Abstract
BEN/SC1/DM-GRASP is a membrane glycoprotein of the immunoglobulin superfamily isolated in the chick by several groups, including ours. Its expression is strictly developmentally regulated in several cell types of the nervous and hemopoietic systems and in certain epithelia. Each of these cell types expresses isoforms of BEN which differ by their level of N-glycosylation and by the presence or absence of the HNK-1 carbohydrate epitope. In the present work, the influence of glycosylation on BEN homophilic binding properties was investigated by two in vitro assays. First, each BEN isoform was covalently coupled to microspheres carrying different fluorescent dyes and an aggregation test was performed. We found that homophilic aggregates form indifferently between the same or different BEN isoforms, showing that glycosylation does not affect BEN homophilic binding properties. This was confirmed in the second test, where the BEN-coated microspheres bound to the neurites of BEN- expressing neurons, irrespective of the isoform considered. The transient expression of the BEN antigen on hemopoietic progenitors prompted us to see whether it might play a role in their proliferation and differentiation. When added to hemopoietic progenitor cells in an in vitro colony formation assay anti-BEN immunoglobulin strongly inhibited myeloid, but not erythroid, colony formation although both types of precursors express the molecule.
Full text
PDF
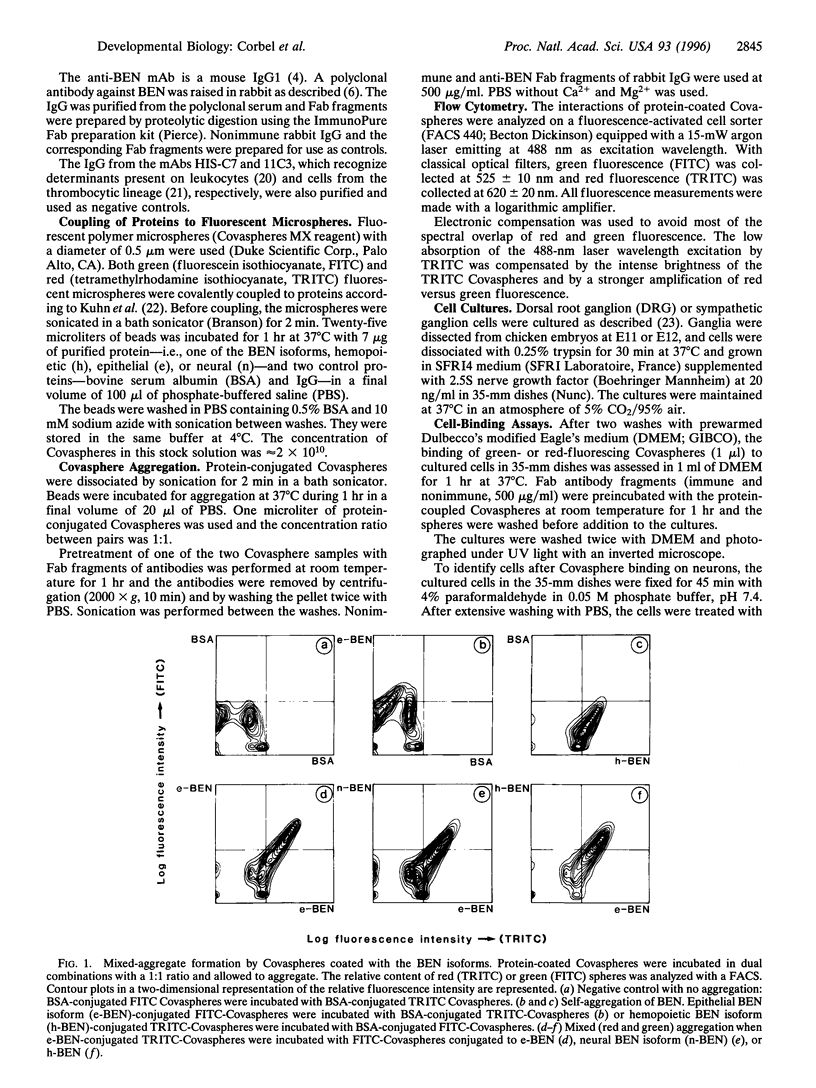
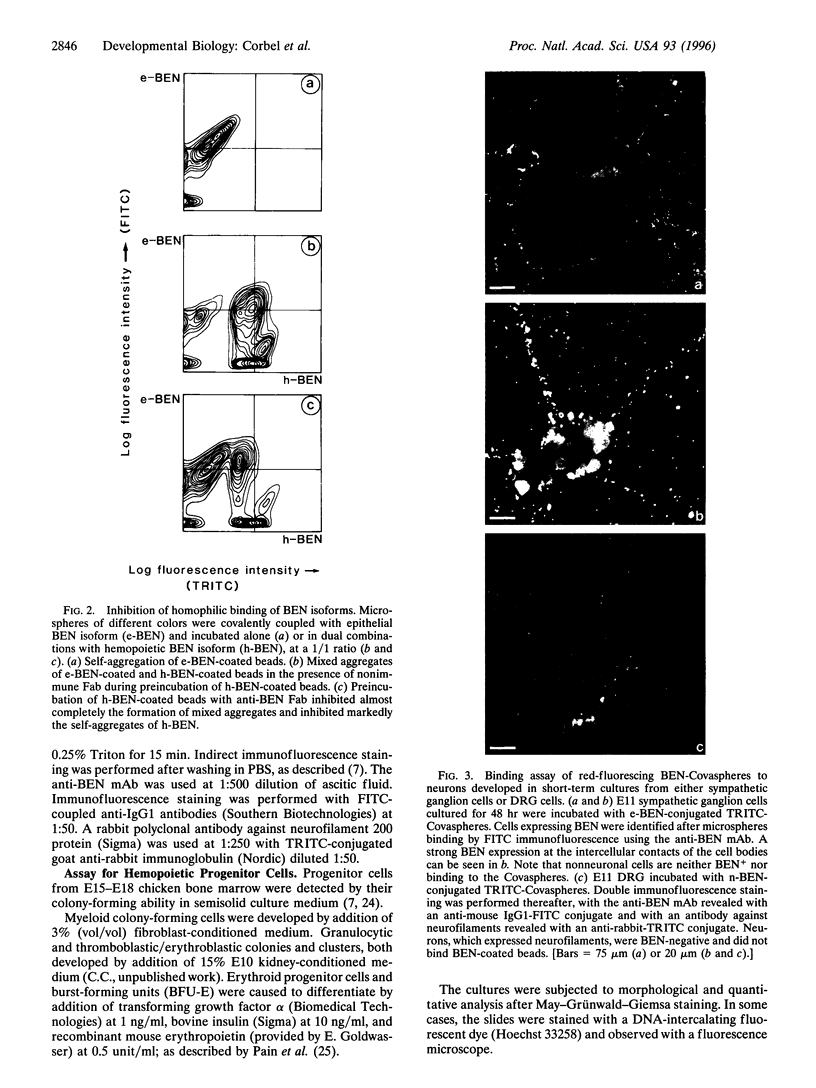



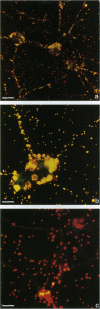
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barbu M., Pourquié O., Vaigot P., Gateau G., Smith J. Phenotypic plasticity of avian embryonic sympathetic neurons grown in a chemically defined medium: direct evidence for noradrenergic and cholinergic properties in the same neurons. J Neurosci Res. 1992 Jul;32(3):350–362. doi: 10.1002/jnr.490320307. [DOI] [PubMed] [Google Scholar]
- Bowen M. A., Patel D. D., Li X., Modrell B., Malacko A. R., Wang W. C., Marquardt H., Neubauer M., Pesando J. M., Francke U. Cloning, mapping, and characterization of activated leukocyte-cell adhesion molecule (ALCAM), a CD6 ligand. J Exp Med. 1995 Jun 1;181(6):2213–2220. doi: 10.1084/jem.181.6.2213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns F. R., von Kannen S., Guy L., Raper J. A., Kamholz J., Chang S. DM-GRASP, a novel immunoglobulin superfamily axonal surface protein that supports neurite extension. Neuron. 1991 Aug;7(2):209–220. doi: 10.1016/0896-6273(91)90259-3. [DOI] [PubMed] [Google Scholar]
- Chédotal A., Pourquié O., Sotelo C. Initial tract formation in the brain of the chick embryo: selective expression of the BEN/SC1/DM-GRASP cell adhesion molecule. Eur J Neurosci. 1995 Feb 1;7(2):198–212. doi: 10.1111/j.1460-9568.1995.tb01056.x. [DOI] [PubMed] [Google Scholar]
- Corbel C., Bluestein H. G., Pourquie O., Vaigot P., Le Douarin N. M. An antigen expressed by avian neuronal cells is also expressed by activated T lymphocytes. Cell Immunol. 1992 Apr 15;141(1):99–110. doi: 10.1016/0008-8749(92)90130-h. [DOI] [PubMed] [Google Scholar]
- Corbel C., Cormier F., Pourquie O., Bluestein H. G. BEN, a novel surface molecule of the immunoglobulin superfamily on avian hemopoietic progenitor cells shared with neural cells. Exp Cell Res. 1992 Nov;203(1):91–99. doi: 10.1016/0014-4827(92)90043-8. [DOI] [PubMed] [Google Scholar]
- Cormier F., Dieterlen-Lièvre F. The wall of the chick embryo aorta harbours M-CFC, G-CFC, GM-CFC and BFU-E. Development. 1988 Feb;102(2):279–285. doi: 10.1242/dev.102.2.279. [DOI] [PubMed] [Google Scholar]
- Jeurissen S. H., Janse E. M., Ekino S., Nieuwenhuis P., Koch G., De Boer G. F. Monoclonal antibodies as probes for defining cellular subsets in the bone marrow, thymus, bursa of fabricius, and spleen of the chicken. Vet Immunol Immunopathol. 1988 Oct;19(3-4):225–238. doi: 10.1016/0165-2427(88)90110-9. [DOI] [PubMed] [Google Scholar]
- Kanki J. P., Chang S., Kuwada J. Y. The molecular cloning and characterization of potential chick DM-GRASP homologs in zebrafish and mouse. J Neurobiol. 1994 Jul;25(7):831–845. doi: 10.1002/neu.480250708. [DOI] [PubMed] [Google Scholar]
- Kuhn T. B., Stoeckli E. T., Condrau M. A., Rathjen F. G., Sonderegger P. Neurite outgrowth on immobilized axonin-1 is mediated by a heterophilic interaction with L1(G4). J Cell Biol. 1991 Nov;115(4):1113–1126. doi: 10.1083/jcb.115.4.1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Künemund V., Jungalwala F. B., Fischer G., Chou D. K., Keilhauer G., Schachner M. The L2/HNK-1 carbohydrate of neural cell adhesion molecules is involved in cell interactions. J Cell Biol. 1988 Jan;106(1):213–223. doi: 10.1083/jcb.106.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lacoste-Eleaume A. S., Bleux C., Quéré P., Coudert F., Corbel C., Kanellopoulos-Langevin C. Biochemical and functional characterization of an avian homolog of the integrin GPIIb-IIIa present on chicken thrombocytes. Exp Cell Res. 1994 Jul;213(1):198–209. doi: 10.1006/excr.1994.1191. [DOI] [PubMed] [Google Scholar]
- Laessing U., Giordano S., Stecher B., Lottspeich F., Stuermer C. A. Molecular characterization of fish neurolin: a growth-associated cell surface protein and member of the immunoglobulin superfamily in the fish retinotectal system with similarities to chick protein DM-GRASP/SC-1/BEN. Differentiation. 1994 Apr;56(1-2):21–29. doi: 10.1046/j.1432-0436.1994.56120021.x. [DOI] [PubMed] [Google Scholar]
- Lehmann J. M., Riethmüller G., Johnson J. P. MUC18, a marker of tumor progression in human melanoma, shows sequence similarity to the neural cell adhesion molecules of the immunoglobulin superfamily. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9891–9895. doi: 10.1073/pnas.86.24.9891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald T. P., Sullivan P. S. Megakaryocytic and erythrocytic cell lines share a common precursor cell. Exp Hematol. 1993 Sep;21(10):1316–1320. [PubMed] [Google Scholar]
- Pain B., Woods C. M., Saez J., Flickinger T., Raines M., Peyrol S., Moscovici C., Moscovici M. G., Kung H. J., Jurdic P. EGF-R as a hemopoietic growth factor receptor: the c-erbB product is present in chicken erythrocytic progenitors and controls their self-renewal. Cell. 1991 Apr 5;65(1):37–46. doi: 10.1016/0092-8674(91)90405-n. [DOI] [PubMed] [Google Scholar]
- Paschke K. A., Lottspeich F., Stuermer C. A. Neurolin, a cell surface glycoprotein on growing retinal axons in the goldfish visual system, is reexpressed during retinal axonal regeneration. J Cell Biol. 1992 May;117(4):863–875. doi: 10.1083/jcb.117.4.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peduzzi J. D., Irwin M. H., Geisert E. E., Jr Distribution and characteristics of a 90 kDa protein, KG-CAM, in the rat CNS. Brain Res. 1994 Mar 21;640(1-2):296–307. doi: 10.1016/0006-8993(94)91885-6. [DOI] [PubMed] [Google Scholar]
- Pourquié O., Coltey M., Thomas J. L., Le Douarin N. M. A widely distributed antigen developmentally regulated in the nervous system. Development. 1990 Aug;109(4):743–752. doi: 10.1242/dev.109.4.743. [DOI] [PubMed] [Google Scholar]
- Pourquié O., Corbel C., Le Caer J. P., Rossier J., Le Douarin N. M. BEN, a surface glycoprotein of the immunoglobulin superfamily, is expressed in a variety of developing systems. Proc Natl Acad Sci U S A. 1992 Jun 15;89(12):5261–5265. doi: 10.1073/pnas.89.12.5261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pourquié O., Hallonet M. E., Le Douarin N. M. Association of BEN glycoprotein expression with climbing fiber axonogenesis in the avian cerebellum. J Neurosci. 1992 Apr;12(4):1548–1557. doi: 10.1523/JNEUROSCI.12-04-01548.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince J. T., Nishiyama A., Healy P. A., Beasley L., Stallcup W. B. Expression of the F84.1 glycoprotein in the spinal cord and cranial nerves of the developing rat. Brain Res Dev Brain Res. 1992 Aug 21;68(2):193–201. doi: 10.1016/0165-3806(92)90061-z. [DOI] [PubMed] [Google Scholar]
- Ramos R. G., Igloi G. L., Lichte B., Baumann U., Maier D., Schneider T., Brandstätter J. H., Fröhlich A., Fischbach K. F. The irregular chiasm C-roughest locus of Drosophila, which affects axonal projections and programmed cell death, encodes a novel immunoglobulin-like protein. Genes Dev. 1993 Dec;7(12B):2533–2547. doi: 10.1101/gad.7.12b.2533. [DOI] [PubMed] [Google Scholar]
- Taira E., Takaha N., Taniura H., Kim C. H., Miki N. Molecular cloning and functional expression of gicerin, a novel cell adhesion molecule that binds to neurite outgrowth factor. Neuron. 1994 Apr;12(4):861–872. doi: 10.1016/0896-6273(94)90338-7. [DOI] [PubMed] [Google Scholar]
- Tanaka H., Matsui T., Agata A., Tomura M., Kubota I., McFarland K. C., Kohr B., Lee A., Phillips H. S., Shelton D. L. Molecular cloning and expression of a novel adhesion molecule, SC1. Neuron. 1991 Oct;7(4):535–545. doi: 10.1016/0896-6273(91)90366-8. [DOI] [PubMed] [Google Scholar]
- el-Deeb S., Thompson S. C., Covault J. Characterization of a cell surface adhesion molecule expressed by a subset of developing chick neurons. Dev Biol. 1992 Jan;149(1):213–227. doi: 10.1016/0012-1606(92)90278-o. [DOI] [PubMed] [Google Scholar]