Abstract
The activity of the enzyme thiopurine methyltransferase (TPMT) is regulated by a common genetic polymorphism. One in 300 individuals lack enzyme activity and 11% are heterozygous for a variant low activity allele and have an intermediate activity. The thiopurine drugs azathioprine, mercaptopurine and thioguanine are substrates for TPMT; these drugs exhibit well documented myelosuppressive effects on haematopoietic cells and have a track record of idiosyncratic drug reactions. The development of severe bone marrow toxicity, in patients taking standard doses of thiopurine drugs, is associated with TPMT deficiency whilst the TPMT heterozygote is at an increased risk of developing myelosuppression. Factors influencing TPMT enzyme activity, as measured in the surrogate red blood cell, are discussed in this review to enable an appreciation of why concordance between TPMT genotype and phenotype is not 100%. This is particularly important for lower/intermediate TPMT activities to avoid misclassification of TPMT status. TPMT testing is now widely available in routine service laboratories. The British National Formulary suggests TPMT testing before starting thiopurine drugs. Dermatologists were quick to adopt routine TPMT testing whilst gastroenterologists do not specifically recommend TPMT screening. TPMT testing is mandatory prior to the use of mercaptopurine in childhood leukaemia. Thiopurine drug dose and other treatment related influences on cell counts explain some of the differing recommendations between clinical specialities. TPMT testing is cost-effective and the major role is in the identification of the TPMT deficient individual prior to the start of thiopurine drugs.
Keywords: azathioprine, childhood leukaemia, mercaptopurine, thioguanine nucleotides, thiopurine methyltransferase, TPMT
Introduction
Individual variations in human red blood cell (RBC) thiopurine methyltransferase (TPMT, E.C.2.1.1.67) activity were first described by Weinshilboum et al. 1 in the late 1970s. Subsequent Caucasian population studies demonstrated that the level of TPMT activity was inherited in an autosomal codominant fashion. The frequency distribution of TPMT activities conformed to Hardy–Weinberg predictions for the inheritance of two alleles one for high (TPMTH) and one for low (TPMTL) enzyme activity. Approximately 89% of a randomly selected population were homozygous for an allele for high RBC TPMT activity, about 11% heterozygous with an intermediate activity and one in every 300 subjects homozygous for an allele for low RBC TPMT activity, the latter lacking detectable TPMT activity 2. It was soon established that the genetic polymorphism controlling RBC TPMT activity also controlled the level of enzyme activity in all other cells and tissues 3–5 but it was over a decade later before the TPMT gene was isolated, sequenced and the variant alleles described at a genetic level 6–8. Controversy remains over various aspects of TPMT genotype/phenotype concordance and whether genotype or phenotype is the most accurate predictor of TPMT status.
The TPMT genetic polymorphism represents a well validated example of the clinical importance of pharmacogenetics 9. Very low, or deficient, TPMT activity is associated with grossly abnormal thiopurine drug metabolism, excess production of cytotoxic metabolites and profound life-threatening myelotoxicity, in patients taking thiopurine drugs. Although this association was reported in the late 1980s 10, there was, initially, a minimal use of TPMT testing prior to the start of thiopurine drug therapy. TPMT analysis was confined to university research centres and not generally available to the clinician. Over the decades TPMT testing has slowly been accepted as a routine test by some specialities and is now widely available 11–13. Testing is recommended on starting thiopurine drugs 9,14,15, but testing is not universally accepted and, when available, how to interpret and apply the TPMT result within a clinical setting is not always clear. Why is this?
Clinical impact of TPMT
The thiopurine drugs, thioguanine, mercaptopurine and azathioprine (a ‘slow-release’ formulation of mercaptopurine), exhibit well-documented myelosuppressive toxic effects on haematopoietic cells. They have a track record of idiosyncratic drug reactions. Severe, life-threatening bone marrow toxicity is due to the excess production of drug derived thioguanine nucleotide (TGN) metabolites 16,17 precipitated by TPMT deficiency 10,18–21. The direct incorporation of TGN derived thioguanine into DNA initiates delayed cytotoxicity 22,23. In addition, the TGNs inhibit intracellular signalling pathways and can induce apoptotic cell death 24–26.
Bone marrow toxicity can also develop in TPMT heterozygotes (‘intermediate’ activity, 11% of subjects 2) when taking standard doses of mercaptopurine 27,28. However, the drug has a narrow therapeutic index. In children with acute lymphoblastic leukaemia (ALL) receiving mercaptopurine chemotherapy, those with lower TPMT activities form higher concentrations of TGN metabolites and have better outcomes 27,29–32. The use of the TPMT genetic polymorphism in the individualization of mercaptopurine therapy in childhood ALL was pioneered by Relling et al. 28,33. Lowering mercaptopurine doses, to predefined TGN concentrations, in TPMT heterozygous and homozygous deficient children, avoided gaps in treatment caused by mercaptopurine-induced neutropenias and enabled the delivery of all other chemotherapeutic agents at maximum tolerated doses 28,33.
Mercaptopurine metabolism is complex (Figure 1). The TGNs are the end-products of a chain of nucleotide metabolites. The initial mercaptopurine nucleotide metabolite can also be methylated by TPMT and the methylmercaptopurine nucleotides (MeMPNs) formed are powerful inhibitors of de novo purine synthesis and promote TGN cytotoxicity 34,35. Thus, in vivo, when the mercaptopurine dose is titrated to target cell counts within ALL chemotherapeutic protocols, intracellular TGN formation differs markedly with TPMT status. For the same target cell count range the TPMT deficient child (on much reduced mercaptopurine dosages) accumulates far higher TGNs than the TPMT heterozygote, and the latter far higher than the TPMT wild-type patient 27,28,36.
Figure 1.
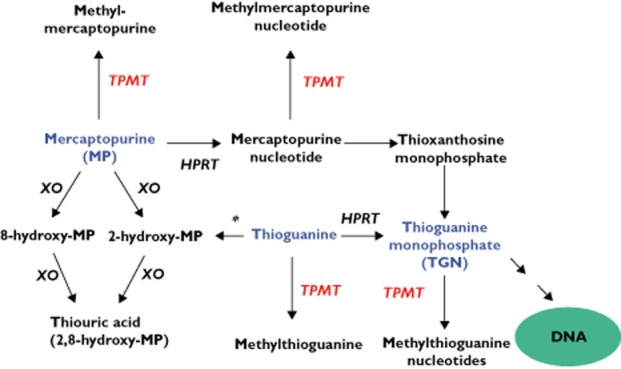
Thiopurine metabolism. Azathioprine is a mercaptopurine pro-drug. Initial nucleotide formation is catalyzed by hypoxanthine phosphoribosyltransferase (HPRT). Mercaptopurine, mercaptopurine nucleotide (thioinosine monophosphate), thioguanine and thioguanine nucleotide (TGN; thioguanosine monophosphate) are methylated by thiopurine methyltransferase (TPMT). The thioguanine nucleotides (TGNs) are the mono-, di-and tri-phosphates of thioguanosine. TGN incorporation into DNA initiates delayed cytotoxicity. TGN cytotoxicity can be promoted by the inhibition of de novo purine synthesis by the methylmercaptopurine nucleotides (MeMPNs). The TGNs also inhibit intracellular signalling pathways. This contributes to thiopurine immunosuppression and can induce apoptotic cell death. Oxidation is catalyzed by xanthine oxidase (XO). Thioguanine requires deamination by guanase (*) before oxidation. The 8-hydroxymercaptopurine metabolite is a good TPMT substrate whilst the 2-hydroxy metabolites (2-hydroxymercaptopurine and 2,8-hydroxymercaptopurine) are potent TPMT inhibitors
Mercaptopurine pharmacogenetics were exposed to a wider clinical audience when the clinical importance of the individualization of thiopurine therapy, underpinned by the TPMT genetic polymorphism, emerged as relevant to thiopurine immunosuppression in, primarily, inflammatory bowel disease (IBD) 37,38. Red cell TGNs were associated with clinical response to mercaptopurine in paediatric and adult IBD 39–41; TPMT heterozygotes accumulated higher TGN concentrations 39. IBD patients with lower TPMT activities had a statistically significant relapse free advantage 42. However, all adverse events (e.g. myelotoxic, gastrointestinal, allergic), were significantly more frequent in IBD patients with lower TPMT activities than those with ‘normal’ activity 43,44. The narrow therapeutic window for mercaptopurine was confirmed and both disease control and adverse events were associated with higher TGN concentrations 39–41,43,44. Thiopurine testing improved the clinical outcome 45. However, it was clear that adverse events were not purely regulated by TPMT. Other factors potentiate these events, but those with lower TPMT activities are at a significantly increased risk of suffering these events.
The TPMT enzyme
TPMT is a cytoplasmic enzyme which catalyzes the S-methylation of aromatic and heterocyclic sulphydryl compounds, but the endogenous function of TPMT is unknown. The catalytic specificity of TPMT was investigated by Weinshilboum's group in the 1990s. Substrates include thiopurine drugs, selenium compounds and the disulfiram metabolite, diethyldithiocarbamate 46,47. Both mercaptopurine and thioguanine (INN, tioguanine) are good substrates for the enzyme and, of their nucleotide metabolites, mercaptopurine nucleotide (thioinosine monophosphate) is a far better substrate than the TGNs. The latter are very poor substrates 48 but are methylated in vivo at concentrations over 500 pmol/8 × 108 red cells 49. Oxidation of the thiopurine ring (via xanthine oxidase, EC 1.11.3.22) produces 8-hydroxymercaptopurine, a good TPMT substrate and the 2-hydroxy products, thioxanthine (2-hydroxymercaptopurine) and thiouric acid (2,8-hydroxymercaptopurine). Both are potent TPMT inhibitors (Figure 1). At very high concentrations (Ki 0.56 mm) the TPMT product methylmercaptopurine also inhibits human kidney TPMT 48. This raises the possibility that, in vivo, thiopurine metabolism may modulate TPMT activity.
TPMT is inhibited by many benzoic acid derivatives. Perhaps the most important are the aspirin metabolite salicylic acid and 5-aminosalicylate medications such as sulphasalazine and mesalazine 50. The latter is widely used in the treatment of IBD, and other immunosuppressive disorders, for which mercaptopurine or azathioprine may be co-administered and drug–drug interactions have been reported. Inhibited TPMT results in the production of elevated TGN concentrations and an increased risk of myelotoxicity 51–53. However, such TPMT inhibition would not be reflected in in vitro measurements of enzyme activity 54.
Measurement of TPMT status
Analytical methods for the measurement of TPMT genotype and phenotypic activity, initially developed in academic medical centres, have been adapted for the routine service sector and a growing number of thiopurine monitoring and TPMT screening services are now available 55–60.
TPMT phenotypic activity
TPMT phenotype is measured in the RBC by radiochemical 1 or chromatographic techniques 57,61–64. These activities are influenced by red cell transfusions. Additionally, in children with ALL, there are disease 65 and treatment-related 27 influences on TPMT activity. For the interpretation of TPMT phenotype, enzyme activities must be measured under constant conditions at a standardized dosage during a specific phase of treatment. These leukaemia-and treatment-related changes do not occur in IBD. TPMT activities measured prior to, and during, thiopurine treatment are the same 66,67. Reporting phenotype in terms of haemoglobin concentration, packed red cells, erythrocyte counts or protein content give similar accuracy and power to predict heterozygous and wild-type individuals 68.
Shifts in the TPMT activity frequency distribution have been widely reported by groups investigating phenotypic activities. These ‘shifts’ are at the most pronounced in children with ALL when the frequency distribution is shifted downwards, well below the range recorded for healthy children, at disease diagnosis, 27,69 whist during chemotherapy the distribution shifts upwards, well above the range recorded for healthy children 27,69,70. Other disease-dependent fluctuations in TPMT activity distributions are reported to be clinically insignificant 71. In healthy populations, children are reported to have higher TPMT activities than adults 72, younger children higher activities than older children 72 with healthy neonates having the highest activities 73. These shifts are probably influenced by the age profiles of the red cell population, younger red cells having higher TPMT activities than older cells 65. Pancytopenia markedly elevates measured red cell TPMT activities 74.
A gender difference has been reported in the distribution of red cell TPMT activities in some 75,76 but not all studies 2,36, with wild-type TPMT infants less than 2 years having a higher activity in boys than girls 72. Hepatic activity (measured in patient surgical biopsy tissue) was significantly higher in men than women; but this difference was not reflected in measurements of red cell TPMT activity 5. In addition, TPMT phenotypic measurements show an interethnic variability. Early studies reported that white Caucasian populations have higher activities than North American Black populations whilst indigenous Norwegian Saami populations have higher activities than Caucasians 77–79. However the TPMT activities measured were similarly polymorphic with a trimodal distribution reported in all three populations. This is not so in Asian populations. Singapore Malay have higher activities than either Singapore ethnic Chinese or Indian populations. The distribution of TPMT activities is binomial in all these populations 80,81 whilst unimodal, normal, distributions are reported in healthy Korean populations 82. These latter reports, emanating from a number of different laboratories but also from studies of diverse populations within the same laboratory, could not be attributed to differences in enzyme activity methodologies. A major factor in these ethnic differences was the inheritance of specific TPMT low activity variant alleles within these populations 83–85. Thus, ethnicity, type of disease, concurrent drug treatment and red cell kinetics and transfusions must be considered when interpreting TPMT activity measurements.
TPMT genotype
Single nucleotide polymorphisms (SNPs) account for the major TPMT low activity variant forms 6,8,9,57,64. Genotyping for the TPMT*3 family of variant alleles will detect over 92% of low activity alleles, inclusion of TPMT*2 pushes this to over 95% 9,57,64. Whilst TPMT*3A is the most common variant allele in Caucasian populations 8,36,64 TPMT*3C is the common mutant allele in African populations 84 with the African-American population having an approximate 50:50 mix of TPMT*3A and TPMT*3C 86. In these populations the variant allele frequency is about 5%. This is in contrast with a frequency of 1 to 3% reported in Asian populations when the dominant allele is TPMT*3C 80,83,87. Over 35 variant low activity TPMT alleles have been reported and, to avoid a duplication of allele numbering, a specific logical nomenclature system has been adopted, for the designation of novel allele names in humans, which is maintained and managed by the TPMT Nomenclature Committee 87,88. The incidence of rare or novel alleles in Caucasian populations has been estimated to be approximately 1 in 200 64,87,88.
TPMT genotype–phenotype concordance
The overall concordance between genotype and phenotype in healthy volunteers is 98.4%, but in the ‘intermediate’ range of TPMT activities this falls to 86%. Approximately 1 in 20 could have novel mutations but 1.6% of individuals with intermediate activities are wild-type in open reading frame 64. A recent systematic review of TPMT testing in adults and children with chronic inflammatory diseases concluded that genotyping specificity approached 100%, but the ability of genotype to identify patients with an intermediate activity was imprecise, ranging from a pooled estimate of 70% to 86% 89. As previously discussed, there are many possible treatment-related, environmental and ethnic influences on phenotypic TPMT activity that could contribute to lack of concordance in the intermediate activity range. In addition, modulation of TPMT activity by tandem repeats within the TPMT promoter has been proposed 90, but larger population studies have shown these effects to be quantitatively small 91.
Neither TPMT genotype nor phenotype alone can be 100% guaranteed to identify the TPMT deficient individual. Genotype tests for the TPMT*3 family and TPMT*2 cover 95% of inactivating alleles and phenotype tests can be used to double-check the TPMT heterozygote for the estimated 1 in 7416 chance of TPMT deficiency due to a rare/novel variant allele 92,93. However, when using phenotypic enzyme activity, as the initial TPMT test, the risk of misclassifying a TPMT deficient patient as one with intermediate activity is higher than the risk of missing the deficient patient by TPMT genotype 93. A comparison of TPMT testing methods in a National Centre showed genotype to be superior to phenotype. Using the phenotype assay 11% of TPMT deficient individuals would have been misclassified as TPMT intermediate activity due to TPMT enzyme activities above the TPMT deficient cut-off value when the TPMT activity was measured in the laboratory under ideal conditions. TPMT genotype was recommended as the primary test 93. Test centres employing phenotypic methodologies frequently use TPMT genotype as an assurance tool to check the intermediate activity cohort for the true positives 92. Although some laboratory forms for the TPMT test request information on prior blood transfusions, it was not stated if this was so for the test centre results previously discussed 92,93 and this could be a major contributory factor for the observed TPMT discordance, particularly for the TPMT deficient phenotype. However, in a study using blood samples taken prior to any transfusions, the accuracy of TPMT genotype was far superior to phenotypic activity measurements in leukaemia patients, a clinical situation in which the latter test is unreliable 36,94.
It has been argued that TPMT genotype analysis will not, as yet, provide that additional information yielded by phenotype, i.e. the identification of those with very high activities who may require escalated dosages to produce an adequate clinical response and those with wild-type genotype with functional ‘intermediate’ activity. However, the latter group mainly consists of false positives produced by variable red cell kinetics due to the underlying disease state or other factors 36 whilst in the former, the preferential production of methylated mercaptopurine metabolites (products of the TPMT reaction) and suboptimal response to thiopurine therapy is not always attributed to very high TPMT activities 95. Frequently, the search for additional information to interpret the clinical picture requires thiopurine metabolite analysis in addition to TPMT status. TPMT testing is a predictor of what could happen during thiopurine therapy, metabolite monitoring is one step nearer the pharmacological action and the latter is a useful adjunct to TPMT status information when compliance with oral therapy is suspected as the cause of drug tolerance 36 or when myelosuppression may be attributed to multiple causes or other toxicity symptoms require investigation.
Cost effectiveness of TPMT testing
Because of life threatening nature of thiopurine drug-related toxicity, prospective identification of patients with TPMT deficiency (homozygous for variant low activity TPMT alleles), prior to the initiation of therapy has increasingly been accepted clinically. The cost of in-patient care for one TPMT deficient patient inadvertently treated with azathioprine has been estimated to cover the cost of over 400 tests for TPMT activity 96. The cost of TPMT testing is offset by the improved patient care and the improved quality of life for the TPMT deficient patient. The cost effectiveness of TPMT screening prior to mercaptopurine or thiopurine drug therapy has been repeatedly stated 45,88,97,98.
Recommendations for, and use of, TPMT testing
The US Federal Drug Administration directed label modifications for 6-mercaptopurine (July 2004) and azathioprine (July 2005) to reflect the pharmacogenetics of thiopurine metabolism and recommend TPMT testing prior to the initiation of thiopurine therapy 9. TPMT testing prior to the prescription of thiopurine drugs is becoming routine clinical practice in Europe 12–15. In the UK, a recent survey indicated that TPMT testing was used by 67% of clinicians prior to azathioprine prescription 99 whilst, worldwide, testing is used by 43% of gastroenterologists in the management of IBD 100. Overall, UK clinical guidelines recommend that patients have their TPMT status checked prior to starting thiopurine drugs. In the UK TPMT testing is mandatory for children and young adults prior to treatment on the ALL2011 trial protocol 101.
A systematic review of TPMT testing in adults and children with chronic inflammatory diseases reported that, compared with a wild-type TPMT genotype, those who were TPMT heterozygotes or TPMT deficient were at an increased risk of developing leucopenia (odds ratios 4.29, 95% CI 2.67, 6.89 and 20.84, 95% CI 3.42, 126.89, respectively). However, there was insufficient evidence to address the effectiveness of TPMT pre-testing with respect to improved patient outcomes 89. A similar meta-analysis of 67 studies reported an odds ratio of 4.06 (95% CI 3.2, 5.48) for intermediate activity patients developing leucopenia compared with patients with high (or ‘normal’) TPMT activity when taking thiopurine medication; 86% of patients with two variant alleles developed myelosuppression 102.
Guidelines to interpret TPMT genotype tests in order to guide the dosing of thiopurines have been developed 14,15. Patients who inherit two variant low activity alleles (TPMT deficiency) will, with 100% certainty, develop life-threatening myelosuppression at conventional thiopurine dosages; drastic dose reduction (e.g. 10% of conventional dose) is required. About 30 to 60% of TPMT heterozygotes will be unable to tolerate full thiopurine doses 14,21,36; TPMT heterozygotes are at a significantly higher risk for toxicity than TPMT wild-type patients 102. Thus a reduced thiopurine dose for the TPMT heterozygote has been recommended at the start of treatment 103. The latter approach risks the under-dosing of those TPMT heterozygous patients who can tolerate full doses, and so a titration upwards approach is advised 14.
The British National Formulary suggests that the clinician should consider measuring TPMT activity before starting thiopurine drugs 104. An initial assessment of TPMT testing in the UK 12 reported that the test was requested by 13 different medical specialities with dermatologists and gastroenterologists the most frequent users. A more recent survey reported that 94% of dermatologists and 60% of gastroenterologists requested TPMT testing 99. The high uptake by dermatologists is reflective of the fact that they were the first speciality in the UK to develop national guidelines advocating the use of TPMT testing 105. Current guidelines for dermatologists review the case for TPMT testing and firmly support routine pre-treatment TPMT testing and emphasize the cost-effectiveness against the intensive support care required for patients with severe and prolonged myelosuppression 106 The guidelines for rheumatologists recommend TPMT testing prior to prescribing azathioprine with the caveat that testing does not replace routine monitoring (of blood cell counts) 107.
In the treatment of autoimmune hepatitis (AIH), where long term azathioprine is the immunosuppressive of choice for the maintenance of remission and is used with prednisolone to induce remission, the UK guidelines state that TPMT measurement ‘should be considered’ to exclude those with TPMT deficiency and TPMT measurement ‘is recommended’ in patients with pre-existing leucopenia 108. The USA AIH guidelines state that the frequency of cytopenia is 46% in azathioprine treated AIH patients but studies to date have shown that this is not predicted by prior knowledge of TPMT status. The most common cause of cytopenia in the AIH patient is hypersplenism associated with underlying cirrhosis. The USA guidelines comment that TPMT deficiency is rare and the azathioprine dose used in conventional treatment is low. Thus they do not support routine TPMT screening but the USA guidelines recommend that TPMT activity should be assessed in patients with cytopenias before or during azathioprine therapy 109.
UK gastroenterologists view TPMT testing with caution, initially not recommending prior to therapy on the basis that decades of experience had shown azathioprine to be a safe drug in the treatment of inflammatory bowel disease 110. The current British Society of Gastroenterology guidelines acknowledge the role of TPMT testing in identifying the 1 in 300 TPMT deficient patient and comment that most patients who develop leucopenia will have a ‘normal’ (high activity, homozygous wild-type genotype) TPMT. TPMT testing is not specifically recommended, although a role is suggested of predicting early events rather than long term control 111. Nonetheless, gastroenterologists are among the most frequent users of TPMT testing in the UK 99.
Despite the widespread use of TPMT testing debate continues with respect to the utility of TPMT testing 112. With a lack of prospective randomized controlled trials the evidence base for routine TPMT testing remains suboptimal 113. The UK Department of Health funded TARGET (TPMT: Azathioprine Response to Genotyping and Enzyme Testing) controlled trial investigated the clinical value and cost-effectiveness of TPMT genotyping in reducing the number of adverse drug reactions associated with azathioprine immunosuppression 114. The recruitment target (n = 500) was not met due to the routine use of TPMT testing in some treatment centres. The study (n = 333) concluded that there was strong evidence for severe neutropenia in the TPMT deficient patient, but TPMT heterozygotes were not at an increased risk of adverse drug reactions at standard doses of azathioprine.
What is clear is that adverse reactions to thiopurine drugs are dose dependent and, of the myriad of adverse reactions attributed to the thiopurine drugs, myelosuppression is more common when thiopurine drugs are used more aggressively, as in the 6-mercaptopurine treatment protocols for childhood ALL, than when used in low dose azathioprine immunosuppressive therapy. In addition, concomitant therapy and the underlying disease process can also influence the susceptibility to myelosuppressive events for all patients. However, unless treated on very low thiopurine doses (in ALL protocols 10%, or less, of the standard protocol mercaptopurine doses are used) the TPMT deficient patient will experience profound myelosuppression when treated with thiopurine drugs. It is cost-effective to routinely perform pre-treatment TPMT testing to identify these individuals alone.
Competing Interests
The author has completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available from request from the corresponding author) and declares support from Leukaemia and Lymphoma Research for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
LL's research group is supported by Leukaemia and Lymphoma Research, London, UK at the University of Sheffield.
References
- 1.Weinshilboum RM, Raymond FA, Pazmino PA. Human erythrocyte thiopurine methyltransferase: radiochemical microassay and biochemical properties. Clin Chim Acta. 1978;85:323–333. doi: 10.1016/0009-8981(78)90311-x. [DOI] [PubMed] [Google Scholar]
- 2.Weinshilboum RM, Sladek SL. Mercaptopurine pharmacogenetics: monogenic inheritance of erythrocyte thiopurine methyltransferase activity. Am J Hum Genet. 1980;32:651–662. [PMC free article] [PubMed] [Google Scholar]
- 3.Van Loon J, Weinshilboum RM. Thiopurine methyltransferase biochemical genetics: human lymphocyte activity. Biochem Genet. 1982;20:637–658. doi: 10.1007/BF00483962. [DOI] [PubMed] [Google Scholar]
- 4.Woodson LC, Dunnette JH, Weinshilboum RM. Pharmacogenetics of human thiopurine methyltransferase: kidney-erythrocyte correlation and immunotitration studies. J Pharmacol Exp Ther. 1982;222:174–181. [PubMed] [Google Scholar]
- 5.Szumlanski CL, Honchel R, Scott MC, Weinshilboum RM. Human liver thiopurine methyltransferase pharmacogenetics: biochemical properties, liver-erythrocyte correlation and presence of isozymes. Pharmacogenetics. 1992;2:148–159. [PubMed] [Google Scholar]
- 6.Krynetski EY, Schuetz JD, Galpin AJ, Pui C-H, Relling MV, Evans WE. A single point mutation leading to loss of catalytic activity in human thiopurine S-methyltransferase. Proc Natl Acad Sci U S A. 1995;92:949–953. doi: 10.1073/pnas.92.4.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Szumlanski C, Otterness D, Her C, Lee D, Brandriff B, Kelsell D, Spurr N, Lennard L, Weiben E, Weinshilboum R. Thiopurine methyltransferase pharmacogenetics: human gene cloning and characterization of a common genetic polymorphism. DNA Cell Biol. 1996;15:17–30. doi: 10.1089/dna.1996.15.17. [DOI] [PubMed] [Google Scholar]
- 8.Otterness D, Szumlanski C, Lennard L, Klemetsdal B, Aarbakke J, Park-Hah JO, Iven H, Schmeigelow K, Branum E, O'Brien J, Weinshilboum RM. Human thiopurine methyltransferase pharmacogenetics: gene sequence polymorphisms. Clin Pharmacol Ther. 1997;62:60–73. doi: 10.1016/S0009-9236(97)90152-1. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Weinshilboum R. Thiopurine S-methyltransferase pharmacogenetics: insights, challenges and future directions. Oncogene. 2006;25:1629–1638. doi: 10.1038/sj.onc.1209372. [DOI] [PubMed] [Google Scholar]
- 10.Lennard L, Van Loon JA, Weinshilboum RM. Pharmacogenetics of acute azathioprine toxicity: relationship to thiopurine methyltransferase genetic polymorphism. Clin Pharmacol Ther. 1989;46:149–154. doi: 10.1038/clpt.1989.119. [DOI] [PubMed] [Google Scholar]
- 11.Flockhart DA, Skaar T, Klein TE, Nguyen AT. Clinically available pharmacogenomics tests. Clin Pharmacol Ther. 2009;86:109–113. doi: 10.1038/clpt.2009.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holme SA, Duley JA, Sanderson J, Routledge PA, Anstey AV. Erythrocyte thiopurine methyltransferase assessment prior to azathioprine use in the UK. Q J Med. 2002;95:439–444. doi: 10.1093/qjmed/95.7.439. [DOI] [PubMed] [Google Scholar]
- 13.Ford LT, Berg JD. ThiopurineS-methyltransferase (TPMT) assessment prior to starting thiopurine drug treatment; a pharmacogenomic test whose time has come. J Clin Pathol. 2010;63:288–295. doi: 10.1136/jcp.2009.069252. [DOI] [PubMed] [Google Scholar]
- 14.Relling MV, Gardner EE, Sandborn WJ, Pui C-H, Stein CM, Carrillo M, Evans WE, Klein TE. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing. Clin Pharmacol Ther. 2011;89:387–391. doi: 10.1038/clpt.2010.320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Relling MV, Gardner EE, Sandborn WJ, Schmiegelow K, Pui C-H, Yee SW, Stein CM, Carrillo M, Evans WE, Hicks JK, Schwab M, Klein TE. Clinical pharmacogenetics implementation consortium guidelines for thiopurine methyltransferase genotype and thiopurine dosing: 2013 update. Clin Pharmacol Ther. 2013;93:324–325. doi: 10.1038/clpt.2013.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lennard L, Murphy MF, Maddocks JL. Severe megaloblastic anaemia associated with abnormal azathioprine metabolism. Br J Clin Pharmacol. 1984;17:171–172. doi: 10.1111/j.1365-2125.1984.tb02333.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maddocks JL, Lennard L, Amess J, Amos R, Meyrick-Thomas R. Azathioprine and severe bone marrow depression. Lancet. 1986;327:156. doi: 10.1016/s0140-6736(86)92291-9. [DOI] [PubMed] [Google Scholar]
- 18.Evans WE, Horner M, Chu YQ, Kalwinsky D, Roberts WM. Altered mercaptopurine metabolism, toxic effects, and dosage requirement in a thiopurine methyltransferase deficient child with acute lymphoblastic leukaemia. J Pediatr. 1991;119:985–989. doi: 10.1016/s0022-3476(05)83063-x. [DOI] [PubMed] [Google Scholar]
- 19.Schutz E, Gummert J, Mohr F, Oellerich M. Azathioprine-induced myelosuppression in thiopurine methyltransferase deficient heart transplant recipient. Lancet. 1993;341:436. doi: 10.1016/0140-6736(93)93028-y. [DOI] [PubMed] [Google Scholar]
- 20.McBride KL, Gilchrist GS, Smithson WA, Weinshilboum RM, Szumlanski CL. Severe 6-thioguanine-induced marrow aplasia in a child with acute lymphoblastic leukaemia and inherited thiopurine methyltransferase deficiency. J Ped Hematol Oncol. 2000;22:441–445. doi: 10.1097/00043426-200009000-00011. [DOI] [PubMed] [Google Scholar]
- 21.Konstantopoulou M, Belgi A, Griffiths KD, Macfarlane AW. Azathioprine-induced pancytopenia in a patient with pompholyx and deficiency of erythrocyte thiopurine methyltransferase. Br Med J. 2005;330:350–351. doi: 10.1136/bmj.330.7487.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tidd DM, Paterson ARP. A biochemical mechanism for the delayed cytotoxic reactions of 6-mercaptopurine. Cancer Res. 1974;34:738–746. [PubMed] [Google Scholar]
- 23.Karran P. Thiopurines, DNA damage, DNA repair and therapy-related cancer. Br Med Bull. 2006;79 and 80:153–170. doi: 10.1093/bmb/ldl020. [DOI] [PubMed] [Google Scholar]
- 24.Tiede I, Fritz G, Strand S, Poppe D, Dvorsky R, Strand D, Lehr HA, Wirtz S, Becker C, Atreya R, Mudter J, Hildner K, Bartsch B, Holtman M, Blumberg R, Walczak H, Iven H, Galle PR, Ahmadian MR, Neurath MF. CD28-dependent Rac1 activation is the molecular target of azathioprine in primary human CD4+ T lymphocytes. J Clin Invest. 2003;111:1133–1145. doi: 10.1172/JCI16432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Poppe D, Tielde I, Fritz G, Becker C, Bartsch B, Wirtz S, Strand D, Tanaka S, Galle PR, Bustelo XR, Neurath MF. Azathioprine suppresses Ezrin-Radixin-Moesin-dependent T cell-APC conjugation through inhibition of Vav guanosine exchange activity on Rac proteins. J Immunol. 2006;176:640–651. doi: 10.4049/jimmunol.176.1.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bourgine J, Garat A, Allorge D, Crunelle-Thibaut A, Lo-Guidice JM, Colombel JF, Brolly F, Billaut-Laden I. Evidence for a functional genetic polymorphism of Rho-GTPase Rac1. Implication in azathioprine response? Pharmacogenet Genomics. 2011;21:313–324. doi: 10.1097/FPC.0b013e3283449200. [DOI] [PubMed] [Google Scholar]
- 27.Lennard L, Lilleyman JS, Van Loon JA. Weinshilboum RM Genetic variation in response to 6-mercaptopurine for childhood acute lymphoblastic leukaemia. Lancet. 1990;336:225–229. doi: 10.1016/0140-6736(90)91745-v. [DOI] [PubMed] [Google Scholar]
- 28.Relling MV, Hancock ML, Rivera GK, Sandlund JT, Ribeiro RC, Krynetski EY, Pui C-H EWE. Mercaptopurine therapy intolerance and heterozygosity at the thiopurine S-methyltransferase gene locus. J Natl Cancer Inst. 1999;91:2001–2008. doi: 10.1093/jnci/91.23.2001. [DOI] [PubMed] [Google Scholar]
- 29.Lennard L, Lilleyman JS. Variable 6-mercaptopurine metabolism and treatment outcome in childhood lymphoblastic leukaemia [published erratum appears in J Clin Oncol 1990; 8: 567] J Clin Oncol. 1989;7:1816–1823. doi: 10.1200/JCO.1989.7.12.1816. [DOI] [PubMed] [Google Scholar]
- 30.Schmiegelow K, Schroder H, Gustafsson G, Kristinsson J, Glomstein A, Salmi T, Wranne L. Risk of relapse in childhood acute lymphoblastic leukaemia is related to RBC methotrexate and mercaptopurine metabolites during maintenance chemotherapy. J Clin Oncol. 1995;13:345–351. doi: 10.1200/JCO.1995.13.2.345. [DOI] [PubMed] [Google Scholar]
- 31.Balis FM Holcenberg JS, Poplack DG, Ge J, Sather HN, Murphy RF, Ames MM, Waskerwitz MJ, Tubergen DG, Zimm S, Gilchrist GS, Bleyer WA. Pharmacokinetics and pharmacodynamics of oral methotrexate and mercaptopurine in children with lower risk acute lymphoblastic leukaemia: a joint Children's Cancer Group and Pediatric Oncology Branch study. Blood. 1998;92:3569–3577. [PubMed] [Google Scholar]
- 32.Schmiegelow K, Bjork O, Glomstein A, Gustafsson G, Keilding N, Kristinsson J, Makipernaa A, Rosthoj S, Szulmlanski C, Sorensen TM, Weinshilboum R. Intensification of mercaptopurine/methotrexate maintenance chemotherapy may increase the risk of relapse for some children with acute lymphoblastic leukaemia. J Clin Oncol. 2003;21:1332–1339. doi: 10.1200/JCO.2003.04.039. [DOI] [PubMed] [Google Scholar]
- 33.Relling MV, Pui C-H, Cheng C, Evans WE. Thiopurine methyltransferase in acute lymphoblastic leukaemia. Blood. 2006;107:843–844. doi: 10.1182/blood-2005-08-3379. [DOI] [PubMed] [Google Scholar]
- 34.Nelson JA. Mechanisms of action of thiopurines used in the curative therapy for childhood leukaemias. Cancer Bull. 1992;44:470–473. [Google Scholar]
- 35.Dervieux T, Brenner TL, Hon YY, Zhou Y, Hancock ML, Sandlund JT, Rivera GK, Ribeiro PC, Boycott JM, Pui C-H, Relling MV, Evans WE. De novo purine synthesis inhibition and antileukaemic effects of mercaptopurine alone or in combination with methotrexate in vivo. Blood. 2002;100:1240–1247. doi: 10.1182/blood-2002-02-0495. [DOI] [PubMed] [Google Scholar]
- 36.Lennard L, Cartwright CS, Wade R, Richards SM, Vora A. Thiopurine methyltransferase genotype-phenotype discordance, and thiopurine active metabolite formation, in childhood acute lymphoblastic leukaemia. Br J Clin Pharmacol. 2013;76:125–136. doi: 10.1111/bcp.12066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Cuffari C, Theoret Y, Latour S, Seidman G. 6-Mercaptopurine metabolism in Crohn's disease: correlation with efficacy and toxicity. Gut. 1996;39:401–406. doi: 10.1136/gut.39.3.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lowry P, Franklin CL, Weaver AL, Gennett Pike M, Mays DC, Tremaine WJ, Lipsky JJ, Sandborn WJ. Measurement of thiopurine methyltransferase activity and azathioprine metabolites in patients with inflammatory bowel disease. Gut. 2001;49:665–670. doi: 10.1136/gut.49.5.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dubinsky MC, Lamothe S, Yang HY, Targan SR, Sinnett D, Theoret Y, Seidman EG. Pharmacogenetics and metabolite measurement for 6-mercaptopurine therapy in inflammatory bowel disease. Gastroenterology. 2000;118:705–713. doi: 10.1016/s0016-5085(00)70140-5. [DOI] [PubMed] [Google Scholar]
- 40.Dubinsky MC, Yang HY, Hassard PV, Seidman EG, Kam LY, Abreu MT, Targan SR, Vasiliauskas EA. 6-MP metabolite profiles provide a biochemical explanation for 6-MP resistance in patients with inflammatory bowel disease. Gastroenterology. 2002;122:904–915. doi: 10.1053/gast.2002.32420. [DOI] [PubMed] [Google Scholar]
- 41.Wright S, Sanders DS, Lobo A, Lennard L. Clinical significance of azathioprine active metabolite concentrations on inflammatory bowel disease. Gut. 2004;53:1123–1128. doi: 10.1136/gut.2003.032896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell S, Kingstone K, Ghosh S. Relevance of thiopurine methyltransferase activity in inflammatory bowel disease patients maintained on low dose azathioprine. Aliment Pharmacol Ther. 2002;16:389–398. doi: 10.1046/j.1365-2036.2002.01177.x. [DOI] [PubMed] [Google Scholar]
- 43.Hindorf U, Lindqvist M, Hildebrand H, Fagerberg U, Almers S. Adverse events leading to modification of therapy in a large cohort of patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2006;24:331–342. doi: 10.1111/j.1365-2036.2006.02977.x. [DOI] [PubMed] [Google Scholar]
- 44.Gisbert JP, Nino P, Rodrigo L, Cara C, Guijarro LG. Thiopurine methyltransferase (TPMT) activity and adverse effects of azathioprine in inflammatory bowel disease: long-term follow-up study of 394 patients. Am J Gastroenterol. 2006;101:2769–2776. doi: 10.1111/j.1572-0241.2006.00843.x. [DOI] [PubMed] [Google Scholar]
- 45.Dubinsky MC, Reyes E, Ofman J, Chiou C-F, Wade S, Sandborn WJ. A cost-effectiveness analysis of alternative disease management strategies in patients with Crohn's disease treated with azathioprine or 6-mercaptopurine. Am J Gastroenterol. 2005;100:2239–2247. doi: 10.1111/j.1572-0241.2005.41900.x. [DOI] [PubMed] [Google Scholar]
- 46.Woodson LC, Ames MM, Selassie CD, Hansch C, Weinshilboum RM. Thiopurine methyltransferase. Aromatic thiol substrates and inhibition by benzoic acid derivatives. Mol Pharmacol. 1983;24:471–478. [PubMed] [Google Scholar]
- 47.Glauser TA, Nelson MD, Zembower DE, Lipsky JJ, Weinshilboum RM. Diethylthiocarbamate S-methylation: evidence for catalysis by human liver thiol methyltransferase and thiopurine methyltransferase. J Pharm Expt Therap. 1993;266:23–32. [PubMed] [Google Scholar]
- 48.Deininger M, Szumlanski CL, Otterness DM, Van Loon J, Ferber W, Weinshilboum RM. Purine substrates for human thiopurine methyltransferase. Biochem Pharmacol. 1994;11:2135–2138. doi: 10.1016/0006-2952(94)90515-0. [DOI] [PubMed] [Google Scholar]
- 49.Rowland K, Lennard L, Lilleyman JS. High performance liquid chromatographic assay of methylthioguanine nucleotides. J Chromatogr B. 1998;705:29–37. doi: 10.1016/s0378-4347(97)00495-7. [DOI] [PubMed] [Google Scholar]
- 50.Szumlanski C, Weinshilboum RM. Sulfsalazine inhibition of thiopurine methyltransferase: possible mechanism for interaction with 6-mercaptopurine. Br J Clin Pharmacol. 1995;39:456–459. doi: 10.1111/j.1365-2125.1995.tb04478.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewis LD, Benin A, Szumlanski CL, Otterness DM, Lennard L, Weinshilboum RM, Nierenberg DW. Olsalazine and 6-mercaptopurine-related bone marrow suppression: a possible drug-drug interaction. Clin Pharmacol Ther. 1997;62:464–475. doi: 10.1016/S0009-9236(97)90125-9. [DOI] [PubMed] [Google Scholar]
- 52.Hande S, Wilson-Rich N, Bousvaros A, Zholudev A, Maurer R, Banks P, Makrauer F, Reddy S, Burakoff R, Friedman S. 5-Aminosalicylate therapy is associated with higher 6-thioguanine levels in adults and children with inflammatory bowel disease in remission on 6-mercaptopurine or azathioprine. Inflamm Bowel Dis. 2006;12:251–257. doi: 10.1097/01.MIB.0000206544.05661.9f. [DOI] [PubMed] [Google Scholar]
- 53.de Boer NKH, Wong DR, Jharap B, de Graaf P, Hooymans PM, Mulder CJJ, Rijmen F, Engels LGJB, van Bodegraven AA. Dose-dependent influence of 5-aminosalicylates on thiopurine metabolism. Am J Gastroenterol. 2007;102:2747–2753. doi: 10.1111/j.1572-0241.2007.01511.x. [DOI] [PubMed] [Google Scholar]
- 54.Shipkova M, Niedmann PD, Armstrong VM, Oellerich M, Wieland E. Determination of thiopurine methyltransferase activity in isolated human erythrocytes does not reflect putative in vivo enzyme inhibition by sulfasalazine. Clin Chem. 2004;50:438–441. doi: 10.1373/clinchem.2003.026096. [DOI] [PubMed] [Google Scholar]
- 55.Thiopurine methyltransferase (TPMT) test. Rochester Test Catalog. Mayo Clinic, Medical Laboratories, Rochester, MN 55095, USA. Available at http://www.mayomedicallaboratories.com/test-catalog/ (last accessed 17 September 2013)
- 56.TPMT testing. Prometheus Laboratories Inc, Therapeutics and Diagnostics, 9410 Carroll Park Drive, San Diego, CA 92121-4203. Available at http://www.prometheuslabs.com/Products_Diagnostics.asp (last accessed 17 September 2013)
- 57.Dervieux T, Meyer G, Barham R, Matsutani M, Barry M, Boulieu R, Neri B, Seidman E. Liquid chromatography-tandem mass spectrometry analysis of erythrocyte thiopurine nucleotides and effects of thiopurine methyltransferase gene variants on these metabolites in patients receiving azathioprine/6-mercaptopurine therapy. Clin Chem. 2005;51:2074–2084. doi: 10.1373/clinchem.2005.050831. [DOI] [PubMed] [Google Scholar]
- 58.TPMT Test. GSTS Pathology, Biochemical Sciences, Purine laboratory, 4th Floor North Wing, St Thomas' Hospital, London SE1 7EH. Available at http://www.gsts.com/a-z-test-index.html (last accessed 17 September 2013)
- 59.Thiopurine S-methyltransferase (TPMT) City assays, Biochemistry Tests, Department of Clinical Biochemistry, City Hospital, Dudley Road, Birmingham, B18 7QH. Available at http://www.cityassays.org.uk/tpmt.html (last accessed 17 September 2013)
- 60.TPMT gene. Royal College of Pathologists of Australia. Catalogue of genetic tests and laboratories. Available at http://genetictesting.rcpa.edu.au/component/gene/genetest/TPMT/ (last accessed 17 September 2013)
- 61.Lennard L, Singleton HJ. High performance liquid chromatographic assay of human red blood cell thiopurine methyltransferase activity. J Chromatogr B. 1994;661:25–33. doi: 10.1016/0378-4347(94)00327-0. [DOI] [PubMed] [Google Scholar]
- 62.Lennard L, Richards S, Cartwright CS, Mitchell C, Lilleyman JS, Vora AJ. The thiopurine methyltransferase genetic polymorphism is associated with thioguanine-related veno-occlusive disease of the liver in children with acute lymphoblastic leukaemia. Clin Pharmacol Ther. 2006;80:375–383. doi: 10.1016/j.clpt.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 63.Kröplin T, Fisher C, Iven H. Inhibition of thiopurine S-methyltransferase activity by impurities in commercially available substrates: a factor for differing results for TPMT measurements. Eur J Clin Pharmacol. 1999;55:285–291. doi: 10.1007/s002280050630. [DOI] [PubMed] [Google Scholar]
- 64.Schaeffeler E, Fisher C, Brockmeier D, Wernet D, Moerike K, Eichelbaum M, Zanger UM, Schwab M. Comprehensive analysis of thiopurine S-methyltransferase phenotype-genotype correlation in a large population of German-caucasians and identification of novel TPMT variants. Pharmacogenetics. 2004;14:407–417. doi: 10.1097/01.fpc.0000114745.08559.db. [DOI] [PubMed] [Google Scholar]
- 65.Lennard L, Chew TS, Lilleyman JS. Human thiopurine methyltransferase activity varies with red blood cell age. Br J Clin Pharmacol. 2001;52:539–546. doi: 10.1046/j.0306-5251.2001.01497.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lindqvist M, Hindorf U, Almer S, Soderkvist P, Strom M, Hjortswang H, Peterson C. No induction of thiopurine methyltransferase during thiopurine treatment in inflammatory bowel disease. Nucleosides Nucleotides Nucleic Acids. 2006;25:1033–1037. doi: 10.1080/15257770600890814. [DOI] [PubMed] [Google Scholar]
- 67.Dilger K, Schaeffeler E, Lukas M, Strauch U, Herfarth H, Muller R, Schwab M. Monitoring of thiopurine methyltransferase activity in postsurgical patients with Crohn's disease during 1 year of treatment with azathioprine. Ther Drug Monit. 2007;29:1–5. doi: 10.1097/FTD.0b013e3180312b9a. [DOI] [PubMed] [Google Scholar]
- 68.Oselin K, Anier K, Tamm R, Kallassalu K, Mäeorg M. Determination of thiopurine S-methyltransferase (TPMT) activity by comparing various normalisation factors: reference values for Estonian population using HPLC-UV. J Chromatog B. 2006;834:77–83. doi: 10.1016/j.jchromb.2006.02.031. assay. [DOI] [PubMed] [Google Scholar]
- 69.Brouwer C, De Abreu RA, Keizer-Garritsen JJ, Lambooy LHJ, Ament K, ter Riet PGJH, van Wering ER, Trijbels FJM, Veerman AJP, Hooherbrugge PM, Bokkerink JPM. Thiopurine methyltransferase in acute lymphoblastic leukaemia: biochemical and molecular biological aspects. Eur J Cancer. 2005;41:613–623. doi: 10.1016/j.ejca.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 70.McLeod HL, Relling MV, Liu Q, Pui C-H, Evans WE. Polymorphic thiopurine methyltransferase in erythrocytes is indicative of activity in leukemic blasts from children with acute lymphoblastic leukemia. Blood. 1995;7:1897–1902. [PubMed] [Google Scholar]
- 71.Gisbert JP, Gomollon F, Cara C, Luna M, Gonzalez-Lama Y, Pajares JM, Mate J, Guijarro LG. Thiopurine methyltransferase activity in Spain: a study of 14,545 patients. Dig Dis Sci. 2007;52:1262–1269. doi: 10.1007/s10620-006-9119-z. [DOI] [PubMed] [Google Scholar]
- 72.Serpe L, Calvo PL, Muntoni E, D'Antico S, Giaccone M, Avagnina A, Baldi M, Barbera B, Curti F, Pera A, Eandi M, Zara GP, Canaparo R. Thiopurine S-methyltransferase pharmacogenetics in a large-scale healthy Italian-Caucasian population: differences in enzyme activity. Pharmacogenomics. 2009;10:1753–1765. doi: 10.2217/pgs.09.103. [DOI] [PubMed] [Google Scholar]
- 73.McLeod HL, Krynetski EY, Williams JA, Evans WE. Higher activity of polymorphic thiopurine S-methyltransferase in erythrocytes from neonates compared to adults. Pharmacogenetics. 1995;5:281–286. doi: 10.1097/00008571-199510000-00003. [DOI] [PubMed] [Google Scholar]
- 74.de Boer NKH, van Bodegraven AA, de Graaf P, van der Hulst RWM, Zoetekouw L, van Kuilenburg ABP. Paradoxical elevated thiopurine S-methyltransferase activity after pancytopenia during azathioprine therapy: potential influence of red blood cell age. Ther Drug Monit. 2008;30:390–393. doi: 10.1097/FTD.0b013e31816c20b3. [DOI] [PubMed] [Google Scholar]
- 75.Klemetsdal B, Wiat E, Aarbakke J. Gender difference in red blood cell thiopurine methyltransferase activity. Scand J Clin Lab Invest. 1993;53:747–749. doi: 10.3109/00365519309092580. [DOI] [PubMed] [Google Scholar]
- 76.Karas-Kuzelicki N, Milek M, Mlinaric-Rascan I. MTHFR and TYMS genotypes influence TPMT activity and its differential modulation in males and females. Clin Biochem. 2010;43:37–42. doi: 10.1016/j.clinbiochem.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 77.McLeod HL, Lin J-S, Scott EP, Pui C-H, Evans WE. Thiopurine methyltransferase activity in American White subjects and Black subjects. Clin Pharmacol Ther. 1994;55:15–20. doi: 10.1038/clpt.1994.4. [DOI] [PubMed] [Google Scholar]
- 78.Klemetsdal B, Tollefsen E, Loennechen T, Johnsen K, Utsi E, Gisholt K, Wist E, Aarbaake J. Interethnic differences in thiopurine methyltransferase activity. Clin Pharmacol Ther. 1992;51:24–31. doi: 10.1038/clpt.1992.4. [DOI] [PubMed] [Google Scholar]
- 79.Klemetsdal B, Straume B, Wist E, Aarbakke J. Identification of factors regulating thiopurine methyltransferase activity in a Norwegian population. Eur J Clin Pharmacol. 1993;44:147–152. doi: 10.1007/BF00315472. [DOI] [PubMed] [Google Scholar]
- 80.Kham SKY, Soh CK, Liu TC, Chan YH, Ariffin H, Tan PL, Yeoh AEJ. Thiopurine S-methyltransferase activity in three major Asian populations: a population-based study in Singapore. Eur J Clin Pharmacol. 2008;64:373–379. doi: 10.1007/s00228-007-0426-x. [DOI] [PubMed] [Google Scholar]
- 81.Kham SKY, Tan PL, Tay AHN, Heng CK, Yeoh AEJ, Quah T-C. Thiopurine methyltransferase polymorphisms in a multiracial Asian population and children with acute lymphoblastic leukaemia. J Pediatr Haematol Oncol. 2002;24:353–359. doi: 10.1097/00043426-200206000-00006. [DOI] [PubMed] [Google Scholar]
- 82.Park-Hah JO, Klementsdal B, Lyssa R, Choi KH, Aarbakke J. Thiopurine emthyltransferase activity in a Korean population sample of children. Clin Pharmacol Ther. 1996;60:68–74. doi: 10.1016/S0009-9236(96)90169-1. [DOI] [PubMed] [Google Scholar]
- 83.Collie-Duguid ESR, Pritchard SC, Powrie RH, Sludden J, Collier DA, McLeod HL. The frequency and distribution of thiopurine methyltransferase alleles in Caucasian and Asian populations. Pharmacogenetics. 1999;9:37–42. doi: 10.1097/00008571-199902000-00006. [DOI] [PubMed] [Google Scholar]
- 84.McLeod HL, Pritchard SC, Githhang J, Indalo A, Ameyaw M-M, Powrie RH, Booth L, Collie-Duguid ESR. Ethnic differences in thiopurine methyltransferase pharmacogenetics: evidence for allele specificity in Caucasian and Kenyan individuals. Pharmacogenetics. 1999;9:773–776. doi: 10.1097/00008571-199912000-00012. [DOI] [PubMed] [Google Scholar]
- 85.McLeod HL, Siva C. The thiopurine S-methyltransferase gene locus – implications for clinical pharmacogenomics. Pharmacogenetics. 2002;3:89–98. doi: 10.1517/14622416.3.1.89. [DOI] [PubMed] [Google Scholar]
- 86.Hon YY, Fessing MY, Pui C-H, Relling MV, Krynerski EY, Evans WE. Polymorphisms of the thiopurine S-methyltransferase gene in African-Americans. Hum Mol Genet. 1999;8:371–376. doi: 10.1093/hmg/8.2.371. [DOI] [PubMed] [Google Scholar]
- 87.Appell ML, Berg J, Duley J, Evans WE, Kennedy MA, Lennard L, Marinaki T, McLeod HL, Relling MV, Schaeffeler E, Schwab M, Weinshilboum R, Yeoh AEJ, McDonagh EM, Hebert JM, Klein TE, Coulthard SA. Nomenclature for alleles of the thiopurine methyltransferase (TPMT) gene. Pharmacogenet Genomics. 2013;23:242–248. doi: 10.1097/FPC.0b013e32835f1cc0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.TPMT Nomenclature Committee . TPMT alleles. Available at http://www.imh.liu.se/tpmtalleles?l=en (last accessed 17 September 2013)
- 89.Booth RA, Ansari MT, Loit E, Tricco AC, Weeks L, Doucette S, Skidmore B, Sears M, Sy R, Karsh J. Assessment of thiopurine S-methyltransferase activity in patients prescribed thiopurines: a systematic review. Ann Intern Med. 2011;154:814–823. doi: 10.7326/0003-4819-154-12-201106210-00009. [DOI] [PubMed] [Google Scholar]
- 90.Spire-Vayron de la Moureyre C, Debuysere H, Fazio F, Sergent E, Bernard C, Sabbagh N, Marez D, Lo Guidice JM, D'halluim J-C, Broly F. Characterization of a variable number tandem repeat region in the thiopurine S-methyltransferase gene promoter. Pharmacogenetics. 1999;9:189–198. [PubMed] [Google Scholar]
- 91.Yan L, Zhang S, Eiff B, Szumlanski CL, Powers M, O'Brien JF, Weinshilboum RM. Thiopurine methyltransferase polymorphic tandem repeat: genotype-phenotype correlation analysis. Clin Pharmacol Ther. 2000;68:210–219. doi: 10.1067/mcp.2000.108674. [DOI] [PubMed] [Google Scholar]
- 92.Ford L, Kampanis P, Berg J. Thiopurine S-methyltransferase genotype-phenotype concordance: used as a quality assurance tool to help control the phenotype assay. Ann Clin Biochem. 2009;46:152–154. doi: 10.1258/acb.2008.008167. [DOI] [PubMed] [Google Scholar]
- 93.Hindorf U, Appell ML. Genotyping should be considered the primary choice for pre-treatment evaluation of thiopurine methyltransferase function. J Crohn's Colitis. 2012;6:655–659. doi: 10.1016/j.crohns.2011.11.014. [DOI] [PubMed] [Google Scholar]
- 94.Fakoury M, Andreu-Gallien J, Mahr A, Medard Y, Azougagh S, Vilmer E, Jacqz-Aigrain E. Should TPMT genotype and activity be used to monitor 6-mercaptopurine treatment in children with acute lymphoblastic leukaemia? J Clin Pharm Ther. 2007;32:633–639. doi: 10.1111/j.1365-2710.2007.00858.x. [DOI] [PubMed] [Google Scholar]
- 95.van Egmond R, Chin P, Zhang M, Sies CW, Barclay ML. High TPMT enyzyme activity does not explain drug resistance due to preferential 6-methylmercaptopurine production in patients on thiopurine treatment. Aliment Pharmacol Ther. 2012;35:1181–1189. doi: 10.1111/j.1365-2036.2012.05084.x. [DOI] [PubMed] [Google Scholar]
- 96.Gardiner S, Gearry RB, Barclay ML, Begg EJ. Two cases of thiopurine methyltransferase (TPMT) deficiency – a lucky save and a near miss with azathioprine. Br J Clin Pharmacol. 2005;62:473–476. doi: 10.1111/j.1365-2125.2005.02474.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Marra CA, Esdaile JM, Anis AH. Practical pharmacogenetics: the cost effectiveness of screening for thiopurine s-methyltransferase polymorphisms in patients with rheumatological conditions treated with azathioprine. J Rheumatol. 2002;29:2507–2512. [PubMed] [Google Scholar]
- 98.Gauba V, Saldanha M, Vize C, Saleh GM. Thiopurine methyltransferase screening before azathioprine therapy. Br J Opthalmol. 2006;90:923–924. doi: 10.1136/bjo.2006.092700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Fargher EA, Tricker K, Newman W, Elliott R, Roberts SA, Shaffer JL, Bruce I, Payne K. Current use of pharmacogenetic testing: a national surveyof thiopurine methyltransferase testing prior to azathioprine prescription. J Clin Pharm Ther. 2007;32:187–195. doi: 10.1111/j.1365-2710.2007.00805.x. [DOI] [PubMed] [Google Scholar]
- 100.Roblin X, Oussalah A, Chevaux J-B, Sparrow M, Peyrin-Biroulet L. Use of thiopurine testing in the management of inflammatory bowel diseases in clinical practice: a worldwide survey of experts. Inflamm Bowel Dis. 2011;17:2480–2487. doi: 10.1002/ibd.21662. [DOI] [PubMed] [Google Scholar]
- 101.UK ALL2011 trial protocol , version 1.0, August 2011. United Kingdom trial for children and young adults with acute lymphoblastic leukaemia and lymphoma. International standard randomised controlled trial number (ISRCTN) 64515327, Section 7.12.2 and Appendix 21.
- 102.Higgs JE, Payne K, Roberts C, Newman WG. Are patients with intermediate TPMT activity at increased risk of myelosuppression when taking thiopurine mediactions? Pharmacogenomics. 2010;11:177–188. doi: 10.2217/pgs.09.155. [DOI] [PubMed] [Google Scholar]
- 103.Meggitt SJ, Gray JC, Reynolds NJ. Azathioprine dosed by thiopurine methyltransferase activity for moderate-to-severe atopic eczema; a double-blind, randomised controlled trial. Lancet. 2006;367:839–846. doi: 10.1016/S0140-6736(06)68340-2. [DOI] [PubMed] [Google Scholar]
- 104. British National Formulary online. Mercaptopurine, section 8.2.1. Available at http://www.medicinescomplete.com/mc/bnf/current/PHP5598-antiproliferative-immunosuppressants.htm#PHP5599 (last accessed 17 September 2013)
- 105.Anstey AV, Wakelin S, Reynolds NJ. Guidelines for prescribing azathioprine in dermatology. Br J Dermatol. 2004;151:1123–1132. doi: 10.1111/j.1365-2133.2004.06323.x. [DOI] [PubMed] [Google Scholar]
- 106.Meggitt SJ, Anstey AV, Mustapa MF, Reynolds NJ, Wakelin S. British Association of Dermatologists' guidelines for the safe and effective prescribing of azathioprine 2011. Br J Dermatol. 2011;165:711–734. doi: 10.1111/j.1365-2133.2011.10575.x. [DOI] [PubMed] [Google Scholar]
- 107.Chakravarty K, McDonald H, Pullar T, Taggart A, Chalmers R, Oliver S, Mooney J, Somerville M, Bosworth A, Kennedy T. BSR/BHPR guideline for disease-modifying anti-rheumatic drug (DMARD) therapy in consultation with the British Association of Dermatologists. Rheumatology. 2008;47:924–925. doi: 10.1093/rheumatology/kel216a. [DOI] [PubMed] [Google Scholar]
- 108.Gleeson D, Heneghan MA. British Society of Gastroenterology (BSG) guidelines for management of autoimmune hepatitis. Gut. 2012;60:1611–1629. doi: 10.1136/gut.2010.235259. [DOI] [PubMed] [Google Scholar]
- 109.Manns MP, Czaja AJ, Gorham JD, Krawitt EL, Mieli-Vergani G, Vergani D, Vierling JM. AASLD Practice Guidelines. Diagnosis and management of autoimmune hepatitis. Hepatology. 2010;51:2193–2213. doi: 10.1002/hep.23584. [DOI] [PubMed] [Google Scholar]
- 110.Carter MJ, Lobo AJ, Travis SPL. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53:v1–16. doi: 10.1136/gut.2004.043372. (Suppl. 5) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Mowat C, Cole A, Windsor A, Ahmad T, Arnott I, Driscoll R, Mitton S, Orchard T, Rutter M, Younge L, Lees C, Ho G, Satsangi J, Bloom S. Guidelines for the management of inflammatory bowel disease in adults. Gut. 2011;60:571–607. doi: 10.1136/gut.2010.224154. [DOI] [PubMed] [Google Scholar]
- 112.Anon. TPMT testing before azathioprine therapy? Drug Ther Bull. 2009;47:9–12. doi: 10.1136/dtb.2008.12.0033. [DOI] [PubMed] [Google Scholar]
- 113.Payne K, Newman W, Fargher E, Tricker K, Bruce IN, Ollier WER. TPMT testing in rheumatology: any better than routine monitoring? Rheumatology. 2007;46:727–729. doi: 10.1093/rheumatology/kel427. [DOI] [PubMed] [Google Scholar]
- 114.Newman WG, Payne K, Tricker K, Roberts SA, Fargher E, Pushpakom S, Alder JE, Sidgwick GP, Payne D, Elliott RA, Heise M, Elles R, Ramsden SC, Andrews J, Houston JB, Qasim F, Shaffer J, Griffiths CEM, Ray DW, Bruce I, Ollier WER. A pragmatic randomised controlled trial of thiopurine methyltransferase genotyping prior to azathioprine treatment: the TARGET study. Pharmacogenomics. 2011;12:815–826. doi: 10.2217/pgs.11.32. [DOI] [PubMed] [Google Scholar]


