Abstract
MicroRNAs (miRNAs) are small noncoding RNAs, which regulate the expression of their target genes post-transcriptionally by RNA interference. They are involved in almost all cellular processes, including proliferation, differentiation, apoptosis, cell survival and the maintenance of tissue specificity. Recent findings also suggest that efflux pumps of the ABC (ATP-binding cassette) transporter family are subject to miRNA-mediated gene regulation. Moreover, it seems that ABC transporters are embedded in a concerted and miRNA-guided network of concurrently regulated proteins that mediate altered drug transport and cell survival in changing environmental conditions. In this review, we summarize recent findings of miRNAs interacting with ABC transporters, which have been connected with drug distribution as well as with drug resistance. Additionally, we specify findings of complex miRNA–protein pathways conferring increased drug export and cell survival.
Keywords: ABC transporter, drug resistance, epigenetics, gene regulation, microRNA
Introduction
The living organism is constantly exposed to various amounts of exogenous substances of different toxicity as well as endogenous products of metabolism. In order not to completely cede the access, distribution and elimination of these compounds exclusively to the physical process of diffusion, evolution developed a filter system consisting of transport proteins, which can transport substrates against their concentration gradient. These membrane-bound transport pumps build a functional border between the body and its environment or between different tissue compartments. In particular, transporter proteins of the ABC (ATP-binding cassette) family play a major ‘doorman’ function 1–3. It is essential for the organism that the expression of these proteins is tightly regulated and can be instantly adapted to changing environmental conditions entailing altered substrate concentrations. Despite transcriptional regulation 4,5 or ‘on-demand’ trafficking of transporter proteins from intracellular storage to the membrane 6,7, it is becoming more obvious that epigenetic mechanisms, such as DNA methylation and histone modifications, modify the expression of ABC transporters and contribute to increased drug efflux and cell survival 8. In the concert of transcriptional and epigenetic gene expression control, microRNAs (miRNAs) play an important linking part, contributing to adaptive regulation of ABC transporters. By RNA interference, miRNAs lead to inhibition of translational processes or messenger RNA (mRNA) degradation of their target genes.
In this review, we provide a brief overview of recent findings about the role of miRNAs in the regulation of human ABC transporters and how this interaction affects intracellular drug levels and drug resistance.
Processing, function and regulation of miRNA transcription
MicroRNAs are small noncoding RNA molecules of 20–24 nucleotides, initially cleaved from transcripts of several hundred nucleotides with multiple hairpin loop structures. Sequential processing, involving the RNase III Drosha, first generates precursor miRNAs (pre-miRs) exhibiting a single hairpin structure and a length of 70–100 nucleotides (nts). These pre-miRNAS are exported from the nucleus into the cytoplasm and are cleaved by RNase III endonuclease dicer into miRNA/miRNA* duplexes. After degradation of one of the RNA strands, the mature single-stranded miRNA guides the RNA-induced silencing complex (RISC) to the 3′-untranslated region (3′-UTR) of the target mRNA. Consequently, the target mRNA is degraded or the translational process inhibited 9–12. It is noteworthy that one single miRNA can target dozens of target genes and one gene can be regulated by dozens of different miRNAs, emphasizing the complexity of the interplay between miRNAs and their target genes.
The miRNA expression profile varies during development as well as between distinct tissue types. Moreover, miRNAs are regulated in a similar manner to protein-coding genes 13,14. There is increasing evidence that miRNAs are regulated by autoregulatory, double-negative or reciprocal negative feedback loops, involving transcription factors and their active downstream cell pathways 15. Transcription factors bind within a 1 kb region upstream of the miRNA genes 16, interacting with cis-regulatory motifs found upstream of the predicted transcription start site, thereby repressing or activating miRNA gene expression.
MicroRNAs targeting ABCB1
The most prominent and so far most intensely investigated member of the ABC transporter family is P-glycoprotein (P-gp), encoded by the ABCB1 gene, also known as multidrug-resistance gene (MDR1). It is apically expressed and exhibits two nucleotide-binding domains (NBDs) and two membrane-spanning domains (MSDs). P-glycoprotein limits the uptake of xenobiotics across membrane barriers, e.g. of the intestine or brain. In the liver and the kidney, P-gp contributes to the elimination of its substrates. In chemotherapeutic-resistant cancer cell lines, P-gp is often observed to be upregulated and is suggested to contribute to the phenomenon of drug resistance 17.
MicroRNAs leading to downregulation of P-glycoprotein in malignant cells
Table 1 and Figure 1 summarize recent findings about miRNAs leading to a downregulation of P-gp expression. In 2008, by comparison of parental MCF-7 breast cancer cells with their doxorubicin-resistant counterpart (MCF-7/DOX), an increased expression of P-gp and anti-apoptotic proteins B-cell CLL/lymphoma 6 (BCL6) and NOTCH1 was observed. In the resistant cell line, the expression level of miR-451 was below the limit of detection. Using 3′-UTR reporter gene assays, the authors could confirm the negatively regulating effect of miRNA-451 on P-gp expression. In turn, transfection of miR-451 re-established the sensitivity of the MCF-7/DOX cells to doxorubicin 18. Also, colon cancer spheres exhibited a decreased expression of miR-144/451 in comparison to parental colorectal cancer cells 19. Transfection of precursor miR-451 conferred a decrease of tumorigenicity and self-renewal to colon spheres. Additionally, macrophage migration inhibitory factor, cyclo-oxygenase-2 and P-gp were found to be downregulated. Macrophage migration inhibitory factor is another confirmed target of miR-451 20 and was shown to increase the expression of cyclo-oxygenase-2 19.
Table 1.
MicroRNAs interfering with ABCB1 expression
| MicroRNA | Effect of miRNA on gene expression | Mechanism | Tissue | Reference |
|---|---|---|---|---|
| MicroRNAs associated with decreased P-glycoprotein expression | ||||
| miR-27a | P-gp↓; | * | K562 chronic myelogenous leukaemia cell line, bone marrow of acute myeloid leukaemia and acute lymphoid leukaemia patients | 21 |
| miR-137 | P-gp↓ | Indirect, probably via Y-box binding protein-1*22 | Human breast cancer cell line MCF-7 | 22 |
| miR-145 | P-gp↓ | * | Human colon carcinoma cell line Caco-2 | 66 |
| miR-200c | P-gp↓ | Direct (in silico predicted binding site) or via BMI-1* 25 and ZEB1* 51 | Human breast cancer cell line MCF-7, breast cancer tissue | 24 |
| miR-298 | P-gp↓ | * | Human breast cancer cell line MDA-MB-231 | 23 |
| miR-331-5p | P-gp↓ | * | Chronic myelogenous leukaemia cell line K562, bone marrow of acute myeloid leukaemia and acute lymphoid leukaemia patients | 21 |
| miR-451 | P-gp↓ | * | Human breast cancer cell line MCF-7 | 18 |
| Macrophage migration inhibitory factor* 20/cyclo-oxygenase-2/P-gp | Spheres of colorectal cancer cell lines DLD1, HT29, LS513 and SW620 | 19 | ||
| miR-1253 | P-gp↓ | * | Human breast cancer cell line MDA-MB-231 | 23 |
| MicroRNAs associated with increased P-glycoprotein expression | ||||
| miR-27a | P-gp↑ | ++ | Human oesophageal squamous cell lines ECA109 and TE-13 | 32 |
| ++ | Human gastric cancer cell line MKN45 | 33 | ||
| ++ | Ovarian cancer cell line A2780 | 31 | ||
| Indirect, maybe via repressing HIPK2 | Ovarian cancer cell line A2780 | 34 | ||
| miR-138 | P-gp↑ | ++ | Human leukaemia cell line HL-60 | 30 |
| miR-296 | P-gp↑ | ++ | Human oesophageal squamous cell line ECA109 | 35 |
| miR-451 | P-gp↑ | ++ | Ovarian cancer cell line A2780 | 31 |
Symbols are as follows: ++, indirect, exact mechanism unknown; and P-gp, P-glycoprotein.
Confirmed mRNA/miRNA interference by 3′-untranslated region (3′-UTR) reporter gene assay.
Figure 1.
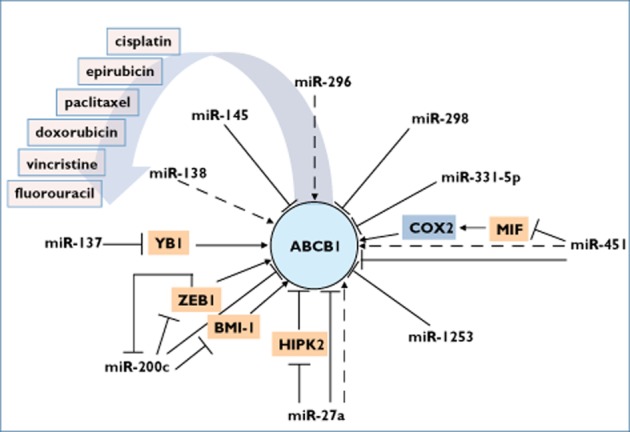
ABCB1 in the complex network of microRNAs (miRNAs) and transcription factors affecting the transport of given drugs. Dashed arrows indicate an indirect upregulating impact of the miRNA on ABCB1 expression, maybe via a repressive transcription factor. Bars indicate direct RNA interference between miRNA and target mRNA. BMI-1, BMI1 polycomb ring finger oncogene; COX2, cyclooxygenase 2; HIPK2, homeodomain interacting protein kinase 2; MIF, macrophage migration inhibitory factor; YB1, Y-box binding protein 1; ZEB1, zinc finger E-box binding homeobox 1
miR-331-5p and miR-27a were inversely correlated with doxorubicin resistance and P-gp expression in K562 chronic myelogenous leukaemia cells 21. Sensitivity to doxorubicin was synergistically increased after transfection of miRNA 27a and miR-331-5p. Moreover, in bone marrow samples obtained from acute myeloid leukaemia and acute lymphoid leukaemia patients with complete remission compared with samples obtained from relapsed patients, both miRNAs showed a lower expression pattern 21.
In doxorubicin-resistant MCF-7 cells, miR-137 was also found to be downregulated and inversely expressed with Y-box binding protein-1 (YB1) and P-gp. miR-137 was confirmed to interact with YB1 by RNA interference. Accordingly, restoration of miR-137 caused a downregulation of YB1 and P-gp, leading to increased sensitivity to doxorubicin, vincristine and paclitaxel. It is noteworthy that YB1 is a transcription factor for ABCB1 and binds to the ABCB1 promoter in a stress response manner 22.
MicroRNA array-based comparison of doxorubicin-resistant human breast cancer cells (MDA-MB-231-R) with their doxorubicin-sensitive counterpart (MDA-MB-231-S) revealed a downregulation of miR-298. At the same time, the MDA-MB-231-R cells showed a higher expression of P-gp. 3′-UTR reporter gene assays identified binding sites for miR-298 and for miR-1253, which both synergistically impair the translation of P-gp. Transfection of miR-298 and miR-1253 caused downregulation of P-gp and increased doxorubicin sensitivity. In turn, transfection of miR-298 inhibitor into MDA-MB-231-S cells conferred doxorubicin resistance and increased P-gp expression. Northern blot experiments showed that the resistant cells expressed pri-miR-298, but compared with sensitive cells, no further processing to mature miR-298 was detected. Although the resistant cells exhibited no difference in Drosha expression, the authors suggested an impaired miR-298 processing as a potential reason for the observed doxorubicin resistance due to a lower expression of Dicer 23.
Breast cancer patients who exhibited a poor response to epirubicin treatment showed lower miR-200c expression compared with patients who showed a good response. In line with these in vivo findings, parental MCF-7 cells revealed a strong downregulation of miR-200c in contrast to their doxorubicin-resistant counterpart, MCF-7/ADR. Restoration of miR-200c induced accumulation and cytotoxicity of doxorubicin in MCF-7/ADR cells, which was linked to a decrease of P-gp expression. Although in vitro confirmation experiments were not performed, the authors reported an in silico predicted miR-200c binding site in the 3′-UTR of ABCB1 24. Because breast cancer stem cells were associated with low expression of the miR-200 family 25 and with increased invasiveness 26–29, these findings suggest P-gp as a further puzzle piece in a miR-200 family-co-ordinated acquisition of chemoresistance in cancer stem cells and in cancer cell lines.
MicroRNAs leading to an upregulation of P-glycoprotein in malignant cells
Table 1 and Figure 1 summarize recent findings about miRNAs leading to an upregulation of P-gp expression. Transfection of miR-138 in vincristine-resistant human leukaemia cell line HL-60/VCR led to a decreased expression of P-gp and the anti-apoptotic protein B-cell CLL/lymphoma 2 (BCL-2). Additionally, the apoptotic protein BCL2-associated X protein (BAX) showed an increased expression pattern. Cotransfection of ABCB1 promoter gene vector construct with miR-138 inhibitor led to a decreased reporter gene activity, suggesting an indirect influence of miR-138 on ABCB1 expression, maybe by RNA interference with a repressive acting transcription factor 30.
Another experiment in doxorubicin-resistant ovarian cancer cell lines (A2780DX5) showed downregulation of P-gp mediated by antagonizing miR-451 and miR-27a 31. Downregulation of miR-27a resulted in a decrease of P-gp expression in oesophageal squamous carcinoma and in gastric cancer cells 32,33.
After transfection of miR-27a inhibitor, Li et al. reported a downregulation of P-gp and an increased sensitivity to paclitaxel in the paclitaxel-resistant ovarian cancer cell line (A2780/Taxol). As antagonism of miR-27a additionally increased the protein expression of co-repressor homeodomain interacting protein kinase 2 (HIPK2), showing an in silico predicted miR-27a binding site, the authors postulated a miRNA-27a/HIPK2/P-gp pathway 34.
However, compared with the reports mentioned above 18,19,21, the partly inconsistent results concerning the influence of miR-451 and miR-27a on P-gp expression and the regulating mechanisms, how P-gp is downregulated by antagonizing miR-27a or miR-451, remain to be clarified.
Oesophageal squamous cell cancer was characterized by an increase in miR-296 expression and, vice versa, lower miR-296 expression was associated with prolonged survival. Transfection of miR-296 inhibitor in human oesophageal squamous cells (ECA109) decreased the IC50 values of vincristine, adriamycin (doxorubicin), 5-fluorouracil and cisplatin. Also, the tumourigenicity of ECA109 cells transfected with miR-296 inhibitor was reduced in athymic nude mice in comparison to cells transfected with negative control. Antagonizing miR-296 in ECA109 cells caused a downregulation of P-gp, which was accompanied by an upregulation of interferon alpha-inducible protein 27 (IFI27; p27) and a decrease of cyclin D1. Moreover, the pro-apoptotic protein BAX showed an increased expression, whereas the anti-apoptotic protein BCL2 was downregulated. Cotransfection of ABCB1 promoter gene vector construct with increasing concentrations of miR-296 inhibitor led to a decreased reporter gene activity, implying an indirect interaction between P-gp and miR-296, maybe via a repressive transcription factor that has not yet been identified 35.
Regarding all reports about miRNA-mediated inhibition of P-gp expression via direct RNA interference, it has to be critically mentioned that the current version of ABCB1 cDNA (accession no. NM_000927.4) exhibits a 3′-UTR sequence of 382 nts, whereas the former version (accession no. NM_000927.3) had a length of 611 nts. Based on NM_000927.4, only miR-145, reported by Ikemura et al. to target ABCB1 3′-UTR 36, may show slight in silico predicted overlapping with the currently published ABCB1 3′-UTR. In these circumstances, investigations to elucidate potential tissue-specific differences in lengths of ABCB1 3′-UTR should be considered.
MircroRNAs targeting ABCG2
Table 2 and Figure 2 summarize miRNAs associated with post-transcriptional regulation of ABCG2 expression. In contrast to ABC transporters of the B and C family, ABCG2 (breast cancer-resistance protein; BCRP) contains a single NBD and a single MSD and is therefore often termed a ‘half transporter’. In order to build a functional transport unit, two ABCG2 proteins have to homodimerize 37. It is apically expressed at blood–tissue barriers of placenta, brain, intestine, liver and kidney. Moreover, it is expressed in stem cells (therefore, also used as a stem cell marker), as well as in drug-resistant cancer cells, where it limits intracellular bioavailability of chemotherapeutics 38–40.
Table 2.
MicroRNAs interfering with ABCG2 expression
| MicroRNA | Effect of miRNA on gene expression | Mechanism | Tissue | Reference |
|---|---|---|---|---|
| miR-200c | ABCG2↓ and ABCG5↓ | Indirect, probably via BMI-1* 25 and ZEB1* 51 | Primary melanomas and metastatic melanomas, human melanoma cell lines (WM35, WM793, WM115A, WM3523A and 1205Lu) | 50 |
| miR-212 | ABCG2↓ | * | Human chronic myelogenous leukaemia cells K562 | 47 |
| miR-328 | ABCG2↓ | * | Human breast cancer cell line MCF-7 | 45,46 |
| * | Human retinoblastoma cell line RB143 | 45 | ||
| ++ | Human chronic myelogenous leukaemia cells K562 | 47 | ||
| * | Side population of colorectal cancer cell lines (SW1116, LoVo, HCT116 and SW480) and colorectal cancer tissue | 49 | ||
| miR-519c | ABCG2↓ | * | Human colon cancer cell line S1 | 41,42 |
| * | Human breast cancer cell line MCF-7 and human retinoblastoma cell line RB143 | 45 | ||
| miR-520h | ABCG2↓ | * * | CD34+CD38− haematopoietic stem cells from human umbilical cord blood | 43 |
| * | Human pancreatic cancer cell line PANC-1 | 44 | ||
| * | Human retinoblastoma cell line RB143 | 45 |
Symbols are as follows: ++, indirect, exact mechanism unknown.
Confirmed mRNA/miRNA interference by 3′-UTR reporter gene assay.
Figure 2.
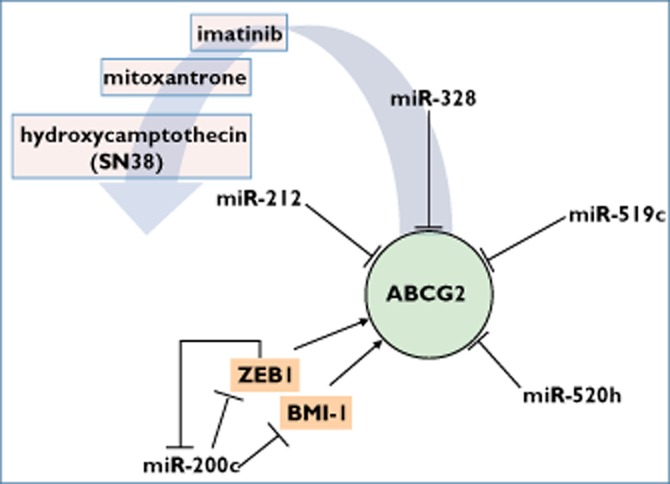
ABCG2 in the network of miRNAs and transcription factors, leading to altered transport of given drugs. Bars indicate direct RNA interference between miRNA and target mRNA. BMI-1, BMI1 polycomb ring finger oncogene; ZEB1, zinc finger E-box binding homeobox 1
In 2008, To et al. identified a miR-519c binding site in the ABCG2 3′-UTR. Interestingly, in comparison to parental S1 colon cancer cells, the mitoxantrone-resistant cell line S1MI80 lost its miR-519c-mediated post-transcriptional control of ABCG2 by expression of shortened ABCG2 3′-UTRs. The binding site of miR-520h, located 5′ upstream of the miR-519c binding site, was not affected by the observed 3′-UTR truncation. However, the drug-resistant cells expressed miR-520h at a lower level than the parental cells, implying another mechanism to upregulate ABCG2 expression and to acquire chemoresistance 41,42. miR-520h was reported earlier to target ABCG2 mRNA by RNA interference and to be involved in haematopoietic stem cell differentiation 43 and in increased ABCG2 expression, migration and invasion of pancreatic cancer cells 44. In 2011, Li et al. reported a proximally located miR-519c binding site, which overlaps the miR-520h binding site 45, confirming the findings of To et al. 41,42, to have an additive negative regulating effect of miR-519c on ABCG2 expression depending on the number of its binding sites.
In mitoxantrone-resistant human breast cancer MCF-7 cells, miR-328 was shown to be inversely regulated with ABCG2. It could also be shown that transfection of miR-328 caused increased sensitivity to mitoxantrone and downregulation of ABCG2 in MCF-7/MX100 cells 45,46. After sorting of the human retinoblastoma cells RB143, the expression of miR-519c, miR-520h and miR-328 was observed to be lower in ABCG2-expressing than in ABCG2-non-expressing cancer cells. Direct binding of miR-328 to ABCG2 3′-UTR was confirmed by reporter gene assays 45,46.
By treating K562 human chronic myelogenous leukaemia cells with increasing concentrations of imatinib, we observed an inverse regulation of ABCG2 with both miR-328 and miR-212 47. In a reporter gene assay, miR-212 was shown to inversely regulate ABCG2, whereas miR-328 could not be confirmed as directly targeting ABCG2 mRNA. Imatinib treatment with concentrations lower than 1 μmol l−1 elevated ABCG2 and reduced miR-212 and miR-328 expressions, whereas concentrations higher than 1 μmol l−1 led to strong recovery of expression of both miRNAs accompanied by declining ABCG2 expression 47. Considering ABCG2 as stem cell marker, this observation could be of value in avoiding selection of drug-resistant stem-like cancer cells by incorrect dosing of imatinib 48.
A subgroup of colorectal cancer cells known as side population cells exhibited not only stem cell-like properties, such as tumourigenicity, self-renewal, invasiveness and chemoresistance against hydroxycamptothecin, but also significantly lower miR-328 expression levels than their non-side population counterpart. Moreover, ABCG2 and matrix metalloprotease 16 were confirmed to be controlled by miR-328 through RNA interference, underlining the crucial role of miR-328 in the maintenance of stem cell properties and invasiveness of cancer cells 49.
In melanomas, compared with melanocytic naevi, the stem cell factor BMI-1, as well as the ABC transporters ABCG2, ABCG5 and ABCB1, were recently described to be expressed inversely with miR-200c and E-cadherin 50. While Chen et al. reported an in silico predicted binding site of miR-200c in ABCB1 3′-UTR 24, it may additionally be speculated that miR-200c mediates an indirect regulation of the efflux transporters ABCG2, ABCG5 and ABCB1, as well as the process of epithelial–mesenchymal transition. This process may be managed via its direct targets, BMI-1 (a member of the polycomb group proteins conferring chromatin modifications and responsible for maintenance of stem cell features by suppressing differentiation and apoptosis) 25 and ZEB1 (zinc finger E-box binding homeobox 1; a transcriptional repressor of E-cadherin) 51. Moreover, it seems that ZEB1 acts as repressive transcription factor for stem cell properties, inhibiting miRNAs including the miR-200 family, suggesting a reciprocal negative feedback loop between miR-200c and ZEB1 52,53. Taken together, these findings could be summarized as miR-200c/BMI-1/ABCG2, ABCG5, ABCB1 and miR-200c/ZEB1/E-cadherin pathways, both probably conferring in many cancers a progressive, invasive and chemoresistant phenotype.
MicroRNAs targeting members of the ABC C family
Table 3 and Figure 3 summarize recent findings on miRNAs interfering with the expression of members of the ABC C and E family. Further important ABC transporters involved in disposition and efflux of many endo-and xenobiotics, as well as in the phenomenon of drug resistance, belong to the ABC C family. Members of this protein family are comprised of two NBDs and two MSDs (ABCC4, ABCC5, ABCC11 and ABCC12) or three MSDs (ABCC1, ABCC2, ABCC3, ABCC6 and ABCC10) 36.
Table 3.
MicroRNAs interfering with the expression of transporters of the ABC C and E family
| ABC transporter | MicroRNA | Effect of miRNA on gene expression | Mechanism | Tissue | Reference |
|---|---|---|---|---|---|
| ABCC1 | miR-326 | ABCC1↓ | * | MCF-7 human breast cancer cells, breast cancer tissue | 54 |
| miR-199a/b and miR-296 | ABCC1↓ | * | Hepatocellular carcinoma | 58 | |
| ABCC2 | miR-297 | ABCC2↓ | * | Human ileocaecal colorectal adenocarcinoma cell line HCT-8 and human colorectal cancer cell line HCT-116 | 55 |
| miR-379 | ABCC2↓ | * | HepG2 human hepatoblastoma cells | 56 | |
| ABCC3 | miR-9* | ABCC3↓ | Indirect, via ID4 and SOX2* | Glioma cell lines (A172, A1207, LN18 and LN229) and human glioma stem cells derived from patients with glioma | 57 |
| ABCC4 | miR-125a/b | ABCC4↓ | *(weak) | Hepatocellular carcinoma | 58 |
| ABCC5 | miR-101, miR-125a and let-7a | ABCC5↓ | * | Hepatocellular carcinoma | 58 |
| ABCC6 | miR-9* | ABCC6↓ | Indirect, via ID4 and SOX2* | Glioma cell lines (A172, A1207, LN18 and LN229) and human glioma stem cells derived from patients with glioma | 57 |
| ABCC10 | let-7a/e | ABCC10↓ | * | Hepatocellular carcinoma | 58 |
| ABCE1 | miR-26a, miR-135b and miR-145 | ABCE1↓ | * | Hepatocellular carcinoma | 58 |
ID4, inhibitor of differentiation 4; SOX2, SRY (sex determining region Y)-box 2. Symbols are as follows: ++, indirect, exact mechanism unknown.
Confirmed mRNA/miRNA interference by 3′-UTR reporter gene assay.
Figure 3.
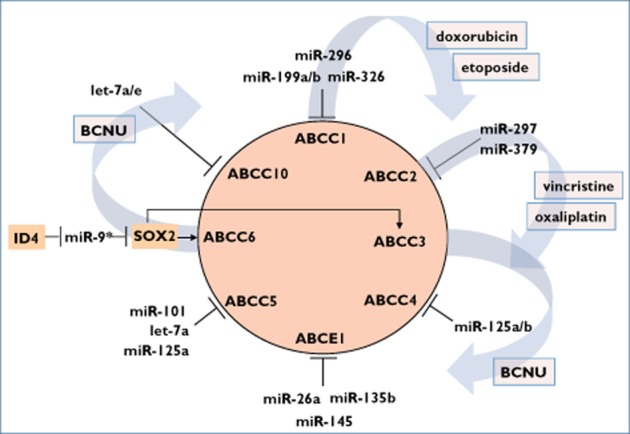
MicroRNAs and transcription factors associated with regulation of transport proteins of the ABC C and E family. Drugs are given, which have been described to be altered in their intracellular accumulation by this network. Bars indicate direct RNA interference between miRNA and target mRNA. ID4, inhibitor of DNA binding 4 (also known as inhibitor of differentiation 4); SOX2, SRY (sex determining region Y)-box 2; BCNU, 1,3-bis(2-chloroethyl)-1-nitrosourea
In contrast to P-gp and ABCG2, ABCC1 was found to be overexpressed in human breast cancer cells resistant to etoposide (MCF-7/VP-16) in comparison to its parental cell line, MCF-7. A microarray study revealed miR-326 to be downregulated in resistant cells. Transfection of miR-326 into MCF-7/VP-16 cells downregulated ABCC1 expression and increased the sensitivity to etoposide and doxorubicin, but not to mitoxantrone. Interestingly, ABCG2-overexpressing and mitoxantrone-resistant MCF-7 cells were not affected in their etoposide sensitivity by miR-326 transfection 54. Moreover, in normal breast tissue, early breast cancer tissue without metastasis and advanced breast cancer tissue with metastasis, a continuous decline of miR-326 expression could be observed, leading inversely to highest ABCC1 expression in advanced breast cancer tissues. Direct miRNA/mRNA interaction was additionally confirmed by 3′-UTR reporter gene assays 54.
In human colorectal cancer tissue samples, an inverse stage-dependent correlation of miR-297 and ABCC2 mRNA and protein expression was observed. miR-297 is able to downregulate ABCC2 expression by directly targeting ABCC2 3′-UTR. Transfection of miR-297 in vitro increases chemosensitivity in colorectal cancer cell lines resistant to vincristine (HCT-8/VCR) or oxaliplatin (HCT-116/L-OHP) as well as in vivo in HCT-116/L-OHP cells after being injected in nude mice 55. ABCC2 mRNA was also identified to be targeted by miR-379 through RNA interference in HepG2 human hepatoblastoma cells. Interestingly, the PXR-ligand rifampicin mediated an upregulation of miR-379, suggesting an initial PXR/miR-379/ABCC2 negative feedback mechanism preventing cells from ABCC2 protein overexpression 56.
Jeon et al. reported that ID4 (inhibitor of differentiation 4) inhibits the expression of miR-9* in glioma cells and that miR-9* targets the stem cell marker SRY (sex determining region Y)-box 2 (SOX2) by direct RNA interference. SOX2 in turn transcriptionally induces the ABC transporter ABCC3 and ABCC6, thereby contributing to chemoresistance against 1,3-bis(2-chloroethyl)-1-nitrosourea (BCNU) and stem cell maintenance in glioma stem cells. These data suggest a possible ID4/miR-9*/SOX2/ABCC3, ABCC6 pathway to be active 57.
By conducting an mRNA and miRNA expression study in hepatocellular carcinoma and adjacent healthy liver tissues followed by reporter gene-based target gene validation, Borel et al. identified ABCC1 as a target of miR-199a/b and miR-296, ABCC4 as a target of miR-125a/b, ABCC5 as a target of miR-101, miR-125a and let-7a, ABCC10 as a target of let-7a/e, and ABCE1 as a target of miR-26a, miR-135b and miR-145 58. However, the functional influence of the observed miRNA/mRNA associations in hepatocellular carcinoma remains to be elucidated.
Summary
Efflux pumps of the ABC transporter family are key players in distribution and elimination of endo-and xenobiotics. Several studies describe ABC transporters concurrently regulated with proteins related to apoptosis, cell cycle, stress response, stem-cell-ness, invasiveness and tumourigenicity. There is increasing evidence that miRNAs are crucially involved in co-ordinating and fine-tuning this complex network of proteins mediating increased drug efflux and cell survival. MicroRNAs therefore play an important role not only in altered drug distribution, but also in the phenomenon of drug resistance 59. Furthermore, it may be speculated that in reaction to (long-term) treatment almost every drug could generate a distinct cellular miRNA expression pattern, leading to a drug-specific survival mechanism and specific ABC transporter expression. In this context, it also has to be mentioned that genetic mutations or polymorphisms might have an interindividual influence on miRNA-mediated gene regulation by leading to altered seed sequences 60,61 or mRNA secondary structures. The increasing knowledge about the function of miRNAs makes them interesting candidates as biomarkers of diseases and in the management of drug therapy 62–64. Moreover, the challenging question is whether miRNAs can be delivered in vivo in a targeted manner and therefore, if miRNAs can be used as drugs in the correction of pathogenic deregulated protein networks or in the optimization of classic therapeutic regimens 65,66.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Begley DJ. ABC transporters and the blood-brain barrier. Curr Pharm Des. 2004;10:1295–1312. doi: 10.2174/1381612043384844. [DOI] [PubMed] [Google Scholar]
- 2.Dietrich CG, Geier A, Oude Elferink RP. ABC of oral bioavailability: transporters as gatekeepers in the gut. Gut. 2003;52:1788–1795. doi: 10.1136/gut.52.12.1788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ho RH, Kim RB. Transporters and drug therapy: implications for drug disposition and disease. Clin Pharmacol Ther. 2005;78:260–277. doi: 10.1016/j.clpt.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 4.Kohle C, Bock KW. Coordinate regulation of human drug-metabolizing enzymes, and conjugate transporters by the Ah receptor, pregnane X receptor and constitutive androstane receptor. Biochem Pharmacol. 2009;77:689–699. doi: 10.1016/j.bcp.2008.05.020. [DOI] [PubMed] [Google Scholar]
- 5.Pascussi JM, Gerbal-Chaloin S, Duret C, Daujat-Chavanieu M, Vilarem MJ, Maurel P. The tangle of nuclear receptors that controls xenobiotic metabolism and transport: crosstalk and consequences. Annu Rev Pharmacol Toxicol. 2008;48:1–32. doi: 10.1146/annurev.pharmtox.47.120505.105349. [DOI] [PubMed] [Google Scholar]
- 6.Kipp H, Arias IM. Trafficking of canalicular ABC transporters in hepatocytes. Annu Rev Physiol. 2002;64:595–608. doi: 10.1146/annurev.physiol.64.081501.155793. [DOI] [PubMed] [Google Scholar]
- 7.Kipp H, Pichetshote N, Arias IM. Transporters on demand: intrahepatic pools of canalicular ATP binding cassette transporters in rat liver. J Biol Chem. 2001;276:7218–7224. doi: 10.1074/jbc.M007794200. [DOI] [PubMed] [Google Scholar]
- 8.Chen KG, Sikic BI. Molecular pathways: regulation and therapeutic implications of multidrug resistance. Clin Cancer Res. 2012;18:1863–1869. doi: 10.1158/1078-0432.CCR-11-1590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ghildiyal M, Zamore PD. Small silencing RNAs: an expanding universe. Nat Rev Genet. 2009;10:94–108. doi: 10.1038/nrg2504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Racz Z, Kaucsar T, Hamar P. The huge world of small RNAs: regulating networks of microRNAs (review) Acta Physiol Hung. 2011;98:243–251. doi: 10.1556/APhysiol.98.2011.3.1. [DOI] [PubMed] [Google Scholar]
- 11.Skalsky RL, Cullen BR. Viruses, microRNAs, and host interactions. Annu Rev Microbiol. 2010;64:123–141. doi: 10.1146/annurev.micro.112408.134243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wiemer EA. The role of microRNAs in cancer: no small matter. Eur J Cancer. 2007;43:1529–1544. doi: 10.1016/j.ejca.2007.04.002. [DOI] [PubMed] [Google Scholar]
- 13.Neilson JR, Zheng GX, Burge CB, Sharp PA. Dynamic regulation of miRNA expression in ordered stages of cellular development. Genes Dev. 2007;21:578–589. doi: 10.1101/gad.1522907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Obernosterer G, Leuschner PJ, Alenius M, Martinez J. Post-transcriptional regulation of microRNA expression. RNA. 2006;12:1161–1167. doi: 10.1261/rna.2322506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hobert O. Common logic of transcription factor and microRNA action. Trends Biochem Sci. 2004;29:462–468. doi: 10.1016/j.tibs.2004.07.001. [DOI] [PubMed] [Google Scholar]
- 16.Lee EJ, Baek M, Gusev Y, Brackett DJ, Nuovo GJ, Schmittgen TD. Systematic evaluation of microRNA processing patterns in tissues, cell lines, and tumors. RNA. 2008;14:35–42. doi: 10.1261/rna.804508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhou SF. Structure, function and regulation of P-glycoprotein and its clinical relevance in drug disposition. Xenobiotica. 2008;38:802–832. doi: 10.1080/00498250701867889. [DOI] [PubMed] [Google Scholar]
- 18.Kovalchuk O, Filkowski J, Meservy J, Ilnytskyy Y, Tryndyak VP, Chekhun VF, Pogribny IP. Involvement of microRNA-451 in resistance of the mcf-7 breast cancer cells to chemotherapeutic drug doxorubicin. Mol Cancer Ther. 2008;7:2152–2159. doi: 10.1158/1535-7163.MCT-08-0021. [DOI] [PubMed] [Google Scholar]
- 19.Bitarte N, Bandres E, Boni V, Zarate R, Rodriguez J, Gonzalez-Huarriz M, Lopez I, Javier Sola J, Alonso MM, Fortes P, Garcia-Foncillas J. MicroRNA-451 is involved in the self-renewal, tumorigenicity, and chemoresistance of colorectal cancer stem cells. Stem Cells. 2011;29:1661–1671. doi: 10.1002/stem.741. [DOI] [PubMed] [Google Scholar]
- 20.Bandres E, Bitarte N, Arias F, Agorreta J, Fortes P, Agirre X, Zarate R, Diaz-Gonzalez JA, Ramirez N, Sola JJ, Jimenez P, Rodriguez J, Garcia-Foncillas J. MicroRNA-451 regulates macrophage migration inhibitory factor production and proliferation of gastrointestinal cancer cells. Clin Cancer Res. 2009;15:2281–2290. doi: 10.1158/1078-0432.CCR-08-1818. [DOI] [PubMed] [Google Scholar]
- 21.Feng DD, Zhang H, Zhang P, Zheng YS, Zhang XJ, Han BW, Luo XQ, Xu L, Zhou H, Qu LH, Chen YQ. Down-regulated miR-331-5p and miR-27a are associated with chemotherapy resistance and relapse in leukaemia. J Cell Mol Med. 2011;15:2164–2175. doi: 10.1111/j.1582-4934.2010.01213.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu X, Li Y, Shen H, Li H, Long L, Hui L, Xu W. MiR-137 restoration sensitizes multidrug-resistant mcf-7/ADM cells to anticancer agents by targeting YB-1. Acta Biochim Biophys Sin (Shanghai) 2013;45:80–86. doi: 10.1093/abbs/gms099. [DOI] [PubMed] [Google Scholar]
- 23.Bao L, Hazari S, Mehra S, Kaushal D, Moroz K, Dash S. Increased expression of P-glycoprotein and doxorubicin chemoresistance of metastatic breast cancer is regulated by miR-298. Am J Pathol. 2012;180:2490–2503. doi: 10.1016/j.ajpath.2012.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Tian W, Cai H, He H, Deng Y. Down-regulation of microRNA-200c is associated with drug resistance in human breast cancer. Med Oncol. 2012;29:2527–2534. doi: 10.1007/s12032-011-0117-4. [DOI] [PubMed] [Google Scholar]
- 25.Shimono Y, Zabala M, Cho RW, Lobo N, Dalerba P, Qian D, Diehn M, Liu H, Panula SP, Chiao E, Dirbas FM, Somlo G, Pera RA, Lao K, Clarke MF. Downregulation of miRNA-200c links breast cancer stem cells with normal stem cells. Cell. 2009;138:592–603. doi: 10.1016/j.cell.2009.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- 27.Gregory PA, Bracken CP, Smith E, Bert AG, Wright JA, Roslan S, Morris M, Wyatt L, Farshid G, Lim YY, Lindeman GJ, Shannon MF, Drew PA, Khew-Goodall Y, Goodall GJ. An autocrine TGF-beta/ZEB/miR-200 signaling network regulates establishment and maintenance of epithelial-mesenchymal transition. Mol Biol Cell. 2011;22:1686–1698. doi: 10.1091/mbc.E11-02-0103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lim Y, Wright JA, Attema JL, Gregory PA, Bert AG, Smith E, Thomas D, Drew PA, Khew-Goodall Y, Goodall GJ. Epigenetic modulation of the miR-200 family is associated with transition to a breast cancer stem-cell-like state. J Cell Sci. 2013;126:2256–2266. doi: 10.1242/jcs.122275. [DOI] [PubMed] [Google Scholar]
- 29.Park SM, Gaur AB, Lengyel E, Peter ME. The miR-200 family determines the epithelial phenotype of cancer cells by targeting the E-cadherin repressors ZEB1 and ZEB2. Genes Dev. 2008;22:894–907. doi: 10.1101/gad.1640608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhao X, Yang L, Hu J, Ruan J. miR-138 might reverse multidrug resistance of leukemia cells. Leuk Res. 2010;34:1078–1082. doi: 10.1016/j.leukres.2009.10.002. [DOI] [PubMed] [Google Scholar]
- 31.Zhu H, Wu H, Liu X, Evans BR, Medina DJ, Liu CG, Yang JM. Role of microRNA miR-27a and miR-451 in the regulation of MDR1/P-glycoprotein expression in human cancer cells. Biochem Pharmacol. 2008;76:582–588. doi: 10.1016/j.bcp.2008.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zhang H, Li M, Han Y, Hong L, Gong T, Sun L, Zheng X. Down-regulation of miR-27a might reverse multidrug resistance of esophageal squamous cell carcinoma. Dig Dis Sci. 2010;55:2545–2551. doi: 10.1007/s10620-009-1051-6. [DOI] [PubMed] [Google Scholar]
- 33.Zhao X, Yang L, Hu J. Down-regulation of miR-27a might inhibit proliferation and drug resistance of gastric cancer cells. J Exp Clin Cancer Res. 2011;30:55. doi: 10.1186/1756-9966-30-55. doi: 10.1186/1756-9966-30-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Li Z, Hu S, Wang J, Cai J, Xiao L, Yu L, Wang Z. MiR-27a modulates MDR1/P-glycoprotein expression by targeting HIPK2 in human ovarian cancer cells. Gynecol Oncol. 2010;119:125–130. doi: 10.1016/j.ygyno.2010.06.004. [DOI] [PubMed] [Google Scholar]
- 35.Hong L, Han Y, Zhang H, Li M, Gong T, Sun L, Wu K, Zhao Q, Fan D. The prognostic and chemotherapeutic value of miR-296 in esophageal squamous cell carcinoma. Ann Surg. 2010;251:1056–1063. doi: 10.1097/SLA.0b013e3181dd4ea9. [DOI] [PubMed] [Google Scholar]
- 36.Ikemura K, Yamamoto M, Miyazaki S, Mizutani H, Iwamoto T, Okuda M. MicroRNA-145 post-transcriptionally regulates the expression and function of P-glycoprotein in intestinal epithelial cells. Mol Pharmacol. 2013;83:399–405. doi: 10.1124/mol.112.081844. [DOI] [PubMed] [Google Scholar]
- 37.Haimeur A, Conseil G, Deeley RG, Cole SP. The MRP-related and BCRP/ABCG2 multidrug resistance proteins: biology, substrate specificity and regulation. Curr Drug Metab. 2004;5:21–53. doi: 10.2174/1389200043489199. [DOI] [PubMed] [Google Scholar]
- 38.Mo W, Zhang JT. Human ABCG2: structure, function, and its role in multidrug resistance. Int J Biochem Mol Biol. 2012;3:1–27. [PMC free article] [PubMed] [Google Scholar]
- 39.Scharenberg CW, Harkey MA, Torok-Storb B. The ABCG2 transporter is an efficient Hoechst 33342 efflux pump and is preferentially expressed by immature human hematopoietic progenitors. Blood. 2002;99:507–512. doi: 10.1182/blood.v99.2.507. [DOI] [PubMed] [Google Scholar]
- 40.van Herwaarden AE, Schinkel AH. The function of breast cancer resistance protein in epithelial barriers, stem cells and milk secretion of drugs and xenotoxins. Trends Pharmacol Sci. 2006;27:10–16. doi: 10.1016/j.tips.2005.11.007. [DOI] [PubMed] [Google Scholar]
- 41.To KK, Robey RW, Knutsen T, Zhan Z, Ried T, Bates SE. Escape from hsa-miR-519c enables drug-resistant cells to maintain high expression of ABCG2. Mol Cancer Ther. 2009;8:2959–2968. doi: 10.1158/1535-7163.MCT-09-0292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.To KK, Zhan Z, Litman T, Bates SE. Regulation of ABCG2 expression at the 3′ untranslated region of its mRNA through modulation of transcript stability and protein translation by a putative microRNA in the S1 colon cancer cell line. Mol Cell Biol. 2008;28:5147–5161. doi: 10.1128/MCB.00331-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liao R, Sun J, Zhang L, Lou G, Chen M, Zhou D, Chen Z, Zhang S. MicroRNAs play a role in the development of human hematopoietic stem cells. J Cell Biochem. 2008;104:805–817. doi: 10.1002/jcb.21668. [DOI] [PubMed] [Google Scholar]
- 44.Wang F, Xue X, Wei J, An Y, Yao J, Cai H, Wu J, Dai C, Qian Z, Xu Z, Miao Y. Hsa-miR-520h downregulates abcg2 in pancreatic cancer cells to inhibit migration, invasion, and side populations. Br J Cancer. 2010;103:567–574. doi: 10.1038/sj.bjc.6605724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li X, Pan YZ, Seigel GM, Hu ZH, Huang M, Yu AM. Breast cancer resistance protein BCRP/ABCG2 regulatory microRNAs (hsa-miR-328,-519c and-520h) and their differential expression in stem-like ABCG2+ cancer cells. Biochem Pharmacol. 2011;81:783–792. doi: 10.1016/j.bcp.2010.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pan YZ, Morris ME, Yu AM. MicroRNA-328 negatively regulates the expression of breast cancer resistance protein (BCRP/ABCG2) in human cancer cells. Mol Pharmacol. 2009;75:1374–1379. doi: 10.1124/mol.108.054163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Turrini E, Haenisch S, Laechelt S, Diewock T, Bruhn O, Cascorbi I. MicroRNA profiling in K-562 cells under imatinib treatment: influence of miR-212 and miR-328 on ABCG expression. Pharmacogenet Genomics. 2012;22:198–205. doi: 10.1097/FPC.0b013e328350012b. [DOI] [PubMed] [Google Scholar]
- 48.Corbin AS, Agarwal A, Loriaux M, Cortes J, Deininger MW, Druker BJ. Human chronic myeloid leukemia stem cells are insensitive to imatinib despite inhibition of BCR-ABL activity. J Clin Invest. 2011;121:396–409. doi: 10.1172/JCI35721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Xu XT, Xu Q, Tong JL, Zhu MM, Nie F, Chen X, Xiao SD, Ran ZH. MicroRNA expression profiling identifies miR-328 regulates cancer stem cell-like sp cells in colorectal cancer. Br J Cancer. 2012;106:1320–1330. doi: 10.1038/bjc.2012.88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Liu S, Tetzlaff MT, Cui R, Xu X. MiR-200c inhibits melanoma progression and drug resistance through down-regulation of BMI-1. Am J Pathol. 2012;181:1823–1835. doi: 10.1016/j.ajpath.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tryndyak VP, Beland FA, Pogribny IP. E-cadherin transcriptional down-regulation by epigenetic and microRNA-200 family alterations is related to mesenchymal and drug-resistant phenotypes in human breast cancer cells. Int J Cancer. 2010;126:2575–2583. doi: 10.1002/ijc.24972. [DOI] [PubMed] [Google Scholar]
- 52.Brabletz S, Brabletz T. The ZB/miR-200 feedback loop – a motor of cellular plasticity in development and cancer? EMBO Rep. 2010;11:670–677. doi: 10.1038/embor.2010.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schuler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T. The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol. 2009;11:1487–1495. doi: 10.1038/ncb1998. [DOI] [PubMed] [Google Scholar]
- 54.Liang Z, Wu H, Xia J, Li Y, Zhang Y, Huang K, Wagar N, Yoon Y, Cho HT, Scala S, Shim H. Involvement of miR-326 in chemotherapy resistance of breast cancer through modulating expression of multidrug resistance-associated protein 1. Biochem Pharmacol. 2010;79:817–824. doi: 10.1016/j.bcp.2009.10.017. [DOI] [PubMed] [Google Scholar]
- 55.Xu K, Liang X, Shen K, Cui D, Zheng Y, Xu J, Fan Z, Qiu Y, Li Q, Ni L, Liu J. MiR-297 modulates multidrug resistance in human colorectal carcinoma by down-regulating MRP-2. Biochem J. 2012;446:291–300. doi: 10.1042/BJ20120386. [DOI] [PubMed] [Google Scholar]
- 56.Haenisch S, Laechelt S, Bruckmueller H, Werk A, Noack A, Bruhn O, Remmler C, Cascorbi I. Down-regulation of ATP-binding cassette C2 protein expression in HEPG2 cells after rifampicin treatment is mediated by microRNA-379. Mol Pharmacol. 2011;80:314–320. doi: 10.1124/mol.110.070714. [DOI] [PubMed] [Google Scholar]
- 57.Jeon HM, Sohn YW, Oh SY, Kim SH, Beck S, Kim S, Kim H. ID4 imparts chemoresistance and cancer stemness to glioma cells by derepressing miR-9*-mediated suppression of SOX2. Cancer Res. 2011;71:3410–3421. doi: 10.1158/0008-5472.CAN-10-3340. [DOI] [PubMed] [Google Scholar]
- 58.Borel F, Han R, Visser A, Petry H, van Deventer SJ, Jansen PL, Konstantinova P. Adenosine triphosphate-binding cassette transporter genes up-regulation in untreated hepatocellular carcinoma is mediated by cellular microRNAs. Hepatology. 2012;55:821–832. doi: 10.1002/hep.24682. [DOI] [PubMed] [Google Scholar]
- 59.Haenisch S, Cascorbi I. miRNAs as mediators of drug resistance. Epigenomics. 2012;4:369–381. doi: 10.2217/epi.12.39. [DOI] [PubMed] [Google Scholar]
- 60.Landi D, Gemignani F, Landi S. Role of variations within microRNA-binding sites in cancer. Mutagenesis. 2012;27:205–210. doi: 10.1093/mutage/ger055. [DOI] [PubMed] [Google Scholar]
- 61.Saunders MA, Liang H, Li WH. Human polymorphism at microRNAs and microRNA target sites. Proc Natl Acad Sci U S A. 2007;104:3300–3305. doi: 10.1073/pnas.0611347104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Kong YW, Ferland-McCollough D, Jackson TJ, Bushell M. MicroRNAs in cancer management. Lancet Oncol. 2012;13:e249–258. doi: 10.1016/S1470-2045(12)70073-6. [DOI] [PubMed] [Google Scholar]
- 63.Wittmann J, Jack HM. Serum microRNAs as powerful cancer biomarkers. Biochim Biophys Acta. 2010;1806:200–207. doi: 10.1016/j.bbcan.2010.07.002. [DOI] [PubMed] [Google Scholar]
- 64.Rukov JL, Shomron N. Microrna pharmacogenomics: post-transcriptional regulation of drug response. Trends Mol Med. 2011;17:412–423. doi: 10.1016/j.molmed.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 65.Janssen HL, Reesink HW, Lawitz EJ, Zeuzem S, Rodriguez-Torres M, Patel K, van der Meer AJ, Patick AK, Chen A, Zhou Y, Persson R, King BD, Kauppinen S, Levin AA, Hodges MR. Treatment of HCV infection by targeting microRNA. N Engl J Med. 2013;368:1685–1694. doi: 10.1056/NEJMoa1209026. [DOI] [PubMed] [Google Scholar]
- 66.Uchino K, Ochiya T, Takeshita F. RNAi therapeutics and applications of microRNAs in cancer treatment. Jpn J Clin Oncol. 2013;43:596–607. doi: 10.1093/jjco/hyt052. [DOI] [PubMed] [Google Scholar]


