (1.43 ± 0.08, n = 3) and did not increase the number of capillary tubes (1.41 ± 0.1 mm/mm2, n = 6) compared with mRNA levels (1.0 ± 0.09, n = 3) and capillary tubes (1.2 ± 0.2 mm/mm2, n = 6) in the control group in which MBECs were incubated with the supernatant harvested from neural progenitor cells from ΔEPOR mice without rhEPO treatment.
Collectively, these results suggest that VEGF secreted by rhEPO-treated neural progenitor cells interacts with upregulated VEGFR2 in MBECs to stimulate in vitro angiogenesis.
Discussion
Our in vitro data show that rhEPO stimulated neural progenitor cells to secrete VEGF through endogenous EPOR and blockage of the PI3K/Akt and ERK1/2 signaling pathways with pharmacological inhibitors abolished VEGF secretion enhanced by rhEPO. Moreover, the supernatant harvested from neural progenitor cells treated with rhEPO upregulated VEGFR2 expression in MBECs and augmented capillary tube-like formation, whereas blockage of VEGFR2 in MBECs suppressed the effect of the supernatant on in vitro angiogenesis. Therefore, this study suggests that the PI3K/Akt and ERK1/2 signaling pathways mediate VEGF secretion by interaction of exogenous EPO with EPOR in neural progenitor cells and that VEGF secreted by neural progenitor cells along with VEGFR2 in cerebral endothelial cells promotes angiogenesis.
Adult SVZ neural progenitor cells express many angiogenic factors, including VEGF (Deleyrolle et al, 2006; Liu et al, 2007). This study shows that exogenous EPO stimulated the neural progenitor cells to secrete VEGF in a dose-dependent manner, suggesting a specific effect of exogenous EPO on neural progenitor cells. However, it remains to be determined whether rhEPO-increased VEGF protein results from increases of transcription and/or the stability of VEGF mRNA. The effect of EPO on VEGF requires EPOR in neural progenitor cells because increases in VEGF protein levels were not detected in the supernatant harvested from neural progenitor cells derived from ΔEPOR mice after incubation with rhEPO. These in vitro data are consistent with previous in vivo findings that treatment of stroke with EPO increased brain VEGF levels (Wang et al, 2004).
Erythropoietin interacts with its receptor and activates many signaling pathways, including two important kinase cascades, the PI3K/Akt and ERK1/2 signaling pathways (Arcasoy and Jiang, 2005). Consistent with published studies, this study shows that treatment of neural progenitor cells with rhEPO activated Akt and its downstream target GSK3α/β, and ERK1/2 (Dong et al, 2001; Wang et al, 2006a, b). Blockage of the PI3K/Akt and ERK1/2 pathways with pharmacological inhibitors abolished rhEPO-augmented VEGF. A time-course western blot analysis showed that activation of Akt by rhEPO occurs before phosphorylation of ERK1/2. These data suggest that exogenous EPO activates the PI3K/Akt and ERK1/2 signaling pathways that promote neural progenitor cells to secrete VEGF and the PI3K/Akt pathway may trigger activation of the ERK1/2 pathway.
Vascular endothelial growth factor mediates neurogenesis by augmenting proliferation and neuronal differentiation of neural progenitor cells (Jin et al, 2002; Meng et al, 2006). However, whether VEGF secreted by neural progenitor cells has any effects on angiogenesis has not been fully investigated (Teng et al, 2008). Using an in vitro angiogenesis assay, this study shows that the supernatant harvested from neural progenitor cells treated with rhEPO augmented capillary tube-like formation in MBECs, whereas the supernatant obtained from neural progenitor cells treated with rhEPO in the presence of inhibitors for PI3K/Akt and ERK1/2 did not increase capillary tube formation. In addition, the supernatant harvested from neural progenitor cells treated with rhEPO upregulated VEGFR2 expression in MBECs. Furthermore, blockage of VEGFR2 with SU1498, a VEGFR2 tyrosine kinase inhibitor, or attenuation of endogenous VEGFR2 expression in MBECs with siRNA against VEGFR2 substantially reduced capillary tube formation augmented by the supernatant harvested from rhEPO-treated neural progenitor cells. Although MBECs constitutively express VEGFR2, the specific effect of rhEPO on upregulation of VEGFR2 in MBECs is confirmed by showing that the supernatant harvested from ΔEPOR neural progenitor cells treated with rhEPO abolished EPO-enhanced VEGFR2 expression and capillary tube formation. Collectively, these data suggest that in addition to VEGF, the supernatant harvested from neural progenitor cells treated with rhEPO could upregulate VEGFR2 expression in endothelial cells and that VEGF in the supernatant interacts with upregulated VEGFR2 in the endothelial cells to promote angiogenesis. However, this study does not identify which factor(s) in the supernatant upregulates VEGFR2 in MBECs and we also cannot rule out that other angiogenic factors such as angiopoietin family genes may also be involved. Further studies are warranted.
In summary, our results show that VEGF secreted by rhEPO-treated neural progenitor cells promotes angiogenesis and that the PI3K/Akt and ERK1/2 pathways mediate upregulation of VEGF in neural progenitor cells whereas the supernatant harvested from rhEPO-treated neural progenitor cells increases VEGFR2 levels in cerebral endothelial cells.
Acknowledgments
This work was supported by NINDS Grants PO1 NS23393, PO1 NS42345, RO1NS43324, and RO1HL 64766
References
- Arcasoy MO, Jiang X. Co-operative signalling mechanisms required for erythroid precursor expansion in response to erythropoietin and stem cell factor. Br J Haematol. 2005;130:121–129. doi: 10.1111/j.1365-2141.2005.05580.x. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Chen ZY, Asavaritikrai P, Prchal JT, Noguchi CT. Endogenous erythropoietin signaling is required for normal neural progenitor cell proliferation. J Biol Chem. 2007;282:25875–25883. doi: 10.1074/jbc.M701988200. [DOI] [PubMed] [Google Scholar]
- Chiasson BJ, Tropepe V, Morshead CM, van der Kooy D. Adult mammalian forebrain ependymal and subependymal cells demonstrate proliferative potential, but only subependymal cells have neural stem cell characteristics. J Neurosci. 1999;19:4462–4471. doi: 10.1523/JNEUROSCI.19-11-04462.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deleyrolle L, Marchal-Victorion S, Dromard C, Fritz V, Saunier M, Sabourin JC, Tran Van Ba C, Privat A, Hugnot JP. Exogenous and fibroblast growth factor 2/epidermal growth factor-regulated endogenous cytokines regulate neural precursor cell growth and differentiation. Stem Cells. 2006;24:748–762. doi: 10.1634/stemcells.2005-0138. [DOI] [PubMed] [Google Scholar]
- Dong G, Chen Z, Li ZY, Yeh NT, Bancroft CC, Van Waes C. Hepatocyte growth factor/scatter factor-induced activation of MEK and PI3K signal pathways contributes to expression of proangiogenic cytokines interleukin-8 and vascular endothelial growth factor in head and neck squamous cell carcinoma. Cancer Res. 2001;61:5911–5918. [PubMed] [Google Scholar]
- Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9:669–676. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- Fukazawa H, Noguchi K, Murakami Y, Uehara Y. Mitogen-activated protein/extracellular signal-regulated kinase kinase (MEK) inhibitors restore anoikis sensitivity in human breast cancer cell lines with a constitutively activated extracellular-regulated kinase (ERK) pathway. Mol Cancer Ther. 2002;1:303–309. [PubMed] [Google Scholar]
- Gotts JE, Chesselet MF. Vascular changes in the subventricular zone after distal cortical lesions. Exp Neurol. 2005;194:139–150. doi: 10.1016/j.expneurol.2005.02.001. [DOI] [PubMed] [Google Scholar]
- Haralabopoulos GC, Grant DS, Kleinman HK, Lelkes PI, Papaioannou SP, Maragoudakis ME. Inhibitors of basement membrane collagen synthesis prevent endothelial cell alignment in matrigel in vitro and angiogenesis in vivo. Lab Invest. 1994;71:575–582. [PubMed] [Google Scholar]
- Jiang BH, Zheng JZ, Aoki M, Vogt PK. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc Natl Acad Sci USA. 2000;97:1749–1753. doi: 10.1073/pnas.040560897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Minami M, Lan JQ, Mao XO, Batteur S, Simon RP, Greenberg DA. Neurogenesis in dentate sub-granular zone and rostral subventricular zone after focal cerebral ischemia in the rat. Proc Natl Acad Sci USA. 2001;98:4710–4715. doi: 10.1073/pnas.081011098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin K, Zhu Y, Sun Y, Mao XO, Xie L, Greenberg DA. Vascular endothelial growth factor (VEGF) stimulates neurogenesis in vitro and in vivo. Proc Natl Acad Sci USA. 2002;99:11946–11950. doi: 10.1073/pnas.182296499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu XS, Zhang ZG, Zhang RL, Gregg S, Morris DC, Wang Y, Chopp M. Stroke induces gene profile changes associated with neurogenesis and angiogenesis in adult subventricular zone progenitor cells. J Cereb Blood Flow Metab. 2007;27:564–574. doi: 10.1038/sj.jcbfm.9600371. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2 method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Louissaint A, Jr, Rao S, Leventhal C, Goldman SA. Coordinated interaction of neurogenesis and angiogenesis in the adult songbird brain. Neuron. 2002;34:945–960. doi: 10.1016/s0896-6273(02)00722-5. [DOI] [PubMed] [Google Scholar]
- Matsumoto T, Claesson-Welsh L. VEGF receptor signal transduction. Sci STKE. 2001;2001:RE21. doi: 10.1126/stke.2001.112.re21. [DOI] [PubMed] [Google Scholar]
- Meng H, Zhang Z, Zhang R, Liu X, Wang L, Robin AM, Chopp M. Biphasic effects of exogenous VEGF on VEGF expression of adult neural progenitors. Neurosci Lett. 2006;393:97–101. doi: 10.1016/j.neulet.2005.09.044. [DOI] [PubMed] [Google Scholar]
- Morshead CM, Reynolds BA, Craig CG, McBurney MW, Staines WA, Morassutti D, Weiss S, van der Kooy D. Neural stem cells in the adult mammalian forebrain: a relatively quiescent subpopulation of subependymal cells. Neuron. 1994;13:1071–1082. doi: 10.1016/0896-6273(94)90046-9. [DOI] [PubMed] [Google Scholar]
- Mu H, Ohashi R, Yan S, Chai H, Yang H, Lin P, Yao Q, Chen C. Adipokine resistin promotes in vitro angiogenesis of human endothelial cells. Cardiovasc Res. 2006;70:146–157. doi: 10.1016/j.cardiores.2006.01.015. [DOI] [PubMed] [Google Scholar]
- Ohab JJ, Fleming S, Blesch A, Carmichael ST. A neurovascular niche for neurogenesis after stroke. J Neurosci. 2006;26:13007–13016. doi: 10.1523/JNEUROSCI.4323-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer TD, Willhoite AR, Gage FH. Vascular niche for adult hippocampal neurogenesis. J Comp Neurol. 2000;425:479–494. doi: 10.1002/1096-9861(20001002)425:4<479::aid-cne2>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Parent JM, Vexler ZS, Gong C, Derugin N, Ferriero DM. Rat forebrain neurogenesis and striatal neuron replacement after focal stroke. Ann Neurol. 2002;52:802–813. doi: 10.1002/ana.10393. [DOI] [PubMed] [Google Scholar]
- Rikitake Y, Hirata K, Kawashima S, Ozaki M, Takahashi T, Ogawa W, Inoue N, Yokoyama M. Involvement of endothelial nitric oxide in sphingosine-1-phosphate-induced angiogenesis. Arterioscler Thromb Vasc Biol. 2002;22:108–114. doi: 10.1161/hq0102.101843. [DOI] [PubMed] [Google Scholar]
- Schweigerer L, Neufeld G, Friedman J, Abraham JA, Fiddes JC, Gospodarowicz D. Capillary endothelial cells express basic fibroblast growth factor, a mitogen that promotes their own growth. Nature. 1987;325:257–259. doi: 10.1038/325257a0. [DOI] [PubMed] [Google Scholar]
- Sengupta TK, Talbot ES, Scherle PA, Ivashkiv LB. Rapid inhibition of interleukin-6 signaling and Stat3 activation mediated by mitogen-activated protein kinases. Proc Natl Acad Sci USA. 1998;95:11107–11112. doi: 10.1073/pnas.95.19.11107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shingo T, Sorokan ST, Shimazaki T, Weiss S. Erythropoietin regulates the in vitro and in vivo production of neuronal progenitors by mammalian forebrain neural stem cells. J Neurosci. 2001;21:9733–9743. doi: 10.1523/JNEUROSCI.21-24-09733.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Studer L, Csete M, Lee SH, Kabbani N, Walikonis J, Wold B, McKay R. Enhanced proliferation, survival, and dopaminergic differentiation of CNS precursors in lowered oxygen. J Neurosci. 2000;20:7377–7383. doi: 10.1523/JNEUROSCI.20-19-07377.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taguchi A, Soma T, Tanaka H, Kanda T, Nishimura H, Yoshikawa H, Tsukamoto Y, Iso H, Fujimori Y, Stern DM, Naritomi H, Matsuyama T. Administration of CD34+ cells after stroke enhances neurogenesis via angiogenesis in a mouse model. J Clin Invest. 2004;114:330–338. doi: 10.1172/JCI20622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng H, Zhang ZG, Wang L, Zhang RL, Zhang L, Morris D, Gregg SR, Wu Z, Jiang A, Lu M, Zlokovic BV, Chopp M. Coupling of angiogenesis and neurogenesis in cultured endothelial cells and neural progenitor cells after stroke. J Cereb Blood Flow Metab. 2008;28:764–771. doi: 10.1038/sj.jcbfm.9600573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai PT, Ohab JJ, Kertesz N, Groszer M, Matter C, Gao J, Liu X, Wu H, Carmichael ST. A critical role of erythropoietin receptor in neurogenesis and post-stroke recovery. J Neurosci. 2006;26:1269–1274. doi: 10.1523/JNEUROSCI.4480-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Chopp M. Activation of the PI3-K/Akt pathway mediates cGMP enhanced-neurogenesis in the adult progenitor cells derived from the subventricular zone. J Cereb Blood Flow Metab. 2005;25:1150–1158. doi: 10.1038/sj.jcbfm.9600112. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Gregg SR, Zhang RL, Jiao Z, LeTourneau Y, Liu X, Feng Y, Gerwien J, Torup L, Leist M, Noguchi CT, Chen ZY, Chopp M. The Sonic hedgehog pathway mediates carbamylated erythropoietin-enhanced proliferation and differentiation of adult neural progenitor cells. J Biol Chem. 2007;282:32462–32470. doi: 10.1074/jbc.M706880200. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Wang Y, Zhang RL, Chopp M. Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke. 2004;35:1732–1737. doi: 10.1161/01.STR.0000132196.49028.a4. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Gregg SR, Hozeska-Solgot A, LeTourneau Y, Wang Y, Chopp M. Matrix metalloproteinase 2 (MMP2) and MMP9 secreted by erythropoietin-activated endothelial cells promote neural progenitor cell migration. J Neurosci. 2006a;26:5996–6003. doi: 10.1523/JNEUROSCI.5380-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhang ZG, Zhang RL, Jiao ZX, Wang Y, Pourabdollah-Nejad DS, LeTourneau Y, Gregg SR, Chopp M. Neurogenin 1 mediates erythropoietin enhanced differentiation of adult neural progenitor cells. J Cereb Blood Flow Metab. 2006b;26:556–564. doi: 10.1038/sj.jcbfm.9600215. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Wang L, Zhang L, Chen J, Zhu Z, Zhang ZG, Chopp M. Nitric oxide enhances angiogenesis via the synthesis of vascular endothelial growth factor and cGMP after stroke in the rat. Circ Res. 2003;92:308–313. doi: 10.1161/01.res.0000056757.93432.8c. [DOI] [PubMed] [Google Scholar]
- Zhang RL, Zhang ZG, Zhang L, Chopp M. Proliferation and differentiation of progenitor cells in the cortex and the subventricular zone in the adult rat after focal cerebral ischemia. Neuroscience. 2001;105:33–41. doi: 10.1016/s0306-4522(01)00117-8. [DOI] [PubMed] [Google Scholar]